Seed Priming Enhances Seed Germination and Morphological Traits of Lactuca sativa L. under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planting Materials and Priming Treatment
2.2. Salt Treatment and Germination
2.3. Germination Parameters and Morphological Traits
2.3.1. Germination Parameters
2.3.2. Seeds’ Morphological Traits
2.4. Electrolyte Leakage
2.5. Data Analysis
3. Results
3.1. Germination Parameters
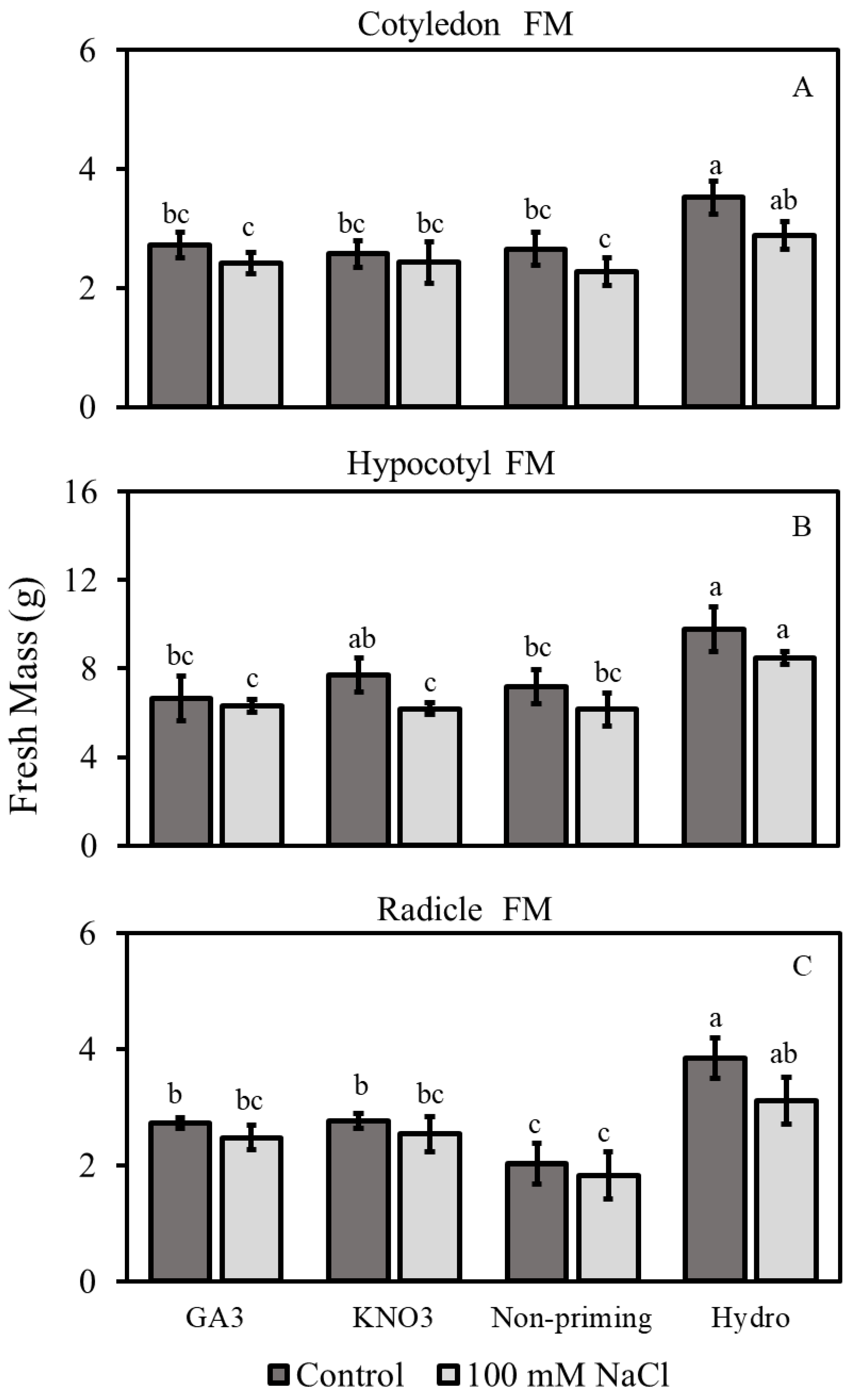
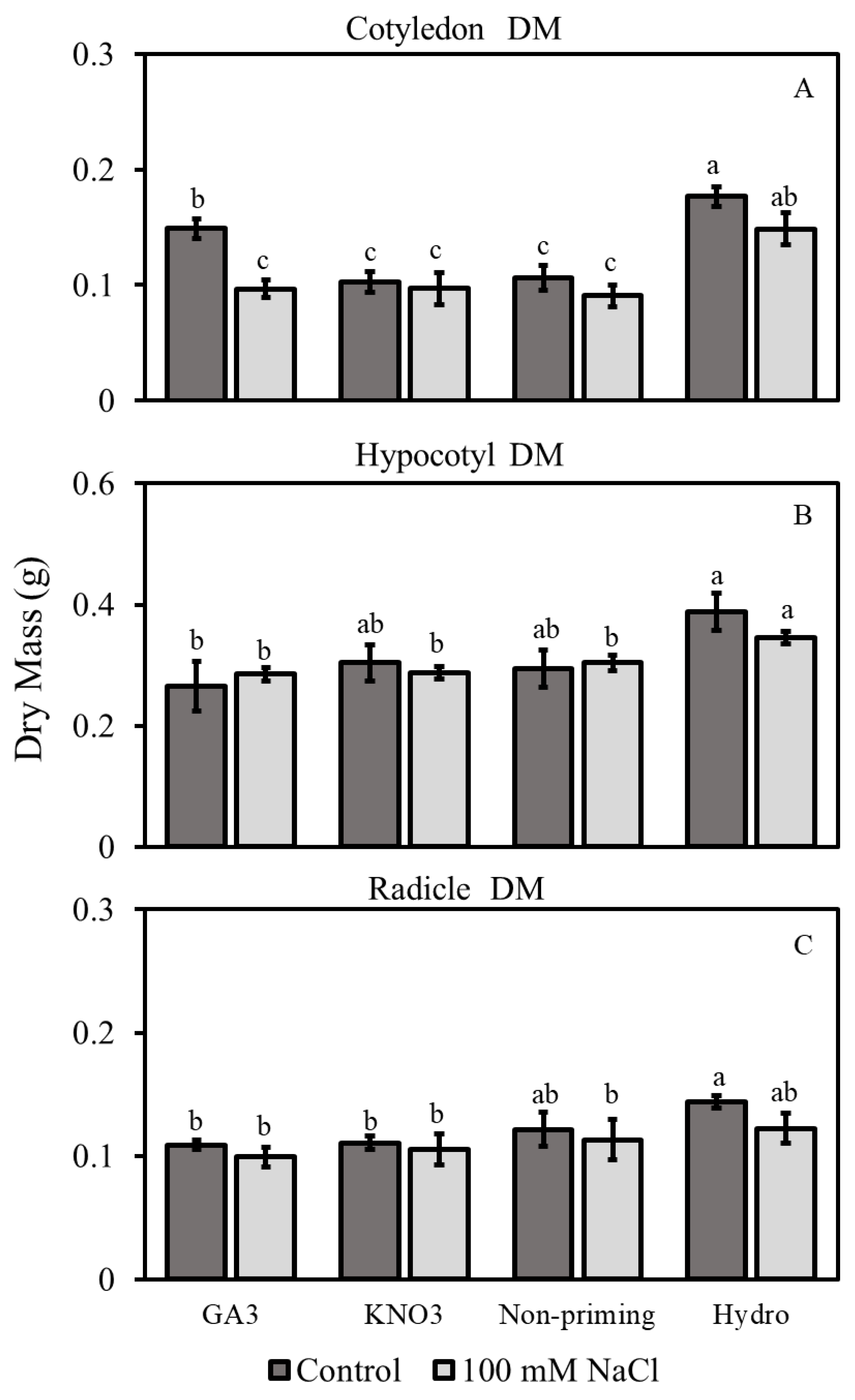
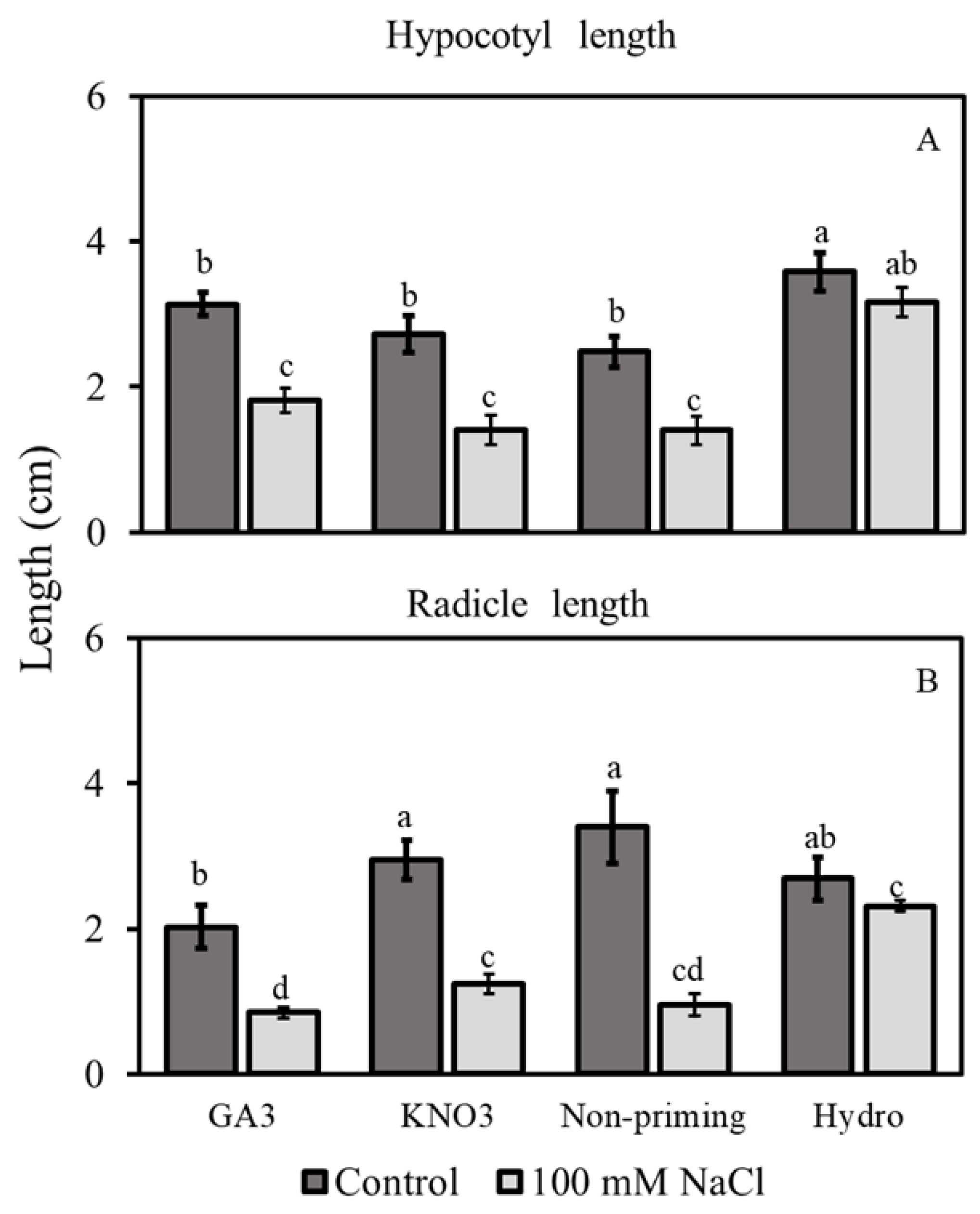
3.2. Morphological Traits
3.2.1. Fresh Mass
3.2.2. Dry Mass
3.2.3. Hypocotyl and Radicle Lengths
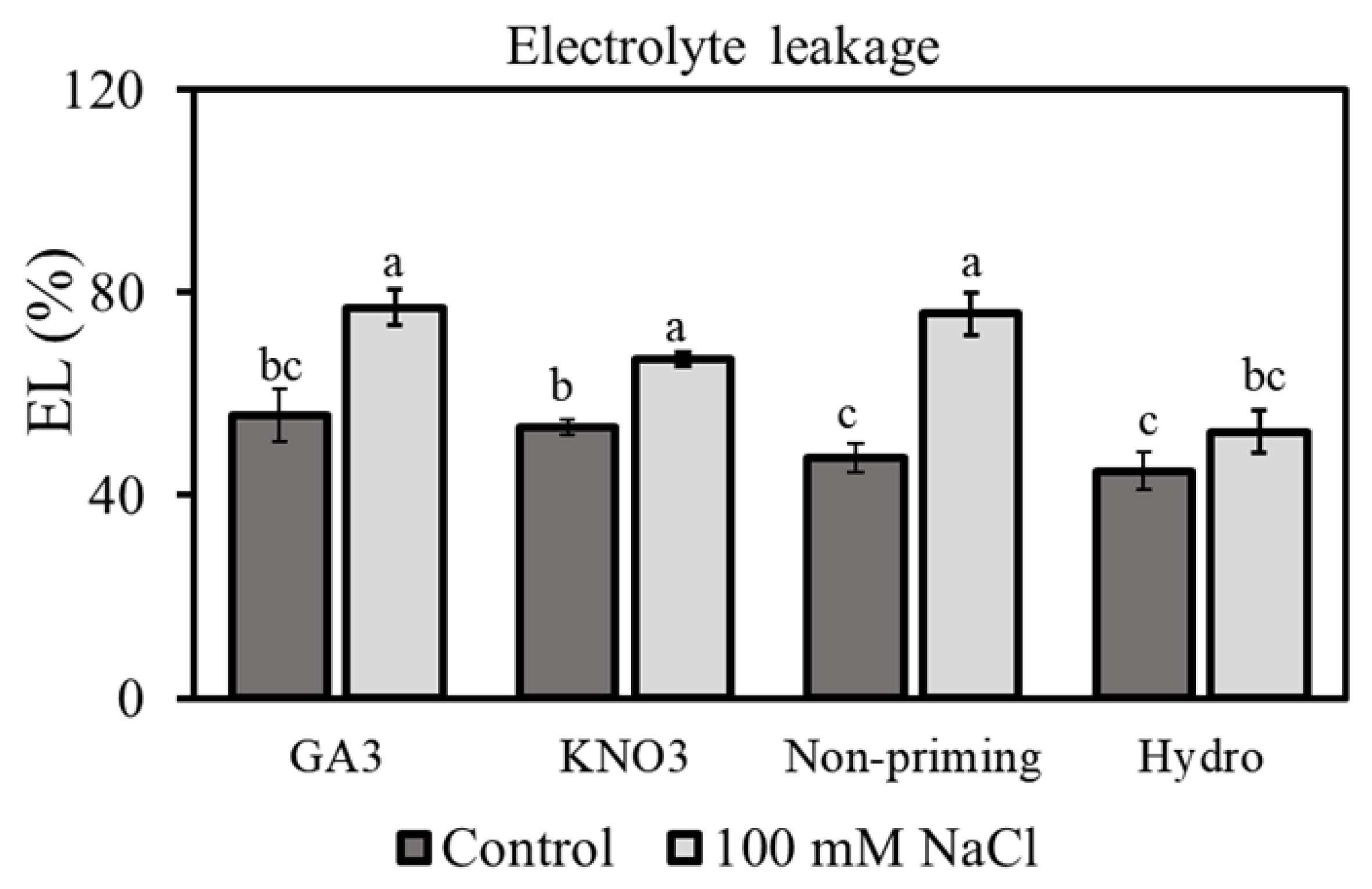
3.3. Electrolyte Leakage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Ahmad, S.; Hussain, S.; Lal, R.; Ul-Allah, S.; Nawaz, A. Rice in saline soils: Physiology, biochemistry, genetics, and management. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 148, pp. 231–287. [Google Scholar]
- Zaman, M.; Shahid, S.A.; Pharis, R.P. Salinity a Serious Threat to Food Security—Where Do We Stand? Int. At. Energy Agency Bull. 2016, 39, 9–10. [Google Scholar]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A New Insight of Salt Stress Signaling in Plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef] [PubMed]
- WMO. State of the Global Climate 2020: Provisional Report; WMO: Geneva, Switzerland, 2021; ISBN 4146702018922. [Google Scholar]
- Rouhi, H.R.; Aboutalebian, M.A.; Sharif-Zadeh, F. Effects of hydro and osmopriming on drought stress tolerance during germination in four grass species. Int. J. AgriScience 2011, 1, 107–114. [Google Scholar]
- Jamil, M.; Lee, C.C.; Rehman, S.U.; Lee, D.B.; Ashraf, M.; Rha, E.S. Salinity (NaCl) tolerance of Brassica species at germination and early seedling growth. Electron. J. Environ. Agric. Food Chem. 2005, 4, 970–976. [Google Scholar]
