Hydrogen Peroxide Imbibition Following Cold Stratification Promotes Seed Germination Rate and Uniformity in Peach cv. GF305
Abstract
:1. Introduction
2. Materials and Methods
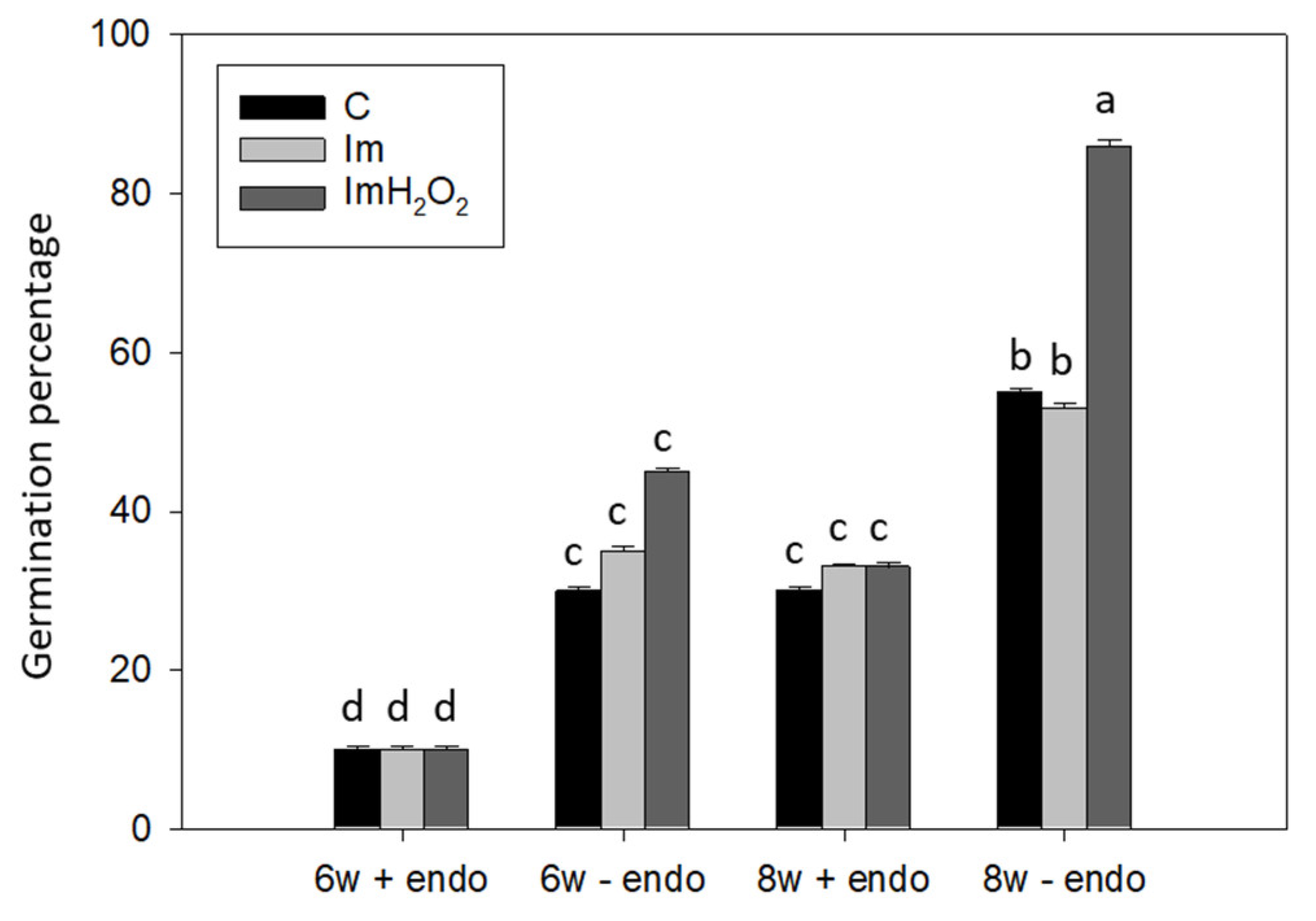
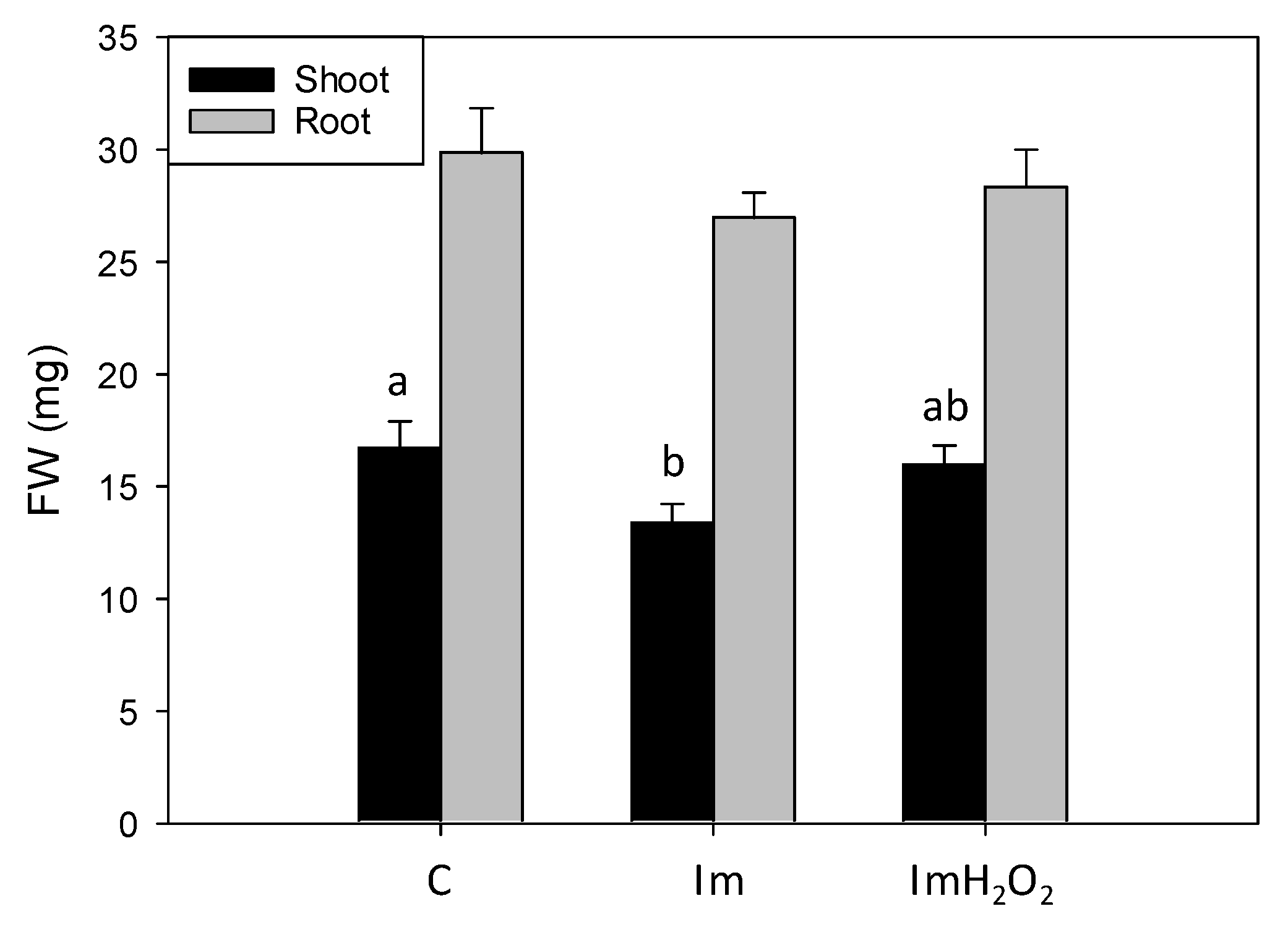
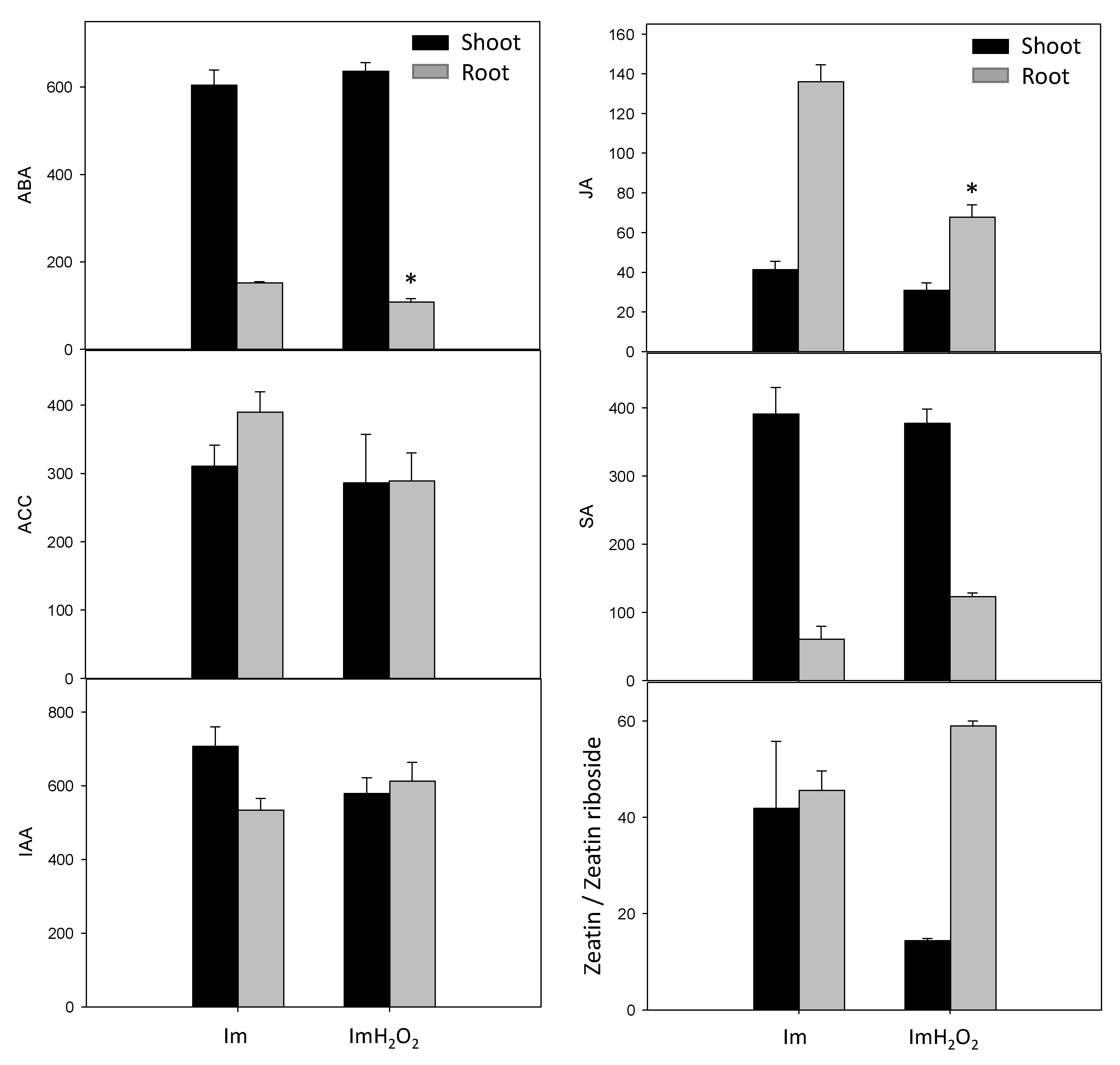
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, M.; Serban, C.; McCord, P. Exogenous ethylene precursors and hydrogen peroxide aid in early seed dormancy release in sweet cherry. J. Am. Soc. Hortic. Sci. 2021, 146, 50–55. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Dicenta, F. Mechanisms of dormancy in seeds of peach (Prunus persica (L.) Batsch) cv. GF305. Sci. Hortic. 2001, 91, 51–58. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Pérez-Alfocea, F.; Hernández, J.A. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vivancos, P.; Barba-Espin, G.; Hernandez, J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013, 32, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171. [Google Scholar] [CrossRef] [PubMed]
- Katzman, L.S.; Taylor, A.G.; Langhans, R.W. Seed enhancements to improve spinach germination. HortScience 2001, 36, 501–523. [Google Scholar] [CrossRef]
- Ogawa, K.; Iwabuchi, M. A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 2001, 42, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Barba-Espín, G.; Diaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernández, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef]
