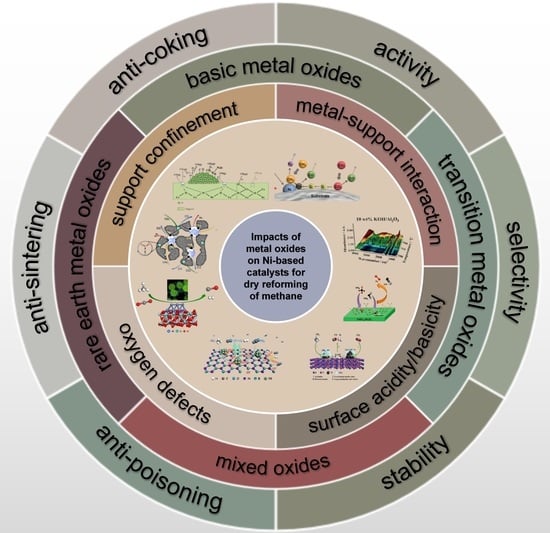
Modification Strategies of Ni-Based Catalysts with Metal Oxides for Dry Reforming of Methane
Abstract
:1. Introduction
2. Reaction and Deactivation Mechanisms
2.1. Catalytic Reaction Mechanism
2.2. Deactivation Mechanisms
2.2.1. Coking
2.2.2. Sintering
2.2.3. Poisoning
3. Impacts of Metal Oxides
3.1. Support Confinement
3.2. Metal–Support Interaction
3.3. Oxygen Defects
3.4. Surface Acidity/Basicity
4. Conclusive Remarks and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashok, J.; Ang, M.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2016, 281, 304–311. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Hongmanorom, P.; Ashok, J.; Zhang, G.; Bian, Z.; Wai, M.H.; Zeng, Y.; Xi, S.; Borgna, A.; Kawi, S. Enhanced performance and selectivity of CO2 methanation over phyllosilicate structure derived Ni-Mg/SBA-15 catalysts. Appl. Catal. B Environ. 2020, 282, 119564. [Google Scholar] [CrossRef]
- Oh, T.H. Carbon capture and storage potential in coal-fired plant in Malaysia—A review. Renew. Sustain. Energy Rev. 2010, 14, 2697–2709. [Google Scholar] [CrossRef]
- Alves, H.J.; Junior, C.B.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrogen Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Gao, X.; Lin, X.; Xie, X.; Li, J.; Wu, X.; Li, Y.; Kawi, S. Modification strategies of heterogeneous catalysts for water–gas shift reactions. React. Chem. Eng. 2022, 7, 551–565. [Google Scholar] [CrossRef]
- Faramawy, S.; Zaki, T.; Sakr, A.A.E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Chen, S. The effects of hydrogen dilution, carbon monoxide poisoning for a Pt–Ru anode in a proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2016, 41, 20680–20692. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Poly-chronopoulou, K.; Goula, M.A. The relationship between reaction temperature and carbon deposition on nickel catalysts based on Al2O3, ZrO2 or SiO2 supports during the biogas dry reforming reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef] [Green Version]
- Charisiou, N.; Tzounis, L.; Sebastian, V.; Hinder, S.; Baker, M.; Polychronopoulou, K.; Goula, M. Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Lin, Z.; Li, T.; Huang, L.; Zhang, J.; Askari, S.; Dewangan, N.; Jangam, A.; Kawi, S. Recent Developments in Dielectric Barrier Discharge Plasma-Assisted Catalytic Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2021, 11, 455. [Google Scholar] [CrossRef]
- Gao, X.; Ashok, J.; Kawi, S. A review on roles of pretreatment atmospheres for the preparation of efficient Ni-based catalysts. Catal. Today 2021, 397–399, 581–591. [Google Scholar] [CrossRef]
