Seed Germination Trials and Ex Situ Conservation of Local Prioritized Endemic Plants of Crete (Greece) with Commercial Interest
Abstract
1. Introduction
2. Materials and Methods
2.1. Focal Local Endemic Plants of Crete (Greece)
2.2. Seed Collections
2.3. Seed Germination Trials
2.4. Experimental Design and Statistical Analysis
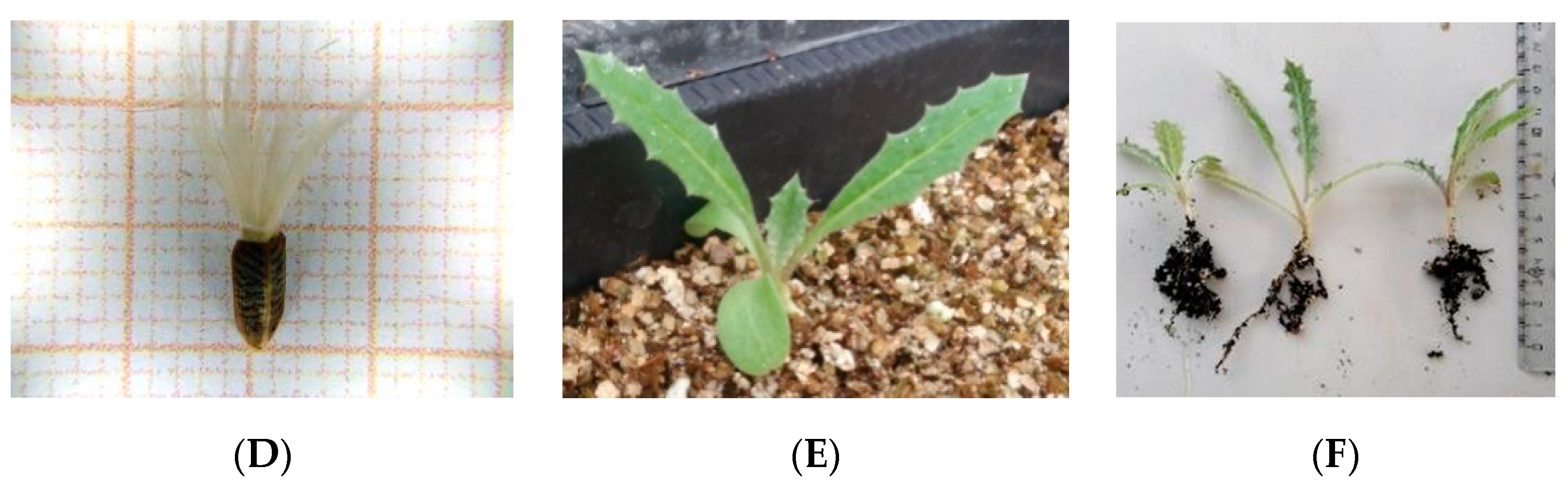
3. Results
3.1. Seed Germination
4. Discussion
4.1. From Seed Germination to Ex Situ Conservation and Sustainable Exploitation
4.2. Re-Evaluation of Feasibility and Readiness Timescale for Sustainable Exploitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maxted, N.; Ford-Lloyd, B.V.; Hawkes, J.G. Complementary conservation strategies. In Plant Genetic Conservation; Maxted, N., Ford-Lloyd, B.V., Hawkes, J.G., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 15–39. [Google Scholar] [CrossRef]
- Krigas, N.; Menteli, V.; Vokou, D. Analysis of the ex situ conservation of the Greek endemic flora at national European and global scales and of its effectiveness in meeting GSPC Target 8. Plant Biosyst. 2016, 150, 573–582. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-facilitated effective propagation protocols of the Endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex situ conservation needs and its future sustainable exploitation as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean Coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the Vulnerable Cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12, 94. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Maloupa, E.; Tsoktouridis, G. Vegetative propagation and ex-situ conservation of Acantholimon androsaceum and Limonium chersonesum, two promising local endemics of Crete (Greece) available for floricultural and pharmaceutical sustainable exploitation. Not. Bot. Hort. Agrobot. Cluj-Napoca 2021, 49, 12261. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; El Haissoufi, M.; Pipinis, E.; Kostas, S.; Libiad, M.; Khabbach, A.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; et al. GIS-Facilitated seed germination and multifaceted evaluation of the Endangered Abies marocana Trab. (Pinaceae) enabling conservation and sustainable exploitation. Plants 2021, 10, 2606. [Google Scholar] [CrossRef]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; et al. Facilitating conservation and bridging gaps for the sustainable exploitation of the Tunisian local endemic Plant Marrubium aschersonii (Lamiaceae). Sustainability 2022, 14, 1637. [Google Scholar] [CrossRef]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Bourgou, S.; Ben Haj Jilani, I.; Ben Othman, W.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; et al. DNA barcoding, GIS-facilitated seed germination and pilot cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian local endemic with potential medicinal and ornamental value. Biology 2022, 11, 462. [Google Scholar] [CrossRef]
- Mohammad Esmaeili, M.; Sattarian, A.; Bonis, A.; Bouzillé, J. Ecology of seed dormancy and germination of Carex divisa Huds.: Effects of stratification, temperature and salinity. Intern. J. Plant Product. 2012, 3, 27–40. [Google Scholar] [CrossRef]
- Zarghani, H.; Mijani, S.; Nasrabadi, S.E.; Ghias-Abadi, M.; Khorramdel, S.; Azimi, R. Temperature effects on the seed germination of some perennial and annual species of Asteraceae family. Plant Breed. Seed Sci. 2014, 69, 3–14. [Google Scholar] [CrossRef]
- Asha Rani, N.S.; Prasad, M.P. In-vitro studies on the germination of Atropa belladonna seeds under different conditions. Intern. J. Sci. Res. 2014, 3, 552–555. Available online: https://www.ijsr.net/get_abstract.php?paper_id=OCT14184 (accessed on 20 May 2022).