- Omid, A.; Farzad, S.-Z. Osmo and hydro priming improvement germination characteristics and enzyme activity of Mountain Rye (Secale montanum) seeds under drought stress. J. Stress Physiol. Biochem. 2012, 8, 253–261. [Google Scholar]
- Francois, L.E.; Maas, E.V. Crop response and management of salt-affected soils. In Handbook of Plant and Crop Stress; Marcel Dekker Press Inc.: New York, NY, USA, 1999; pp. 169–201. [Google Scholar]
- Mahmoudi, H.; Kaddour, R.; Huang, J.; Nasri, N.; Olfa, B.; M’Rah, S.; Hannoufa, A.; Lachaâl, M.; Ouerghi, Z. Varied tolerance to NaCl salinity is related to biochemical changes in two contrasting lettuce genotypes. Acta Physiol. Plant. 2011, 33, 1613–1622. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Powell, A.A.; Bingham, I.J. The interaction between salinity stress and seed vigour during germination of soyabean seeds. Seed Sci. Technol. 2003, 31, 715–725. [Google Scholar] [CrossRef]
- Jenks, M.A.; Hasegawa, P.M.; Jain, S.M. Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007; ISBN 1402055773. [Google Scholar]
- Tekrony, D.M.; Egli, D.B. Accumulation of seed vigour during development and maturation. In Basic and Applied Aspects of Seed Biology; Springer: Berlin/Heidelberg, Germany, 1997; pp. 369–384. [Google Scholar]
- Walters, C.; Towill, L.; Collins, C.O. Seeds and pollen. In Commercial Storage Fruits, Vegetables, and Florist and Nursery Stocks; United States Department of Agriculture: Washington, DC, USA, 2004; p. 735. [Google Scholar]
- Shaban, M. Review on physiological aspects of seed deterioration. Int. J. Agric. Crop Sci. 2013, 6, 627–631. [Google Scholar]
- Yadav, P.V.; Maya, K.; Zakwan, A. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Res. J. Seed Sci. 2011, 4, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, M.; Germida, J.; Vujanovic, V. Fungal endophytes improve wheat seed germination under heat and drought stress. Botany 2012, 90, 137–149. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar]