- Leida, C.; Conejero, A.; Arbona, V.; Gómez-Cadenas, A.; Llácer, G.; Badenes, M.L.; Ríos, G. Chilling-dependent release of seed and bud dormancy in peach associates to common changes in gene expression. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Imani, A.; Rasouli, M.; Tavakoli, R.; Zarifi, E.; Fatahi, R.; Barba-ESPÍN, G.; Martínez-Gómez, P. Optimization of seed germination in Prunus species combining hydrogen peroxide or gibberellic acid pre-treatment with stratification. Seed Sci. Technol. 2011, 39, 204–207. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Bernal-Vicente, A.; Cantabella, D.; Petri, C.; Hernández, J.A. Metabolomics and biochemical approaches link salicylic acid biosynthesis to cyanogenesis in peach plants. Plant Cell Physiol. 2017, 58, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Pellny, T.K.; Locato, V.; Vivancos, P.D.; Markovic, J.; De Gara, L.; Pallardo, F.V.; Foyer, C.H. Pyridine nucleotide cycling and control of intracellular redox state in relation to poly (ADP-Ribose) polymerase activity and uuclear localization of glutathione during exponential growth of Arabidopsis cells in culture. Mol. Plant 2009, 2, 442–456. [Google Scholar] [CrossRef] [Green Version]