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Statwick, J.M. Germination pretreatments to break hard-seed dormancy in Astragalus cicer L. (Fabaceae). Peer J. 2016, 4, e2621. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, Z.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2019, 249, 291–303. [Google Scholar] [CrossRef]
- Rivera, D.; Obón, C.; Heinrich, M.; Inocencio, C.; Verde, A.; Fajardo, J. Gathered Mediterranean food plants—Ethnobotanical investigations and historical development. In Local Mediterranean Food Plants and Nutraceuticals; Heinrich, M., Müller, W.E., Galli, C., Eds.; Karger Publishing: Basel, Switzerland, 2006; Volume 59, pp. 18–74. [Google Scholar]
- Baldermann, S.; Blagojevic, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Ngwene, B.; Norkeweit, J.; Schröter, A.; Schröter, D.; et al. Are neglected plants the food for the future? Crit. Rev. Plant Sci. 2016, 35, 106–119. [Google Scholar] [CrossRef]
- Scariot, V.; Seglie, L.; Gaino, W.; Devecchi, M. Evaluation of European native bluebells for sustainable floriculture. Acta Hortic. 2012, 937, 273–280. [Google Scholar] [CrossRef]
- Omotayo, A.O.; Ijatuyi, E.J.; Ogunniyi, A.I.; Aremu, A.O. Exploring the resource value of Transvaal Red Milk Wood (Mimusops zeyheri) for food security and sustainability: An appraisal of existing evidence. Plants 2020, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Georghiou, K.; Delipetrou, P. Patterns and traits of the endemic plants of Greece. Bot. J. Linn. Soc. 2010, 162, 130–422. [Google Scholar] [CrossRef]
- Menteli, V.; Krigas, N.; Avramakis, E.; Turland, N.; Vokou, D. Endemic plants of Crete in electronic trade and wildlife tourism: Current patterns and implications for conservation. J. Biol. Res. Thessalon. 2019, 26, 10. [Google Scholar] [CrossRef] [PubMed]
- Kloukina, C.; Tomou, E.M.; Krigas, N.; Sarropoulou, V.; Madesis, P.; Maloupa, E.; Skaltsa, H. Non-polar secondary metabolites and essential oil of ex-situ propagated and cultivated Sideritis syriaca L. subsp. syriaca (Lamiaceae) with consolidated identity (DNA Barcoding): Towards a potential new industrial crop. Ind. Crop. Prod. 2020, 158, 112957. [Google Scholar] [CrossRef]
- Fournaraki, C. Conservation of Threatened Plants of Crete—Seed Ecology, Operation and Management of a Gene Bank. Ph.D. Thesis, National and Kapodistrian University of Athens, Faculty of Biology, Department of Botany, Athens, Greece, 2010. (In Greek with English Abstract). [Google Scholar]
- Geneve, L.R. Some common misconceptions about seed dormancy. Comb. Proc. Int. Plant Propag. Soc. 2005, 55, 9–12. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Gkika, P.I.; Krigas, N.; Menexes, G.; Eleftherohorinos, I.E.; Maloupa, E. Effect of temperature and light on seed germination of Erysimum naense and Erysimum krendlii. Cent. Eur. J. Biol. 2013, 8, 1194–1203. [Google Scholar] [CrossRef][Green Version]
- Grigoriadou, K.; Krigas, N.; Maloupa, E. GIS-facilitated ex situ conservation of the rare Greek endemic Campanula incurva Aucher: Seed germination requirements and effect of growth regulators on in vitro proliferation and rooting. Plant Biosyst. 2014, 148, 1169–1177. [Google Scholar] [CrossRef]
- Krigas, Ν.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Krigas, N.; Karapatzak, E.; Panagiotidou, M.; Sarropoulou, V.; Samartza, I.; Karydas, A.; Damianidis, C.K.; Najdovski, B.; Teofilovski, A.; Mandzukovski, D.; et al. Prioritizing plants around the cross-border area of Greece and the Republic of North Macedonia: Integrated conservation actions and sustainable exploitation potential. Diversity 2022, 14, 570. [Google Scholar] [CrossRef]
- Strid, A. Atlas of the Aegean Flora Part 1 (Text & Plates) & Part 2 (Maps), 1st ed.; Botanic Garden and Botanical Museum and Freie Universität: Berlin, Germany, 2016; Volume 33. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Ahmad, N.; Hafeez, K. Thermal hardening: A new seed vigor enhancement tool in rice. J. Integr. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]
- Probert, R.J. The role of temperature in the regulation of seed dormancy and germination. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 261–292. [Google Scholar] [CrossRef]
- Farina, E.; Dalla Guda, C. Campanula. Guida Pratica Alla Coltivazione; Ace International: Vernasca (PC), Italy, 2006; pp. 240. [Google Scholar]
- Joung, Y.H.; Liao, M.S.; Roh, M.S.; Kamo, K.; Song, J.S. In vitro propagation of Campanula glomerata ‘Aucalis’ from leaf blade explants. Sci. Hortic. 2002, 92, 137–146. [Google Scholar] [CrossRef]
- Blionis, G.J.; Vokou, D. Reproductive attributes of Campanula populations from Mt Olympos, Greece. Plant Ecol. 2005, 178, 77–88. [Google Scholar] [CrossRef]
- Mork, E.K.; Sriskandarajah, S.; Serek, M. Influence of seed germination conditions on regenerative ability in Campanula carpatica. Europ. J. Hortic. Sci. 2005, 70, 173–176. Available online: https://www.pubhort.org/ejhs/2005/file_29850.pdf (accessed on 13 September 2022).