- Taylor, A.G.; Harman, G.E. Concepts and technologies of selected seed treatments. Annu. Rev. Phytopathol. 1990, 28, 321–339. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.A.; Farooq, M.; Nawaz, A. Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int. J. Agric. Biol. 2006, 8, 23–28. [Google Scholar]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Parera, C.A.; Cantliffe, D.J. Presowing seed priming. Hortic. Rev. 1994, 16, 109–141. [Google Scholar]
- Iqbal, M.; Ashraf, M.; Jamil, A.; Ur-Rehman, S. Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J. Integr. Plant Biol. 2006, 48, 181–189. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.K.; Kaur, N. Effect of osmo-and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. Plant Growth Regul. 2002, 37, 17–22. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okçu, G.; Atak, M.; Cıkılı, Y.; Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Casenave, E.C.; Toselli, M.E. Hydropriming as a pre-treatment for cotton germination under thermal and water stress conditions. Seed Sci. Technol. 2007, 35, 88–98. [Google Scholar] [CrossRef]
- Wu, L.; Huo, W.; Yao, D.; Li, M. Effects of solid matrix priming (SMP) and salt stress on broccoli and cauliflower seed germination and early seedling growth. Sci. Hortic. 2019, 255, 161–168. [Google Scholar] [CrossRef]
- Singh, H.; Jassal, R.K.; Kang, J.S.; Sandhu, S.S.; Kang, H.; Grewal, K. Seed priming techniques in field crops—A review. Agric. Rev. 2015, 36, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Ranal, M.A.; de Santana, D.G.; Ferreira, W.R.; Mendes-Rodrigues, C. Calculating germination measurements and organizing spreadsheets. Braz. J. Bot. 2009, 32, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Soltani, E.; Ghaderi-Far, F.; Baskin, C.C.; Baskin, J.M. Problems with using mean germination time to calculate rate of seed germination. Aust. J. Bot. 2015, 63, 631–635. [Google Scholar] [CrossRef]