- Damyanova, S.; Shterevaa, I.; Pawelecb, B.; Mihaylovc, L.; Fierro, J.L.G. Characterization of none and yttrium-modified Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2000, 278, 119335. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.R.; Poerjoto, A.J.; Jie, T.; Ashok, J.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni–La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrogen Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Zheng, M.; Cai, S.; Zhang, J.; Askari, S.; Dewangan, N.; Ashok, J.; Kawi, S. Recent progress in anti-coking Ni catalysts for thermo-catalytic conversion of greenhouse gases. Process Saf. Environ. Prot. 2021, 156, 598–616. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of me-thane to hydrogen production. Renew. Sustain. Energ. Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Atia, H.; Fakeeha, A.H.; Armbruster, U.; Abasaeed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA–15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl. Catal. A Gen. 2018, 567, 102–111. [Google Scholar] [CrossRef]
- Pan, X.Y.; Kuai, P.; Liu, Y.; Ge, Q.; Liu, C.J. Promotion effects of Ga2O3 on CO2 adsorption and conversion over a SiO2-supported Nicatalyst. Energy Environ. Sci. 2010, 3, 1322–1325. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, R.; Hao, X.; Bao, Z.; Wu, T.; Wang, B.; Yu, F. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B Environ. 2019, 254, 612–623. [Google Scholar] [CrossRef]
- Dias, J.; Assaf, J. Influence of calcium content in Ni/CaO/γ-Al2O3 catalysts for CO2-reforming of methane. Catal. Today 2003, 85, 59–68. [Google Scholar] [CrossRef]
- Wei, T.; Jia, L.; Luo, J.-L.; Chi, B.; Pu, J.; Li, J. CO2 dry reforming of CH4 with Sr and Ni co-doped LaCrO3 perovskite catalysts. Appl. Surf. Sci. 2019, 506, 144699. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Cheng, C.K. Catalytic conversion of methane and carbon dioxide (greenhouse gases) into syngas over sa-marium–cobalt-trioxides perovskite catalyst. J. Clean. Prod. 2017, 148, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Li, S.; Liang, X. Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: Role of MgO. Fuel 2020, 284, 119082. [Google Scholar] [CrossRef]
- Oemar, U.; Hidajat, K.; Kawi, S. Pd–Ni catalyst over spherical nanostructured Y2O3 support for oxy-CO2 reforming of methane: Role of surface oxygen mobility. Int. J. Hydrogen Energy 2015, 40, 12227–12238. [Google Scholar] [CrossRef]
- Li, Z.; Kathiraser, Y.; Kawi, S. Facile Synthesis of Multi-Ni-Core@Ni Phyllosilicate@CeO2 Shell Hollow Spheres with High Oxygen Vacancy Concentration for Dry Reforming of CH4. ChemCatChem 2018, 10, 2994–3001. [Google Scholar] [CrossRef]
- Guo, D.; Lu, Y.; Ruan, Y.Z.; Zhao, Y.F.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. Effects of extrinsic defects originating from the inter-facial reaction of CeO2–x–nickel silicate on catalytic performance in methane dry reforming. Appl. Catal. B Environ. 2020, 277, 119278. [Google Scholar] [CrossRef]