- Tanaka, N.; Yamada, Y.; Shimomura, K.; Ishimaru, K. Polyacetilenes in tissue cultures of Campanula glomerata. Plant Tissue Cult. Lett. 1996, 13, 215–217. [Google Scholar] [CrossRef][Green Version]
- Sriskandarajah, S.; Frello, S.; Serek, M. Induction of adventitious shoots in vitro in Campanula carpatica. Plant Cell Tissue Organ Cult. 2001, 67, 295–298. [Google Scholar] [CrossRef]
- Koutsovoulou, K.; Daws, M.I.; Thanos, C.A. Campanulaceae: A family with small seeds that require light for germination. Ann. Bot. 2014, 113, 135–143. [Google Scholar] [CrossRef]
- Jankowska-Blaszczuk, M.; Daws, M.I. Impact of red:far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Funct. Ecol. 2007, 21, 1055–1062. [Google Scholar] [CrossRef]
- Karousou, R.; Kokkini, S.; Bessiere, J.M.; Vokou, D. Calamintha cretica (Lamiaceae), a Cretan endemic: Distribution and essential oil composition. Nord. J. Bot. 1996, 16, 247–251. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hasegawa, Y.; Shirota, K.; Noboru, S.N.; Nakamura, T.; Chomnawang, M.T.; Sato, K. Differentiation inducing effect of piperitenone oxide, a fragrant ingredient of spearmint (Mentha spicata), but not carvone and menthol, against human colon cancer cells. J. Funct. Foods 2014, 8C, 62–67. [Google Scholar] [CrossRef]
- Caneva, G.; Kumbaric, A.; Savo, V.; Casalini, R. Ecological approach in selecting extensive green roof plants: A data-set of Mediterranean plants. Plant Biosyst. 2013, 149, 374–383. [Google Scholar] [CrossRef]
- Casalini, R.; Bartoli, F.; Caneva, G. Investigation of seed germination of twelve Mediterranean wildflowers for evaluating their potential use on extensive green roofs. Acta Hortic. 2017, 1189, 263–266. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. Seed germination, micropropagation from adult and juvenile origin explants and address of hyperhydricity of the Cretan endemic herb Calamintha cretica. Not. Bot. Hort. Agrobot. Cluj Napoca 2020, 48, 1504–1518. [Google Scholar] [CrossRef]
- European Union Herbal Monograph on Origanum majorana L., Herba. European Medicines Agency, Committee on Herbal Medicinal Products, EMA/HMPC/166517/2015. 2 February 2016. Available online: https://www.ema.europa.eu/en (accessed on 15 September 2022).
- Markaki, E. Out of the Place (Ex-Situ) Conservation of Endemic Plants of Crete. Graduate Dissertation, Higher Technological Educational Institution of Crete, Faculty of Agricultural Technology, Department of Plant Production, Heraklion, Greece, 2006. (In Greek). [Google Scholar]
- SID-KEW (Seed Information Database-Royal Botanic Gardens, Kew), Wakehurst Place, Kew Gardens, 592 London, UK.
- Özkum, D. In vitro shoot regeneration of oregano (Origanum minutiflorum O. Schwarz & Davis). Hacet. J. Biol. Chem. 2007, 35, 97–100. [Google Scholar]
- Yildirim, M.U. Micropropagation of Origanum acutidens (Hand.-Mazz.) Ietsw. using stem node explants. Sci. World J. 2013, 2013, 276464. [Google Scholar] [CrossRef]
- Liopa-Tsakalidi, A.; Zakynthinos, G.; Varzakas, T.; Xynias, I.N. Effect of NaCl and GA3 on seed germination and seedling growth of eleven medicinal and aromatic crops. J. Med. Plants Res. 2011, 5, 4065–4073. [Google Scholar] [CrossRef]
- Laghmouchi, Y.; Bouyahya, A.; Senhaji, N.S.; Abrini, J. Effect of temperature, salt stress and pH on seed germination of medicinal plant Origanum compactum. Biocatal. Agric. Biotechnol. 2017, 10, 156–160. [Google Scholar] [CrossRef]
- Kadis, C.C.; Georghiou, K. The germination physiology of the endangered plants of Cyprus, Alyssum akamasicum and Origanum cordifolium. In Proceedings of the Fourth International Workshop on Seeds: Basic and Applied Aspects of Seed Biology, Angers, France, 20–24 July 1992; Volume 2, pp. 461–465. [Google Scholar]
- Thanos, C.A. Physiology of seed germination in marjoram (Origanum majorana L.). ISAFA Comun. Di Ric. 2000, 2, 31–36. [Google Scholar]
- Farashah, H.D.; Afshari, R.T.; Sharifzadeh, F.; Chavoshinasab, S. Germination improvement and α-amylase and β-1,3-glucanase activity in dormant and non-dormant seeds of Oregano (Origanum vulgare). Aust. J. Crop Sci. 2011, 5, 421–427. [Google Scholar]
- Radojević, L.J.; Ćalić-Dragosavac, D.; Špirić, J.; Stevanović, B.; Stevanović, V. In vitro propagation of Dianthus ciliatus ssp. dalmaticus and D. giganteus ssp. croaticus (Caryophyllaceae) from stem segment cultures. Bot. Serb. 2010, 34, 153–161. [Google Scholar]
- Radojević, L.J. Application of in vitro Culture in Flower Production: State and Possibilities of Floriculture Development in Serbia. In Proceedings of the Landscape Horticulture Seminar, Faculty of Forestry, Belgrade, Serbia, 8–9 February 2007; pp. 46–48. (In Serbian). [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T., Jr.; Geneve, R.L. Hartmann and Kester’s Plant Propagation: Principles and Practices, 7th ed.; Prentice Hall Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Papafotiou, M.; Stragas, J. Seed germination and in vitro propagation of Dianthus fruticosus L. Acta Hortic. 2009, 813, 481–484. [Google Scholar] [CrossRef]
- Kootenay Local Agricultural Society. Perennial Seed Germination Information. 2008. Available online: https://docplayer.net/46736765-Perennial-seed-germination-information-kootenay-local-agricultural-society-2008.html (accessed on 30 August 2022).
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar] [CrossRef]
- Alsos, I.G.; Muller, E.; Eidesen, P.B. Germinating seeds or bulbils in 87 of 113 tested Arctic species indicate potential for ex situ seed bank storage. Polar Biol. 2013, 36, 819–830. [Google Scholar] [CrossRef]
- Murray, D.F.; Kelso, S. Chromosome numbers and notes on the taxonomy of selected Alaskan vascular plants. Rhodora 1997, 99, 33–55. Available online: http://www.jstor.org/stable/23313280 (accessed on 20 October 2022).