- Labouriau, L.G. On the frequency of isothermal germination in seeds of Dolichos biflorus L. Plant Cell Physiol. 1978, 19, 507–512. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mavi, K.; Demir, I.; Matthews, S. Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Sci. Technol. 2010, 38, 14–25. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.; Olorunwa, O.J.; Wilson, J.C.; Barickman, T.C. Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress. Stresses 2021, 1, 285–304. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Massoud, R.B.; Baatour, O.; Tarchoune, I.; Salah, I.B.; Nasri, N.; Abidi, W.; Kaddour, R.; Hannoufa, A.; Lachaâl, M. Influence of different seed priming methods for improving salt stress tolerance in lettuce plants. J. Plant Nutr. 2012, 35, 1910–1922. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- AOSA (Association of Official Seed Analysts). SCST (Society of Commercial Seed Technologists). Rules for testing seeds. J. Seed Technol. 1993, 16, 1–113. [Google Scholar]
- Quartacci, M.F.; Glišić, O.; Stevanović, B.; Navari-Izzo, F. Plasma membrane lipids in the resurrection plant Ramonda serbica following dehydration and rehydration. J. Exp. Bot. 2002, 53, 2159–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Ahmad, P.; Azooz, M.M.; Prasad, M.N.V. Salt Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Szepesi, Á. Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt-and osmotic stress. Acta Biol. Szeged. 2005, 49, 123–125. [Google Scholar]
- James, R.A.; Von Caemmerer, S.; Condon, A.G.T.; Zwart, A.B.; Munns, R. Genetic variation in tolerance to the osmotic stress componentof salinity stress in durum wheat. Funct. Plant Biol. 2008, 35, 111–123. [Google Scholar] [CrossRef]
- Gzik, A. Accumulation of proline and pattern of α-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ. Exp. Bot. 1996, 36, 29–38. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015; ISBN 1605353531. [Google Scholar]