- Pino, L.; Italiano, C.; Vita, A.; Laganà, M.; Recupero, V. Ce0.70La0.20Ni0.10O2-δ catalyst for methane dry reforming: Influence of reduction temperature on the catalytic activity and stability. Appl. Catal. B Environ. 2017, 218, 779–792. [Google Scholar] [CrossRef]
- Wang, H.Q.; Dong, X.L.; Zhao, T.T.; Yu, H.R.; Li, M. Dry reforming of methane over bimetallic Ni–Co catalyst prepared from La(CoxNi1−x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B Environ. 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Izadbakhsh, A.; Huang, J.; Zi-Feng, Y. Catalytic performance of cubic ordered mesoporous alumina supported nickel catalysts in dry reforming of methane. Microporous Mesoporous Mater. 2020, 310, 110616. [Google Scholar] [CrossRef]
- Li, K.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Liu, R.; Gong, J. Dry reforming of methane over La2O2CO3-modified Ni/Al2O3 cata-lysts with moderate metal support interaction. Appl. Catal. B Environ. 2020, 264, 118448. [Google Scholar] [CrossRef]
- Jabbour, K.; Saad, A.; Inaty, L.; Davidson, A.; Massiani, P.; Hassan, N.E. Ordered mesoporous Fe–Al2O3 based-catalysts syn-thesized via a direct “one-pot” method for the dry reforming of a model biogas mixture. Int. J. Hydrogen Energy 2019, 144, 14889–14907. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, J.; Xie, X.; Luo, X.; Su, T.; Ji, H. CO2 reforming of CH4 to syngas over nickel-based catalysts. Environ. Chem. Lett. 2020, 18, 997–1017. [Google Scholar] [CrossRef]
- Guharoy, U.; Reina, T.R.; Liu, J.; Sun, Q.; Gu, S.; Cai, Q. A theoretical overview on the prevention of coking in dry reforming of methane using non-precious transition metal catalysts. J. CO2 Util. 2021, 53, 101728. [Google Scholar] [CrossRef]
- Gao, X.; Ge, Z.; Zhu, G.; Wang, Z.; Ashok, J.; Kawi, S. Anti-Coking and Anti-Sintering Ni/Al2O3 Catalysts in the Dry Re-forming of Methane: Recent Progress and Prospects. Catalysts 2021, 11, 1003. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: A review. J. Ind. Eng. Chem. 2022, 107, 20–30. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. Int. J. Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Czaplicka, N.; Rogala, A.; Wysocka, I. Metal (Mo, W, Ti) Carbide Catalysts: Synthesis and Application as Alternative Catalysts for Dry Reforming of Hydrocarbons—A Review. Int. J. Mol. Sci. 2021, 22, 12337. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, M.; Sekine, Y. Effects of alloying for steam or dry reforming of methane: A review of recent studies. Catal. Sci. Technol. 2022, 12, 3387–3411. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Aghaali, M.H.; Firoozi, S. Enhancing the catalytic performance of Co substituted NiAl2O4 spinel by ultrasonic spray pyrolysis method for steam and dry reforming of methane. Int. J. Hydrogen Energy 2020, 46, 357–373. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Dry reforming of methane for syngas production: A review and assessment of catalyst development and efficacy. J. Indian Chem. Soc. 2021, 98, 100002. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Rodríguez-Ramos, I.; Anderson, J.A.; Guerrero-Ruiz, A. Mechanistic aspects of the dry reforming of methane over ruthenium catalysts. Appl. Catal. A Gen. 2000, 202, 183–196. [Google Scholar] [CrossRef]