- Baskin, J.M.; Baskin, C.C. Germination eco-physiology of Draba verna. Bull. Torrey Bot. Club 1970, 97, 209–216. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Effects of relative humidity on after ripening and viability in seeds of the winter annual Draba verna. Bot. Gaz. 1979, 140, 284–287. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The light factor in the germination ecology of Draba verna. Am. J. Bot. 1972, 57, 756–759. [Google Scholar] [CrossRef]
- Csonthos, P.; Tamas, J.; Balogh, L. Thousand-seed weight records of species from the flora of Hungary, II. Dicotyledonopsida. Stud. Bot. Hung. 2007, 38, 179–189. [Google Scholar]
- Cerabolini, B.; Ceriani, R.M.; Caccianiga, M.; De Andreis, R.; Raimondi, B. Seed size, shape and persistence in soil: A test on Italian flora from Alps to Mediterranean coasts. Seed Sci. Res. 2003, 13, 75–85. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Stampfli, A.; Newbery, D.M. Dispersal and microsite limitation in an abandoned calcareous grassland of the southern Prealps. Folia Geobot. 2000, 35, 125–141. [Google Scholar] [CrossRef]
- Frischie, S.; Fernandez-Pascual, E.; Ramirez, C.G.; Toorop, P.; Gonzalez, M.H.; Jimenez-Alfaro, B. Hydrothermal thresholds for seed germination in winter annual forbs from old-field Mediterranean landscapes. Plant Biol. 2018, 21, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Cavers, P.B. Interpopulation variation in germination responses of Scotch thistle, Onopordum acanthium L., to various concentrations of GA3, KNO3, and NaHCO3. Can. J. Bot. 2000, 78, 1156–1163. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B.; Bernards, M.A. Seed bank dynamics of Onopordum acanthium: Emergence patterns and chemical attributes. J. Ecol. 2002, 90, 672–683. [Google Scholar] [CrossRef]
- Wieneke, S.; Prati, D.; Brandl, R.; Stocklin, J.; Auge, H. Genetic variation in Sanguisorba minor after 6 years in situ selection under elevated CO2. Glob. Chang. Biol. 2004, 10, 1389–1401. [Google Scholar] [CrossRef]
- Monsen, S.B. Selection of plants for fire suppression on semiarid sites. In Proceedings—Ecology and Management of Annual Rangelands, Gen. Tech. Rep. INT-GTR-313, Boise, Idaho, 18–22 May 1992; Monsen, S.B., Kitchen, S.G., Eds.; U.S. Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1994; pp. 363–373. [Google Scholar]