- Vivancos, P.D.; Dong, Y.P.; Ziegler, K.; Markovic, J.; Pallardo, F.V.; Pellny, T.K.; Verrier, P.J.; Foyer, C.H. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010, 64, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Zeinalabedini, M.; Majourhat, K.; Khayam-Nekoui, M.; Hernández, J.A.; Martínez-Gómez, P. Breaking seed dormancy in long-term stored seeds from Iranian wild almond species. Seed Sci. Technol. 2009, 37, 267–275. [Google Scholar] [CrossRef]
- Kim, D.H. Practical methods for rapid seed germination from seed coat-imposed dormancy of Prunus yedoensis. Sci. Hortic. 2019, 243, 451–456. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Barba-Espín, G. Potassium nitrate treatment is associated with modulation of seed water uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings. Seeds 2022, 1, 5–15. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Arrigoni, O. The ascorbic acid system in seeds: To protect and to serve. Seed Sci. Res. 2003, 13, 249–260. [Google Scholar] [CrossRef]
- Hernández Cortés, J.A. Seed Science Research: Global trends in seed biology and technology. Seeds 2022, 1, 1–4. [Google Scholar] [CrossRef]
- Wang, M.; Van Der Meulen, R.M.; Visser, K.; Van Schalk, H.P.; Van Duijn, B.; De Boer, A.H. Effects of dormancy-breaking chemicals on ABA levels in barley grain embryos. Seed Sci. Res. 1998, 8, 129–137. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Sun, N.; Liu, H.; Zhao, Y.; Liang, Y.; Zhang, L.; Han, Y. Functional diversity of jasmonates in rice. Rice 2015, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Ma, J.; Hause, B.; Nick, P.; Riemann, M. Jasmonate is required for the response to osmotic stress in rice. Environ. Exp. Bot. 2020, 175, 104047. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Tong, X.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espin, G.; Nicolas, E.; Almansa, M.S.; Cantero-Navarro, E.; Albacete, A.; Hernandez, J.A.; Diaz-Vivancos, P. Role of thioproline on seed germination: Interaction ROS-ABA and effects on antioxidative metabolism. Plant Physiol. Biochem. 2012, 59, 30–36. [Google Scholar] [CrossRef]
- Gidrol, X.; Lin, W.S.; Dégousée, N.; Yip, S.F.; Kush, A. Accumulation of reactive oxygen species and oxidation of cytokinin in germinating soybean seeds. Eur. J. Biochem. 1994, 224, 21–28. [Google Scholar] [CrossRef]
- Hernández, J.A.; Diaz-Vivancos, P.; Barba-Espín, G.; Clemente-Moreno, M.J. On the Role of Salicylic Acid in Plant Responses to Environmental Stresses; Springer: Berlin, Germany, 2017; ISBN 9789811060687. [Google Scholar]
- Ishibashi, Y.; Koda, Y.; Zheng, S.H.; Yuasa, T.; Iwaya-Inoue, M. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Ann. Bot. 2013, 111, 95–102. [Google Scholar] [CrossRef] [Green Version]
| GSH nmol−1 FW | GSS Gnmol−1 FW | GSH/ (GSH+GSSG) | ASC nmol−1 FW | DHA nmol−1 FW | |
|---|---|---|---|---|---|
| IM_SHOOT | 274.2 ± 13.7 | 13.1 ± 0.9 | 0.95 ± 0.01 | 884.4 ± 62.1 | 116.6 ± 26.1 |