- Minh, D.P.; Pham, X.H.; Siang, T.; Vo, D.V.N. Review on the catalytic tri-reforming of methane—Part I: Impact of operating conditions, catalyst deactivation and regeneration. Appl. Catal. A Gen. 2021, 621, 118202. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Ge, Q.; Li, W.; Xu, H. Studies of Carbon Deposition over Rh–CeO2/Al2O3 during CH4/CO2 Reforming. Stud. Surf. Sci. Catal. 2007, 167, 379–384. [Google Scholar]
- Siang, T.J.; Pham, T.L.; Van Cuong, N.; Phuong, P.T.; Phuc, N.H.H.; Truong, Q.D.; Vo, D.-V.N. Combined steam and CO2 reforming of methane for syngas production over carbon-resistant boron-promoted Ni/SBA-15 catalysts. Microporous Mesoporous Mater. 2018, 262, 122–132. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Ashok, J.; Kawi, S. A comprehensive review of anti-coking, anti-poisoning and anti-sintering catalysts for biomass tar reforming reaction. Chem. Eng. Sci. X 2020, 7, 100065. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Jiang, J.; Yan, F.; Li, K.; Tian, S.; Gao, Y.; Zhou, H. Dry Reforming of Model Biogas on a Ni/SiO2 Catalyst: Overall Performance and Mechanisms of Sulfur Poisoning and Regeneration. ACS Sustain. Chem. Eng. 2017, 5, 10248–10257. [Google Scholar] [CrossRef]
- Kang, D.; Lim, H.S.; Lee, J.W. Enhanced catalytic activity of methane dry reforming by the confinement of Ni nanoparticles into mesoporous silica. Int. J. Hydrogen Energy 2017, 42, 11270–11282. [Google Scholar] [CrossRef]
- Qian, L.; Huang, K.; Wang, H.; Kung, M.C.; Kung, H.H.; Li, J.; Chen, G.; Du, Q. Evaluation of the catalytic surface of Ni impregnated meso-microporous silica KIT-6 in CH4 dry reforming by inverse gas chromatography. Microporous Mesoporous Mater. 2016, 243, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Quek, X.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal–support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Deng, J.; Shen, K.; Yu, X.; Qian, W.; Chu, W.; Wei, F. One-pot Synthesis of Ordered Mesoporous NiCeAl Oxide Catalysts and a Study of Their Performance in Methane Dry Reforming. ChemCatChem 2014, 6, 1470–1480. [Google Scholar] [CrossRef]
- Ma, Q.; Han, Y.; Wei, Q.; Makpal, S.; Gao, X.; Zhang, J.; Zhao, T. Stabilizing Ni on bimodal mesoporous-macroporous alumina with enhanced coke tolerance in dry reforming of methane to syngas. J. CO2 Util. 2020, 35, 288–297. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Liu, H.; Wei, Q.; Gong, D.; Mo, L.; Tao, H.; Cui, S.; Wang, L. One-Step Synthesis of Highly Dispersed and Stable Ni Nanoparticles Confined by CeO2 on SiO2 for Dry Reforming of Methane. Energies 2020, 13, 5956. [Google Scholar] [CrossRef]