- Shaw, N.L. Production and use of planting stock. In Restoring Western Ranges and Wildlands; Monsen, S.B., Stevens, R., Shaw, N.L., Eds.; Gen. Tech. Rep. RMRS-GTR-136; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004; Volume 3, pp. 745–768. [Google Scholar]
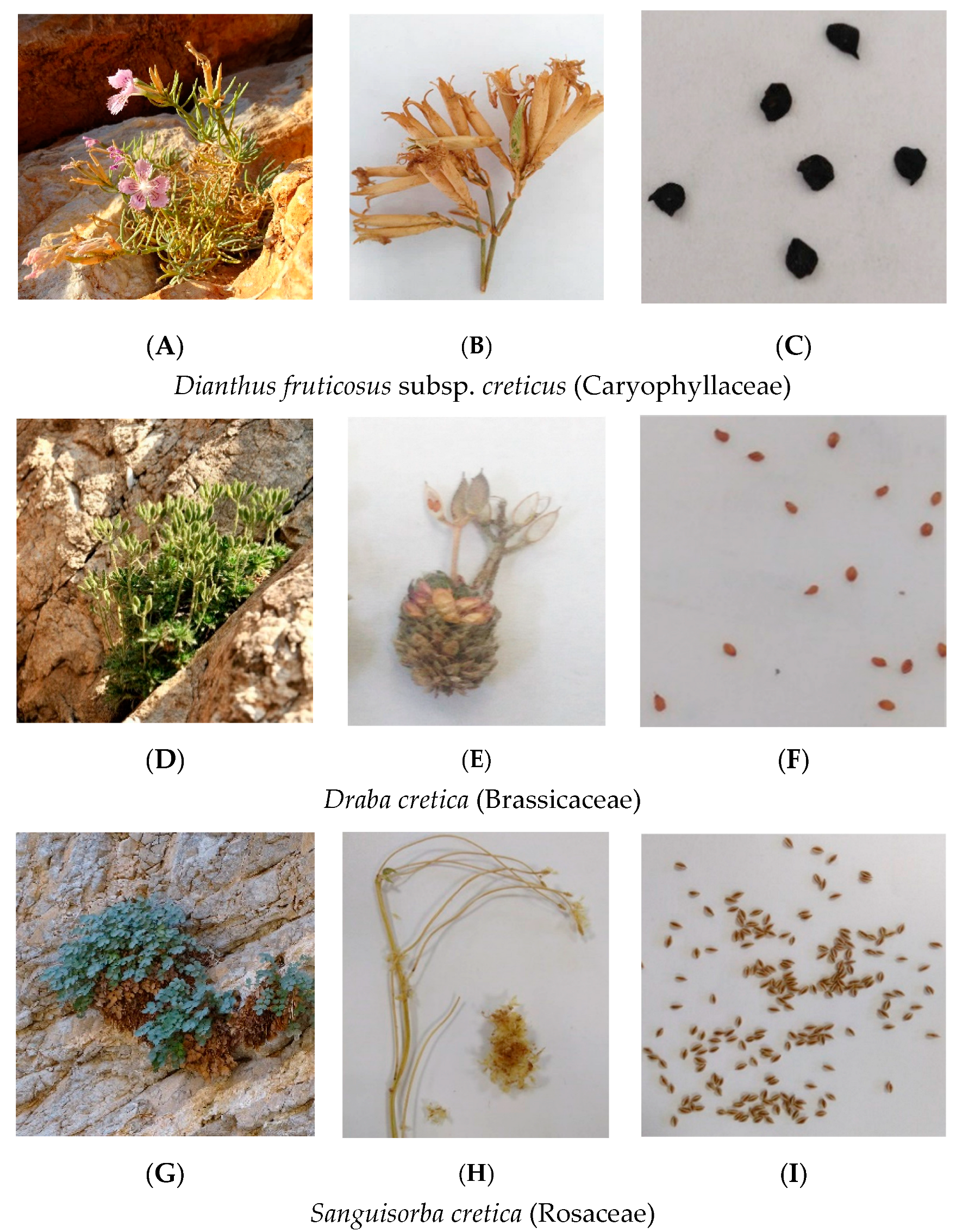



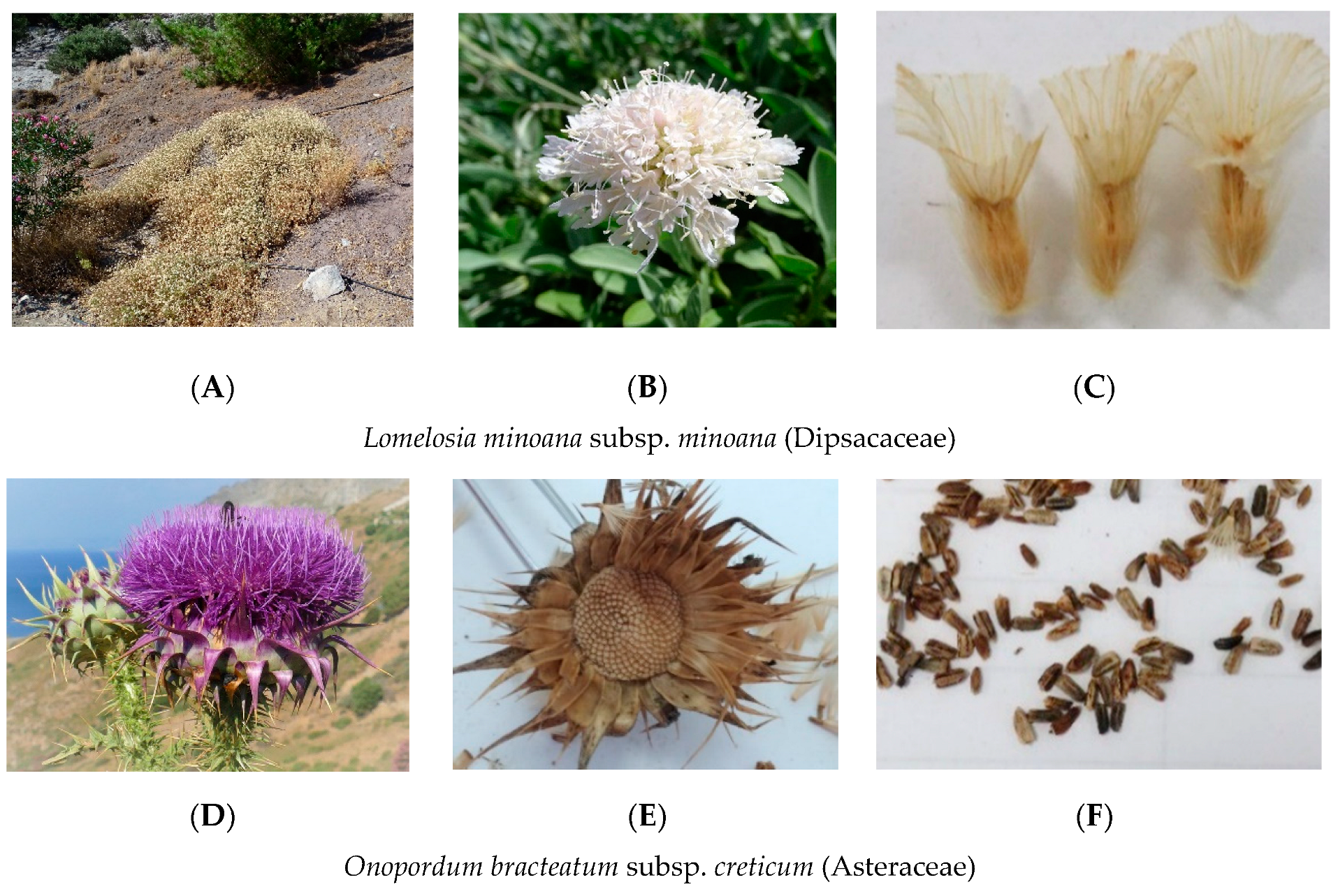
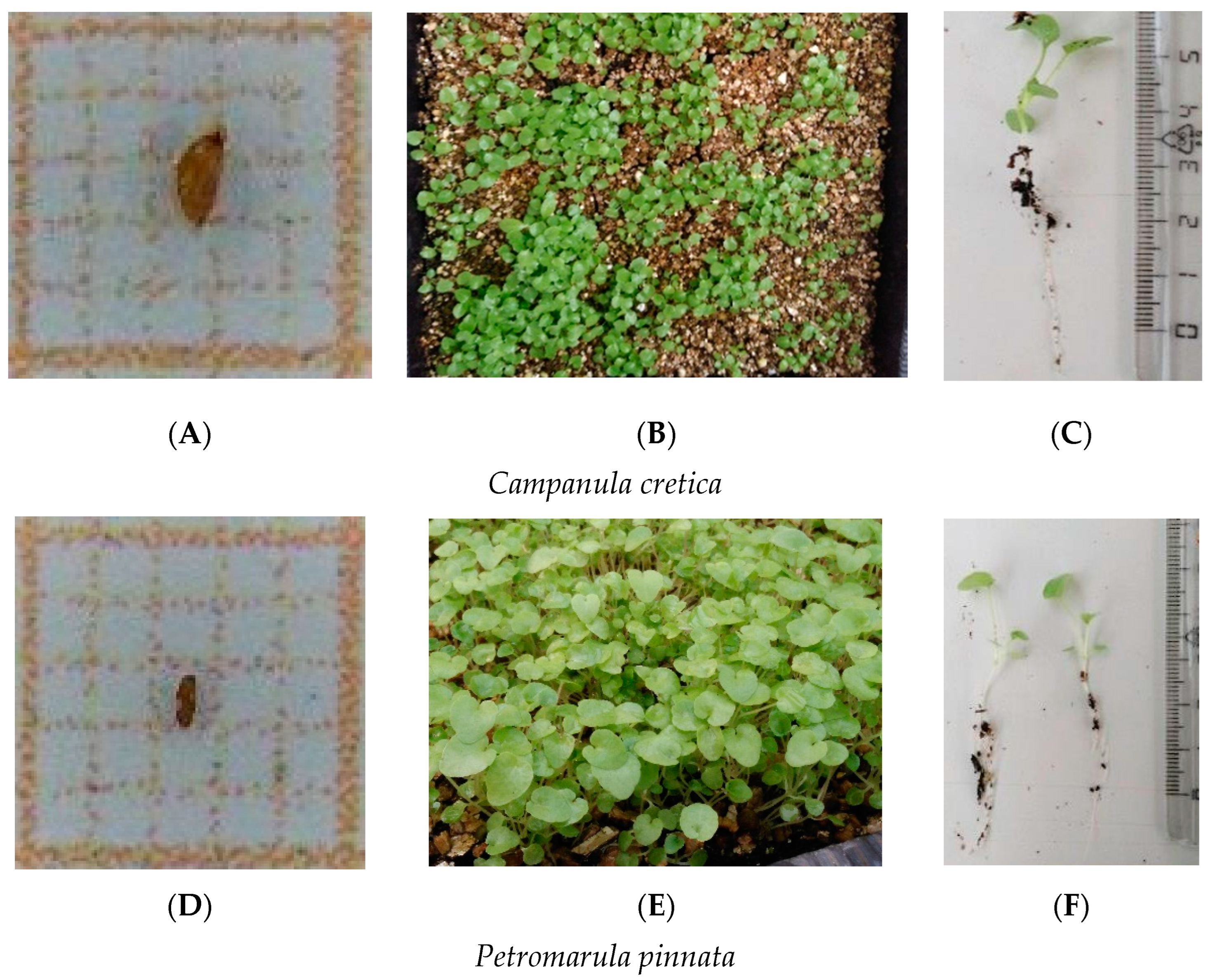



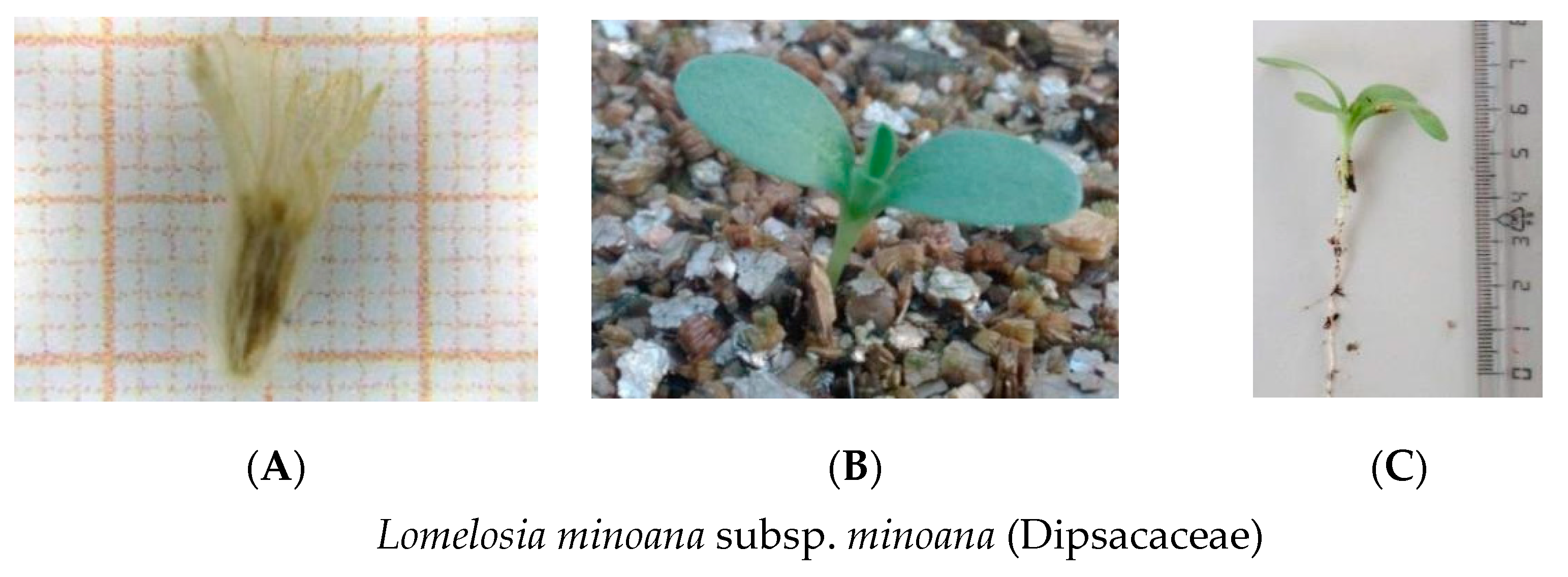

| Extinction Risk | Presidential Decree 67/1981 | Scientific Name | Family | Seed Accessions Stored | IPEN Accession Number Used (Period) | Collection Date | Collection Site | Prefecture | Latitude (North) | Longitude (East) |
|---|---|---|---|---|---|---|---|---|---|---|
| EN | - | Campanula cretica (A. DC.) D. Dietr. | Campanulaceae | 2 | GR-BBGK-1-19,147 (A) | 29/8/2018 | Samaria Gorge | Chania | 35.513536 | 24.017463 |
| CR | YES | Calamintha cretica (L.) Lam. | Lamiaceae | 4 | GR-BBGK-1-19,146 (A,S) | 29/8/2018 | Samaria Gorge | Chania | 35.513536 | 24.017463 |
| EN | - | Dianthus fruticosus L. subsp. creticus (Tausch) Runemark | Caryophyllaceae | 5 | GR-BBGK-1-19,17 (A.S) | 29/8/2018 | Agios Georgios Selinaris | Lasithi | 35.284803 | 25.540993 |
| CR | - | Draba cretica Boiss. & Heldr. | Brassicaceae | 3 | GR-BBGK-1-19,10 (A,S) | 29/8/2018 | Skinakas | Heraklion | 35.21146 | 24.89459 |
| CR | - | Lomelosia minoana (P.H. Davis) Greuter & Burdet subsp. minoana | Dispacaceae | 4 | GR-BBGK-1-19,16 (A) | 7/10/2018 | Ano Viannos | Heraklion | 35.05421 | 25.40998 |
| EN | - | Onopordum bracteatum Boiss. & Heldr. subsp. creticum Franco | Asteraceae | 1 | GR-BBGK-1-19,1 (A,S) | 29/8/2018 | Zomynthos | Rethymno | 35.24257 | 24.88549 |
| CR | - | Origanum microphyllum (Benth.) Vogel | Lamiaceae | 5 | GR-BBGK-1- 19,31 (A.S) | 7/10/2018 | Dikti Mountain | Lasithi | 35.118191 | 25.492043 |
| VU | - | Petromarula pinnata (L.) A. DC. | Campanulaceae | 27 | GR-BBGK-1-19,124 (A) | 29/8/2018 | Gorge of Agia Irini | Chania | 35.2995 | 23.8346 |
| CR | YES | Sanguisorba cretica Hayek | Rosaceae | 4 | GR-BBGK-1-19,154 (A,S) | 29/8/2018 | Samaria Gorge | Chania | 35.51353 | 24.017463 |
| Plant Taxon | Days after Sowing | Germination (%) | t50 (Days) | p-Values |