| IMH2O2_SHOOT | 211.5 ± 6.9 * | 12.2 ± 0.4 | 0.94 ± 0.00 | 1184.6 ± 67.0 | 162.8 ± 39.0 |
| IM_ROOT | 105.7 ± 7.4 | 12.1 ± 0.5 | 0.89 ± 0.01 | nd | nd |
| IMH2O2_ROOT | 148.6 ± 4.9 * | 12.2 ± 0.4 | 0.92 ± 0.00 * | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba-Espín, G.; Hernández, J.A.; Martínez-Andújar, C.; Díaz-Vivancos, P. Hydrogen Peroxide Imbibition Following Cold Stratification Promotes Seed Germination Rate and Uniformity in Peach cv. GF305. Seeds 2022, 1, 28-35. https://doi.org/10.3390/seeds1010004
Barba-Espín G, Hernández JA, Martínez-Andújar C, Díaz-Vivancos P. Hydrogen Peroxide Imbibition Following Cold Stratification Promotes Seed Germination Rate and Uniformity in Peach cv. GF305. Seeds. 2022; 1(1):28-35. https://doi.org/10.3390/seeds1010004
Chicago/Turabian StyleBarba-Espín, Gregorio, José A. Hernández, Cristina Martínez-Andújar, and Pedro Díaz-Vivancos. 2022. "Hydrogen Peroxide Imbibition Following Cold Stratification Promotes Seed Germination Rate and Uniformity in Peach cv. GF305" Seeds 1, no. 1: 28-35. https://doi.org/10.3390/seeds1010004
APA StyleBarba-Espín, G., Hernández, J. A., Martínez-Andújar, C., & Díaz-Vivancos, P. (2022). Hydrogen Peroxide Imbibition Following Cold Stratification Promotes Seed Germination Rate and Uniformity in Peach cv. GF305. Seeds, 1(1), 28-35. https://doi.org/10.3390/seeds1010004