- Alasvandyari, F.; Mahdavi, B.; Hosseini, S.M. Glycine betaine affects the antioxidant system and ion accumulation and reduces salinity-induced damage in safflower seedlings. Arch. Biol. Sci. 2017, 69, 139–147. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol. Biochem. 2017, 118, 362–369. [Google Scholar] [CrossRef]
- Tsegay, B.A.; Andargie, M. Seed priming with gibberellic acid (GA3) alleviates salinity induced inhibition of germination and seedling growth of Zea mays L., Pisum sativum var. abyssinicum A. Braun and Lathyrus sativus L. J. Crop Sci. Biotechnol. 2018, 21, 261–267. [Google Scholar] [CrossRef]
- Imran, M.; Boelt, B.; Mühling, K. Zinc seed priming improves salt resistance in maize. J. Agron. Crop Sci. 2018, 204, 390–399. [Google Scholar] [CrossRef]
- Tahira, T.; Riaz, A.; Muhammad, F.; Basra, S.M.A. Improving salt tolerance in barley by osmopriming and biopriming. Int. J. Agric. Biol. 2018, 20, 2455–2464. [Google Scholar]
- Kim, I.-J.L.; Yun, B.-W.; Jamil, M. GA mediated OsZAT-12 expression improves salt resistance of rice. Int. J. Agric. Biol 2016, 18, 330–336. [Google Scholar]
- Bajehbaj, A.A. The effects of NaCl priming on salt tolerance in sunflower germination and seedling grown under salinity conditions. Afr. J. Biotechnol. 2010, 9, 1764–1770. [Google Scholar]
- Younesi, O.; Moradi, A. Effect of priming of seeds of Medicago sativa ‘bami’with gibberellic acid on germination, seedlings growth and antioxidant enzymes activity under salinity stress. J. Hortic. Res. 2014, 22, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hortic. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Ahmad, N.; Saleem, B.A. Osmopriming improves the germination and early seedling growth of melons (Cucumis melo L.). Pak. J. Agric. Sci. 2007, 44, 529–536. [Google Scholar]
- Sarwar, N.; Yousaf, S.; Jamil, F.F. Induction of salt tolerance in chickpea by using simple and safe chemicals. Pak. J. Bot. 2006, 38, 325. [Google Scholar]
- Nasri, N.; Kaddour, R.; Mahmoudi, H.; Baatour, O.; Bouraoui, N.; Lachaâl, M. The effect of osmopriming on germination, seedling growth and phosphatase activities of lettuce under saline condition. Afr. J. Biotechnol. 2011, 10, 14366–14372. [Google Scholar]