- Li, K.; Chang, X.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Assabumrungrat, S.; Zhao, Z.-J.; Zeng, L.; Gong, J. Ordered mesoporous Ni/La2O3 catalysts with interfacial synergism towards CO2 activation in dry reforming of methane. Appl. Catal. B Environ. 2019, 259, 118092. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Da Costa, P.; Hu, C. Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane. Catalysts 2019, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.Y.; Jang, J.S.; Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, J.S. Reduced perovskite LaNiO3 catalysts modified with Co and Mn for low coke formation in dry reforming of methane. Appl. Catal. A Gen. 2019, 575, 198–203. [Google Scholar] [CrossRef]
- Kim, B.J.; Jeon, K.W.; Na, H.S.; Lee, Y.L.; Ahn, S.Y.; Kim, K.J.; Jang, W.J.; Shim, J.O.; Roh, H.S. Reducible oxide (CeO2, ZrO2, and CeO2–ZrO2) promoted Ni–MgO catalysts for carbon dioxide reforming of methane reaction. Korean J. Chem. Eng. 2020, 37, 1130–1136. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Wirk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Yttrium promoted Ni-based double-layered hydroxides for dry methane Reforming. J. CO2 Util. 2018, 27, 247–258. [Google Scholar]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.-M. Effect of NiAl2O4 Formation on Ni/Al2O3 Stability during Dry Reforming of Me-thane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, B.; Qin, L.; Wang, Y.; Yu, F.; Han, J. Non-thermal plasma-enhanced dry reforming of methane and CO2 over Ce-promoted Ni/C catalysts. Mol. Catal. 2020, 485, 110821. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, L.; Yan, W.; Qi, R.; Tu, W.; Wang, Z. CeO2-Promoted Ni/SiO2 Catalysts for Carbon Dioxide Reforming of Me-thane: The Effect of Introduction Methodologies. Catal. Lett. 2021, 151, 2144–2152. [Google Scholar] [CrossRef]
- Mette, K.; Kühl, S.; Tarasov, A.; Willinger, M.G.; Kröhnert, J.; Wrabetz, S.; Trunschke, A.; Scherzer, M.; Girgsdies, F.; Düdder, H.; et al. High-Temperature Stable Ni Nanoparticles for the Dry Reforming of Methane. ACS Catal. 2016, 6, 7238–7248. [Google Scholar] [CrossRef]
- Lanre, M.S.; Al-Fatesh, A.S.; Fakeeha, A.H.; Kasim, S.O.; Ibrahim, A.A.; Al-Awadi, A.S.; Al-Zahrani, A.A.; Abasaeed, A.E. Catalytic Performance of Lanthanum Promoted Ni/ZrO2 for Carbon Dioxide Reforming of Methane. Processes 2020, 8, 1502. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Al-Fatesh, A.S.; Khan, W.U.; Kasim, S.O.; Abasaeed, A.E.; Fakeeha, A.H.; Bonura, G.; Frusteri, F. Enhanced coke suppression by using phosphate-zirconia supported nickel catalysts under dry methane reforming conditions. Int. J. Hydrogen Energy 2019, 44, 27784–27794. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B Environ. 2018, 244, 427–437. [Google Scholar] [CrossRef]