|---|---|---|---|---|
| Calamintha cretica | 15 | 2.0 b | 24.17 | 0.000 *** |
| 30 | 20.0 a | |||
| 45 | 26.0 a | |||
| 60 | 26.0 a | |||
| Campanula cretica | 15 | 40 c | 30.00 | 0.000 *** |
| 30 | 50 c | |||
| 45 | 70 b | |||
| 60 | 100 a | |||
| Dianthus fruticosus subsp. creticus | 15 | 93 b | 10.00 | 0.001 ** |
| 30 | 100 a | |||
| 45 | 100 a | |||
| 60 | 100 a | |||
| Draba cretica | 15 | 50 b | 12.16 | 0.000 *** |
| 30 | 90 a | |||
| 45 | 90 a | |||
| 60 | 91 a | |||
| Lomelosia minoana subsp. minoana | 15 | 20 b | 10.74 | 0.009 ** |
| 30 | 34 a | |||
| 45 | 36 a | |||
| 60 | 38 a | |||
| Onopordum bracteatum subsp. creticum | 15 | 1.5 a | 10.50 | 1.000 ns |
| 30 | 1.5 a | |||
| 45 | 1.5 a | |||
| 60 | 1.5 a | |||
| Origanum microphyllum | 15 | 8.3 b | 18.14 | 0.017 * |
| 30 | 22.7 a | |||
| 45 | 22.7 a | |||
| 60 | 22.7 a | |||
| Petromarula pinnata | 15 | 50 b | 15.00 | 0.000 *** |
| 30 | 100 a | |||
| 45 | 100 a | |||
| 60 | 100 a | |||
| Sanguisorba cretica | 15 | 6 b | 21.94 | 0.000 *** |
| 30 | 86 a | |||
| 45 | 86 a | |||
| 60 | 86 a |
| Cretan Taxon/ (Pre-Treatment) | Days after Sowing | Germination (%) | t50 (Days) | p-Values |
|---|---|---|---|---|
| Calamintha cretica (dH2O for 24 h) | 15 | 5 b | 22.45 | 0.025 * |
| 30 | 18 a | |||
| 45 | 20 a | |||
| 60 | 22 a | |||
| Calamintha cretica (50 ppm GA3 solution for 24 h) | 15 | 25 b | 9.23 | 0.044 * |
| 30 | 36 a | |||
| 45 | 36 a | |||
| 60 | 36 a | |||
| Dianthus fruticosus subsp. creticus (dH2O for 24 h) | 15 | 79 a | 9.30 | 0.992 ns |
| 30 | 80 a | |||
| 45 | 80 a | |||
| 60 | 80 a | |||
| Draba cretica (dH2O for 24 h) | 15 | 60 b | 13.27 | 0.026 * |
| 30 | 71 a | |||
| 45 | 74 a | |||
| 60 | 74 a | |||
| Onopordum bracteatum subsp. creticum (dH2O for 24 h) | 15 | 18 a | 8.42 | 1.000 ns |
| 30 | 18 a | |||
| 45 | 18 a | |||
| 60 | 18 a | |||
| Onopordum bracteatum subsp. creticum (50 ppm GA3 solution for 24 h) | 15 | 23 a | 10.41 | 0.411 ns |
| 30 | 24 a | |||
| 45 | 24 a | |||
| 60 | 24 a | |||
| Onopordum bracteatum subsp. creticum (250 ppm GA3 solution for 24 h) | 15 | 23 a | 9.61 | 0.323 ns |
| 30 | 24 a | |||
| 45 | 25 a | |||
| 60 | 25 a | |||
| Origanum microphyllum (50 ppm GA3 solution for 24 h) | 15 | 16 a | 12.00 | 0.587 ns |