- Jamil, M.; Ashraf, M.; Rha, E. Alleviation of salt stress using gibberellic acid in Chinese cabbage. Acta Agron. Hung. 2012, 60, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, Y.; Jahufer, Z. Location and chemical composition of semi-permeable layer of forage seeds. Bangladesh J. Bot. 2013, 42, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.; Borgohain, P.; Saha, B.; Moulick, D.; Tanti, B.; Panda, S.K. Seed priming and seedling pre-treatment induced tolerance to drought and salt stress: Recent advances. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019; pp. 253–263. [Google Scholar]
- Sivritepe, N.; Sivritepe, H.O.; Eris, A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci. Hortic. 2003, 97, 229–237. [Google Scholar] [CrossRef]
- Aschermann-Koch, C.; Hofmann, P.; Steiner, A.M. Presowing treatment for improving seed quality in cereals. Germination and vigour. Seed Sci. Technol. 1992, 20, 435–440. [Google Scholar]
- McDonald, M.B. Seed priming. In Seed Technology and Its Biological Basis; Sheffild Academic Press: Sheffild, UK, 2000; pp. 287–325. [Google Scholar]
- Adetunji, A.E.; Varghese, B.; Pammenter, N.W. Effects of inorganic salt solutions on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. Plants 2020, 9, 1164. [Google Scholar] [CrossRef]
- Al-Maskri, A.; Khan, M.M.; Al-Manther, O.; Al-Habsi, K. Effect of accelerated aging on lipid peroxidation, leakage and seedling vigor (RGR) in cucumber (Cucumis sativus L.) seeds. Pak. J. Agric. Sci. 2002, 39, 330–337. [Google Scholar]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Maathuis, F. Ion Channels and Plant Stress Responses; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 3642104940. [Google Scholar]