- Shah, M.; Bordoloi, A.; Nayak, A.K.; Mondal, P. Effect of Ti/Al ratio on the performance of Ni/TiO2–Al2O3 catalyst for methane reforming with CO2. Fuel Process. Technol. 2019, 192, 21–35. [Google Scholar] [CrossRef]
- Xu, G.; Shi, K.; Gao, Y.; Xu, H.; Wei, Y. Studies of reforming natural gas with carbon dioxide to produce synthesis gas: X. The role of CeO2 and MgO promoters. J. Mol. Catal. A Chem. 1999, 147, 47–54. [Google Scholar] [CrossRef]
- Jin, B.T.; Shang, Z.Y.; Li, S.G.; Jiang, Y.B.; Gu, X.H.; Liang, X.H. Reforming of methane with carbon dioxide over cerium oxide promoted nickel nanoparticles deposited on 4-channel hollow fibers by atomic layer deposition. Catal. Sci. Technol. 2020, 10, 3212–3222. [Google Scholar] [CrossRef]
- Jawad, A.; Rezaei, F.; Rownaghi, A.A. Highly Efficient Pt/Mo–Fe/Ni-Based Al2O3–CeO2 Catalysts for Dry Reforming of Me-thane. Catal. Today 2019, 350, 80–90. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G.; Xue, Q.; Chen, L.; Lu, Y. High carbon-resistance Ni/CeAlO3–Al2O3 catalyst for CH4/CO2 reforming. Appl. Catal. B Environ. 2013, 136–137, 260–268. [Google Scholar] [CrossRef]
- Faria, E.C.; Neto, R.C.R.; Colman, R.C.; Noronha, F.B. Hydrogen production through CO2 reforming of methane over Ni/CeZrO2/Al2O3 catalysts. Catal. Today 2014, 228, 138–144. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Nishino, T.; Ayas, N.; Vo, D.V.N. Coke-resistant Y2O3-promoted cobalt supported on mesoporous alumina for enhanced hydrogen production. J. Energy Inst. 2021, 94, 272–284. [Google Scholar] [CrossRef]
- Taherian, Z.; Gharahshiran, V.S.; Fazlikhani, F.; Yousefpour, M. Catalytic performance of Samarium-modified Ni catalysts over Al2O3–CaO support for dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 7254–7262. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhou, Z.; Chen, S.; Zhang, T.; Song, F.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst. Appl. Catal. B Environ. 2019, 264, 118522. [Google Scholar] [CrossRef]
- Figueredo, G.P.; Medeiros, R.L.; Macedo, H.P.; de Oliveira, A.; Braga, R.M.; Mercury, J.M.; Melo, M.A.; Melo, D.M. A comparative study of dry reforming of methane over nickel catalysts supported on perovskite-type LaAlO3 and commercial α-Al2O3. Int. J. Hydrogen Energy 2018, 43, 11022–11037. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.; Yoon, S.; Müller, C.R. Dry-reforming of methane over bimetallic Ni–M/La2O3 (M = Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Van der Heide, P.A.W. Systematic x-ray photoelectron spectroscopic study of La1-xSrx-based perovskite-type oxides. Surf. Interface Anal. 2002, 33, 414–425. [Google Scholar] [CrossRef]