| 30 | 17 a | |||
| 45 | 17 a | |||
| 60 | 21 a | |||
| Sanquisorba cretica (dH2O for 24 h) | 30 | 17 a | 20.61 | 0.000 *** |
| 45 | 17 a | |||
| 60 | 21 a | |||
| 60 | 89 a |
| Germination Rate (%) every Forthright (in Days) during Autumn (A)/Spring (S) | ||||||
|---|---|---|---|---|---|---|
| Scientific Name | Pre-Treatment (Spring) | Germination Onset Day A/S | 15th A/S | 30th A/S | 45th A/S | 60th A/S |
| ! Campanula cretica | - | 9/- | 40/- | 50/- | 70/- | 100/- |
| ! Calamintha cretica | 24 h in H2O | -/12 | -/5 | -/18 | -/20 | -/22 |
| 50 ppm GA3 (24 h) | 13/9 | 2/25 | 20/36 | 26/36 | 26/36 | |
| ! Dianthus fruticosus subsp. creticus | 24 h in H2O | 6/5 | 93/79 | 100/80 | 100/80 | 100/80 |
| ! Draba cretica | 24 h in H2O | 9/9 | 50/60 | 90/71 | 90/74 | 91/74 |
| ! Lomelosia minoana subsp. minoana | - | 6/- | 20/- | 34/- | 36/- | 38/- |
| ! Onopordum bracteatum subsp. creticum | 0 ppm GA3 (24 h in H2O) | 6/7 | 1.5/18 | 1.5/18 | 1.5/18 | 1.5/18 |
| 50 ppm GA3 (24 h) | -/6 | -/23 | -/24 | -/24 | -/24 | |
| 250 ppm GA3 (24 h) | -/6 | -/23 | -/24 | -/25 | -/25 | |
| ! Origanum microphyllum | 50 ppm GA3 (24 h) | 6/9 | 8.3/16 | 22.7/17 | 22.7/17 | 22.7/21 |
| Petromarula pinnata | - | 9/- | 50/- | 100/- | 100/- | 100/- |
| ! Sanguisorba cretica | 24 h in H2O | 13/2 | 6/18 | 86/89 | 86/89 | 86/89 |
| Cretan Endemic Plant | General Ornamental Interest [4] | * Special Ornamental Interest [4] | Agro-Alimentary Interest [5] | Medicinal Interest [6] | Level II & III Assessments ** [4] | Sowing Period (Maximum Germination Percentage Achieved) | Increase in Sum of Scores (+) and Upgraded Percentage (%) |
|---|---|---|---|---|---|---|---|
| Calamintha cretica | Low (37.50%) | Below average to very high (31.25%/43.90%/51.61%/63.24%) | High (59.52%) | Below average (46.30%) | 69.44%/Short-term | A, S (36%) | +12 (86.11%)/Achieved |
| Campanula cretica | Average (50.00%) | Average (55.21%/49.09%/46.77%/51.07%) | Low (33.33%) | Low (33.33%) | 36.11%/Long-term | A (100%) | +18 (61.11%)/Short-term |
| Dianthus fruticosus subsp. creticus | Low (36.67%) | Low (23.96%/21.04%/25.81%/28.06%) | Low (35.71%) | Very low(11.11%) | 34.72%/Indeterminable | A, S (100%) | +23 (66.67%)/Short-term |