- Chiu, K.Y.; Wang, C.S.; Sung, J.M. Lipid peroxidation and peroxide-scavenging enzymes associated with accelerated aging and hydration of watermelon seeds differing in ploidy. Physiol. Plant. 1995, 94, 441–446. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fotopoulos, V. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019; ISBN 9789811386244. [Google Scholar]
| Priming | Treatment | MGT 3 | MGR | Z | GI |
|---|---|---|---|---|---|
| GA3 | Control | 2.16 ± 0.03a 1,2 | 0.5 ± 0.01a | 0.72 ± 0.05a | 11.8 ± 0.14a |
| NaCl | 2.23 ± 0.11a | 0.4 ± 0.02a | 0.65 ± 0.11ab | 11.5 ± 0.12a | |
| Hydro | Control | 2.01 ± 0.02a | 0.5 ± 0.03a | 0.32 ± 0.08c | 12.5 ± 0.01a |
| NaCl | 2.04 ± 0.01a | 0.4 ± 0.08a | 0.42 ± 0.03c | 12.3 ± 0.02a | |
| KNO3 | Control | 2.17 ± 0.04a | 0.5 ± 0.01a | 0.71 ± 0.09a | 11.8 ± 0.16a |
| NaCl | 2.37 ± 0.18a | 0.4 ± 0.03a | 0.55 ± 0.13b | 11.2 ± 0.18a | |
| Non-priming | Control | 2.17 ± 0.02a | 0.5 ± 0.01a | 0.71 ± 0.03a | 11.8 ± 0.08a |
| NaCl | 2.32 ± 0.11a | 0.4 ± 0.02a | 0.56 ± 0.18ab | 11.2 ± 0.43a | |
| Priming | *** | *** | *** | *** | |
| Salt | ** | ** | *** | *** | |
| Priming × Salt treatment | NS | NS | ** | NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, B.; Olorunwa, O.J.; Barickman, T.C. Seed Priming Enhances Seed Germination and Morphological Traits of Lactuca sativa L. under Salt Stress. Seeds 2022, 1, 74-86. https://doi.org/10.3390/seeds1020007
Adhikari B, Olorunwa OJ, Barickman TC. Seed Priming Enhances Seed Germination and Morphological Traits of Lactuca sativa L. under Salt Stress. Seeds. 2022; 1(2):74-86. https://doi.org/10.3390/seeds1020007
Chicago/Turabian StyleAdhikari, Bikash, Omolayo J. Olorunwa, and T. Casey Barickman. 2022. "Seed Priming Enhances Seed Germination and Morphological Traits of Lactuca sativa L. under Salt Stress" Seeds 1, no. 2: 74-86. https://doi.org/10.3390/seeds1020007
APA StyleAdhikari, B., Olorunwa, O. J., & Barickman, T. C. (2022). Seed Priming Enhances Seed Germination and Morphological Traits of Lactuca sativa L. under Salt Stress. Seeds, 1(2), 74-86. https://doi.org/10.3390/seeds1020007