- Shahnazi, A.; Firoozi, S. Improving the catalytic performance of LaNiO3 perovskite by manganese substitution via ultrasonic spray pyrolysis for dry reforming of methane. J. CO2 Util. 2021, 45, 101455. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, Y.; Kim, J.H.; Jang, G.Y.; Yoon, S.P.; Yun, J.W. Characteristics of Sr1−xYxTi1−yRuyO3+/−δ and Ru-impregnated Sr1−xYxTiO3+/−δ perovskite catalysts as SOFC anode for methane dry reforming. Appl. Surf. Sci. 2020, 510, 145450. [Google Scholar] [CrossRef]
- Yang, E.-H.; Noh, Y.S.; Hong, G.H.; Moon, D.J. Combined steam and CO2 reforming of methane over La1-xSrxNiO3 perovskite oxides. Catal. Today 2018, 299, 242–250. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Shekhawat, D.; Haynes, D.J.; Spivey, J.J. The effect of La substitution by Sr- and Ca- in Ni substituted Lanthanum Zirconate pyrochlore catalysts for dry reforming of methane. Appl. Catal. A Gen. 2020, 602, 117721. [Google Scholar] [CrossRef]
- Misture, S.T.; McDevitt, K.M.; Glass, K.C.; Edwards, D.D.; Howe, J.Y.; Rector, K.D.; He, H.; Vogel, S.C. Sulfur-resistant and regenerable Ni/Co spinel-based catalysts for methane dry reforming. Catal. Sci. Technol. 2015, 5, 4565–4574. [Google Scholar] [CrossRef]
- Jiang, C.; Loisel, E.; Cullen, D.A.; Dorman, J.A.; Dooley, K.M. On the enhanced sulfur and coking tolerance of Ni-Co-rare earth oxide catalysts for the dry reforming of methane. J. Catal. 2020, 393, 215–229. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Evaluating the performance of Ni catalyst supported on La2O3–MgAl2O4 for dry re-forming of methane in a packed bed dielectric barrier discharge (DBD) plasma reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, G.; Bi, G.; Guo, Y.; Xie, J. Monolithic SiC-foam supported NiLa2O3 composites for dry reforming of methane with enhanced carbon resistance. Fuel Process. Technol. 2021, 212, 106627. [Google Scholar] [CrossRef]
- Cho, E.; Lee, Y.-H.; Kim, H.; Jang, E.J.; Kwak, J.H.; Lee, K.; Ko, C.H.; Yoon, W.L. Ni catalysts for dry methane reforming prepared by A-site exsolution on mesoporous defect spinel magnesium aluminate. Appl. Catal. A Gen. 2020, 602, 117694. [Google Scholar] [CrossRef]

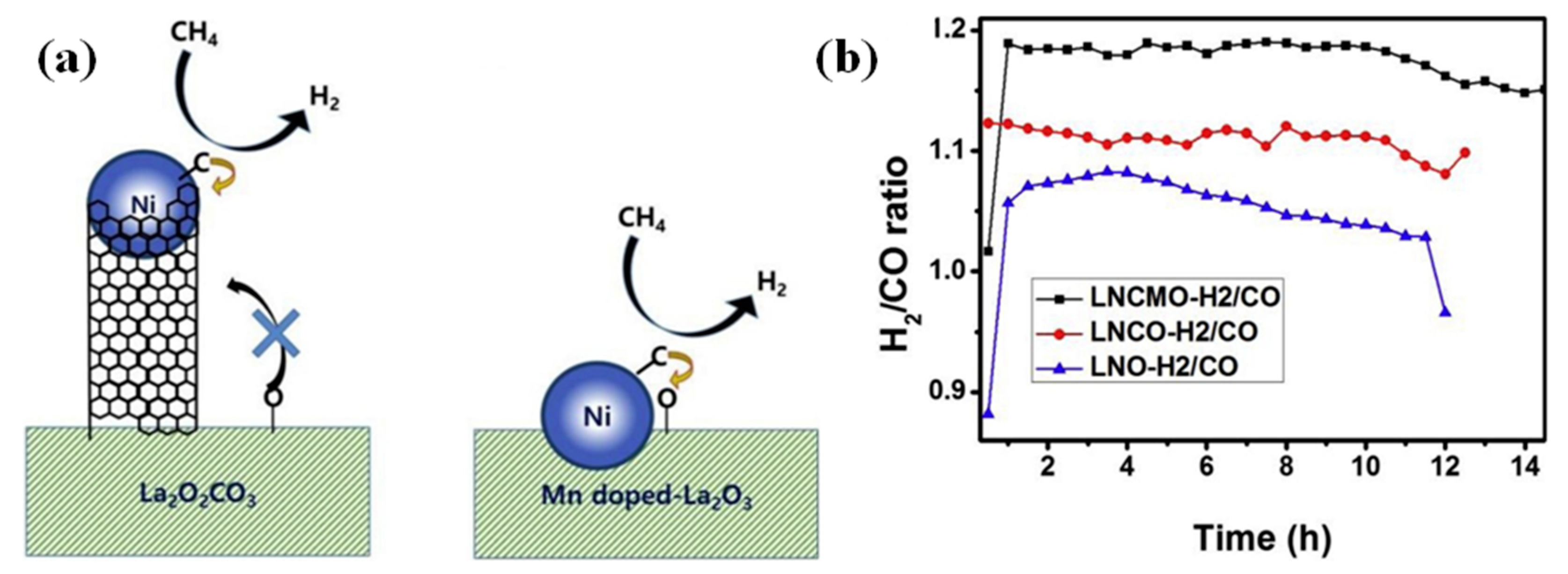
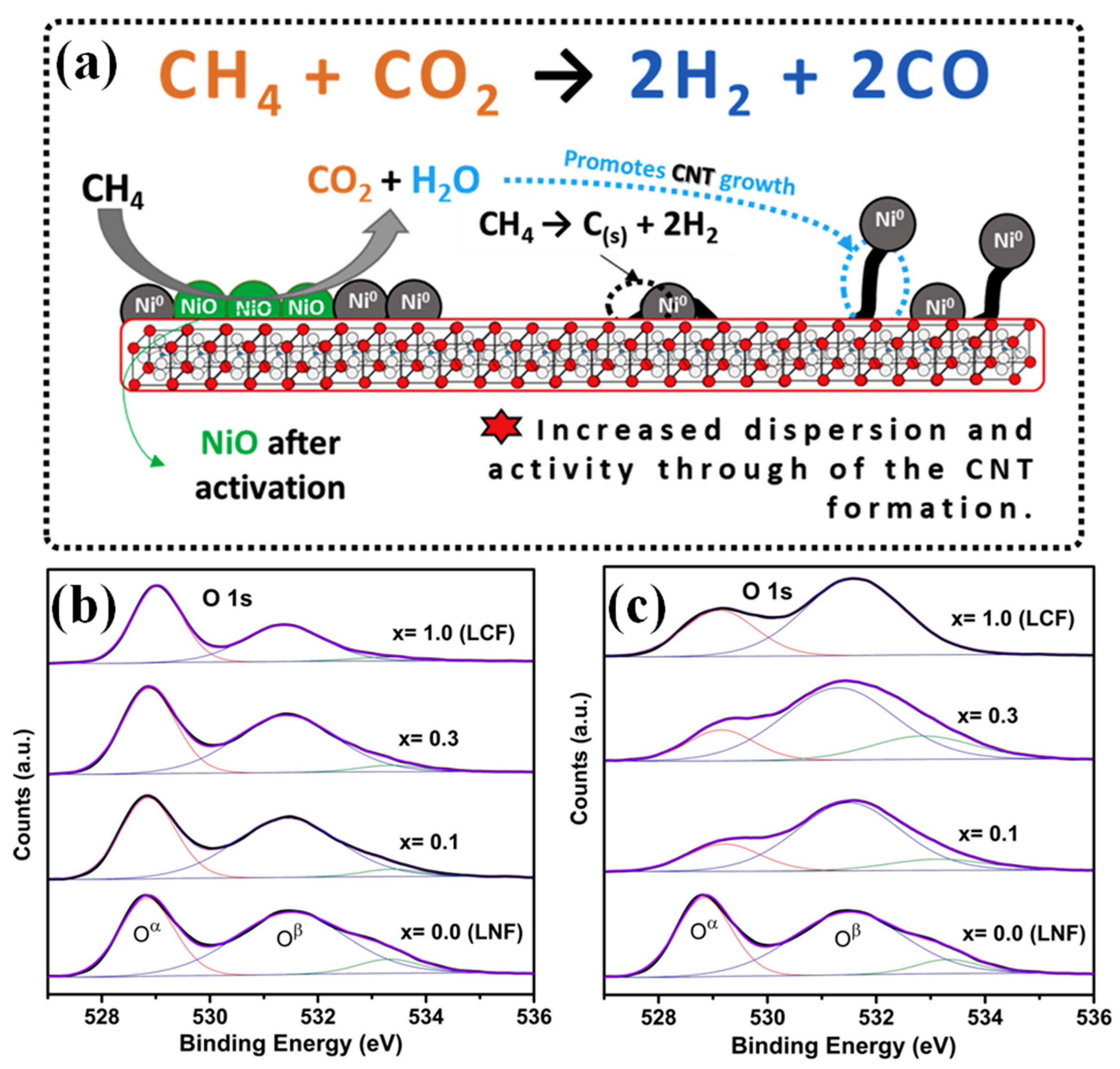
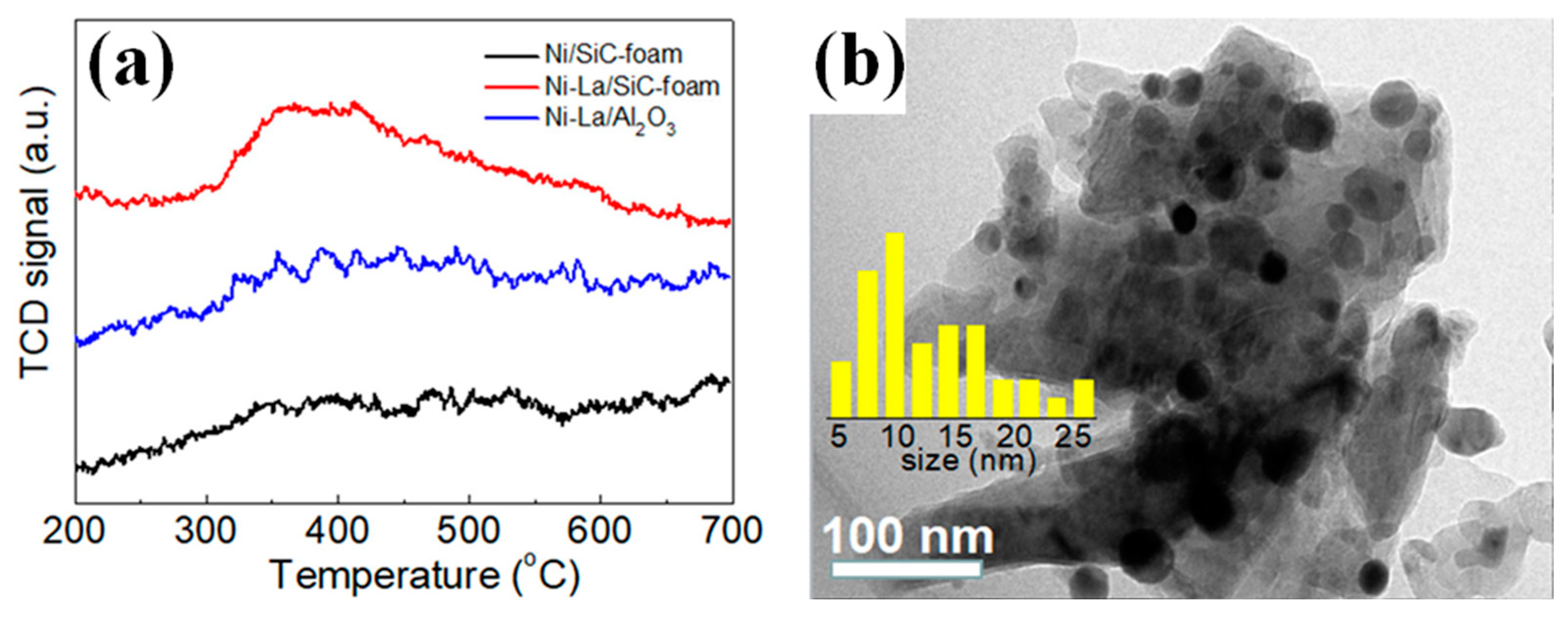
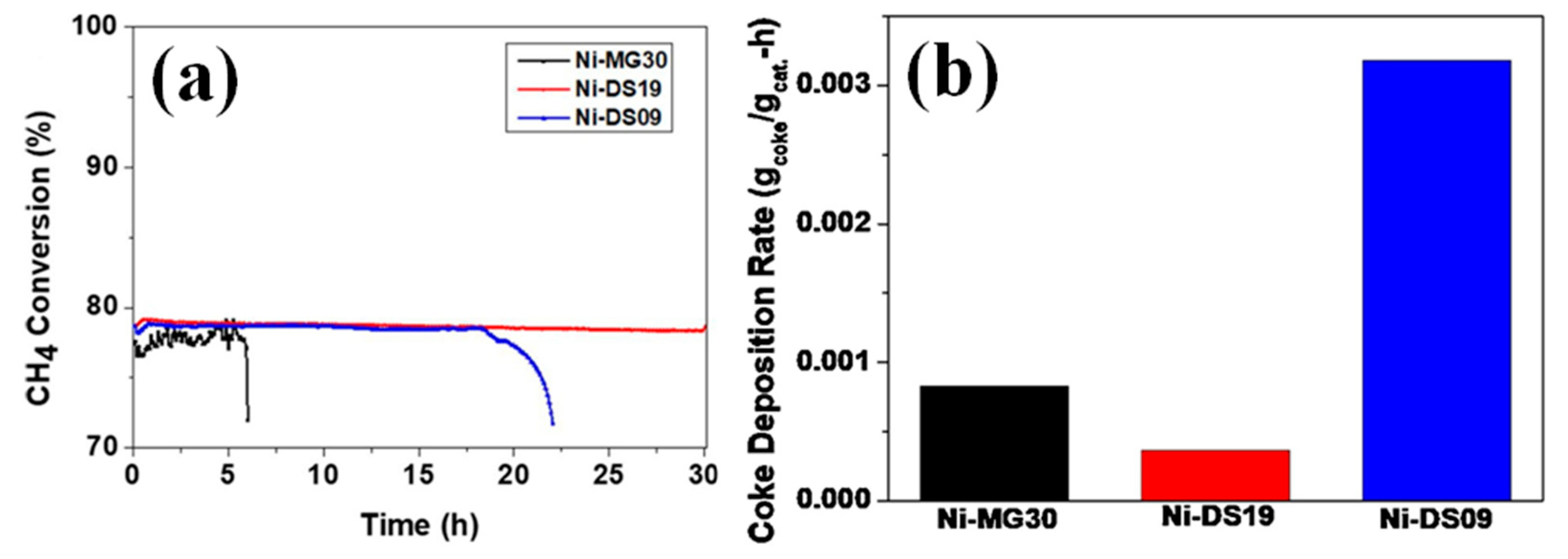
| Catalyst | Temperature (°C) | CH4/CO2 | CH4 Conversion (%) | CO2 Conversion (%) | H2/CO | Remark | Ref |
|---|---|---|---|---|---|---|---|
| Fe5%Ni5%Al2O3 | 700 | 1.8:1 | 50 | 89 | 1.1 | NiFe alloy particles were confined within the ordered mesoporous Al2O3 frameworks. | [33] |
| Ni/Al2O3 | 850 | 1:1 | 99 | 96 | 0.89 | Cubic and mesoporous Al2O3 confined Ni particles and exhibited strong sintering resistance over 210 h. | [31] |
| Ni–CeO2/SiO2 | 700 | 1:1 | 77 | 85 | 0.95 | High Ni dispersion on CeO2 and abundant Ni–CeO2 interfaces enhanced the coke resistance. | [58] |