| Draba cretica | Average (53.33%) | Very high (59.38%/68.83%/67.74%/85.38%) | Very low (14.29%) | Very low (11.11%) | 36.11%/Long-term | A, S (91%) | +24 (69.44%)/Short-term |
| Lomelosia minoana subsp. minoana | Average (51.67%) | Very high (62.50%/62.08%/68.28%/70.75%) | No (0) | Very low (20.37%) | 40.28%/Long-term | A (38%) | +22 (70.83%)/Achieved |
| Onopordum bracteatum subsp. creticum | Low (34.17%) | Low to below average (31.25%/38.70%/45.70%/47.04%) | Low (38.10%) | Below average(46.39%) | 38.89%/Long-term | A, S (25%) | +11 (54.17%)/Medium-term |
| Origanum microphyllum | Below average (44.17%) | Low to average (35.42%/44.42%/47.31%/54.35%) | Very high (80.95%) | High (59.26%) | 52.78%/Medium-term | A, S (22.7%) | +7 (62.50%)/Short-term |
| Petromarula pinnata | Average (52.50%) | Low to average (54.17%/45.45%/50.54%/54.15%) | Below average (42.86%) | Low(37.04%) | 45.83%/Long-term | A (100%) | +12 (62.50%)/Short-term |
| Sanguisorba cretica | Low (32.50%) | Low (31.25%/30.39%/39.25%/38.34) | Below average (45.24%) | Low (27.78%) | 38.89%/Long-term | A, S (89%) | +24 (72.22%)/Achieved |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarropoulou, V.; Krigas, N.; Tsoktouridis, G.; Maloupa, E.; Grigoriadou, K. Seed Germination Trials and Ex Situ Conservation of Local Prioritized Endemic Plants of Crete (Greece) with Commercial Interest. Seeds 2022, 1, 279-302. https://doi.org/10.3390/seeds1040024
Sarropoulou V, Krigas N, Tsoktouridis G, Maloupa E, Grigoriadou K. Seed Germination Trials and Ex Situ Conservation of Local Prioritized Endemic Plants of Crete (Greece) with Commercial Interest. Seeds. 2022; 1(4):279-302. https://doi.org/10.3390/seeds1040024
Chicago/Turabian StyleSarropoulou, Virginia, Nikos Krigas, Georgios Tsoktouridis, Eleni Maloupa, and Katerina Grigoriadou. 2022. "Seed Germination Trials and Ex Situ Conservation of Local Prioritized Endemic Plants of Crete (Greece) with Commercial Interest" Seeds 1, no. 4: 279-302. https://doi.org/10.3390/seeds1040024
APA StyleSarropoulou, V., Krigas, N., Tsoktouridis, G., Maloupa, E., & Grigoriadou, K. (2022). Seed Germination Trials and Ex Situ Conservation of Local Prioritized Endemic Plants of Crete (Greece) with Commercial Interest. Seeds, 1(4), 279-302. https://doi.org/10.3390/seeds1040024