| Ni/La2O3 | 650 | 1:1 | 30 | 68 | 0.9 | Ni agglomeration was alleviated over 50 h due to the La2O3 mesopore confinement. | [59] |
| Ni/Y2O3–ZrO2 | 700 | 1:1 | 67 | 71 | 0.85 | Ni particle size was reduced over 8 h due to re-dispersion and strong MSI with Y2O3 doping. | [60] |
| LaNi0.34Co0.33Mn0.33O3 | 800 | 1:1.05 | 94 | 92.5 | 1.15 | MnO enhanced the interaction between the metal and La2O3 support. | [61] |
| Ni/Al2O3–La2O3CO3 | 650 | 1:1 | 61 | 65 | 0.85 | La2O2CO3 increased the number of Ni active sites by inhibiting the NiAl2O4 formation. | [32] |
| Ni/MgO–ZrO2 | 800 | 1:1 | 68 | 75 | 0.89 | ZrO2 tuned the MSI in Ni/MgO and enhanced the reducibility. | [62] |
| 1.5CeO2−x–NSNT | 750 | 1:1 | 82 | 88 | 0.91 | Ni silicate nanotubes (NSNTs) reacted with CeO2 to produce Ce3+ and oxygen defects, inhibiting the coke formation. | [28] |
| Ce0.70La0.20Ni0.10O2−δ | 750 | 1:1 | 73 | 84 | 0.88 | Oxygen defects and La2O2CO3 contributed to the improved coke resistance. | [29] |
| La(Co0.1Ni0.9)0.5Fe0.5O3 | 750 | 1:1 | 70 | 80 | 0.89 | Co partial substitution generated oxygen vacancies and enhanced the amount of surface oxygen species. | [30] |
| La0.4Ce0.6Ni0.5Fe0.5O3 | 750 | 1:1 | 62 | 72 | 0.91 | Reversible redox reaction and undercoordinated B-site cations increased oxygen defect concentration. | [63] |
| Co–Ni/Sc-SBA–15 | 700 | 1:1 | 72.5 | 79 | 0.91 | More basic sites were generated with the Sc doping, reducing the inert carbon amount. | [19] |
| SmCoO3 | 800 | 1:1 | 93 | 90 | 1.1 | Co activated CH4 and Sm2O2CO3 removed carbon intermediates. | [24] |
| Ni/Al2O3–MgO | 800 | 1:1 | 40 | 52 | 0.7 | MgO enhanced the concentration of medium and strong basic sites, thus alleviating the encapsulated carbon formation. | [25] |
| Y-doped Ni–Mg–Al double-layered hydroxides | 700 | 1:1 | 76.2 | 80.8 | 0.92 | Weak and medium basic sites were introduced by Y2O3, promoting reversible CO2 adsorption and desorption. | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Lin, W.; Ge, Z.; Ge, H.; Kawi, S. Modification Strategies of Ni-Based Catalysts with Metal Oxides for Dry Reforming of Methane. Methane 2022, 1, 139-157. https://doi.org/10.3390/methane1030012
Gao X, Lin W, Ge Z, Ge H, Kawi S. Modification Strategies of Ni-Based Catalysts with Metal Oxides for Dry Reforming of Methane. Methane. 2022; 1(3):139-157. https://doi.org/10.3390/methane1030012
Chicago/Turabian StyleGao, Xingyuan, Weihao Lin, Zhiyong Ge, Hongming Ge, and Sibudjing Kawi. 2022. "Modification Strategies of Ni-Based Catalysts with Metal Oxides for Dry Reforming of Methane" Methane 1, no. 3: 139-157. https://doi.org/10.3390/methane1030012
APA StyleGao, X., Lin, W., Ge, Z., Ge, H., & Kawi, S. (2022). Modification Strategies of Ni-Based Catalysts with Metal Oxides for Dry Reforming of Methane. Methane, 1(3), 139-157. https://doi.org/10.3390/methane1030012