Potential Hypoglycemic Secondary Metabolites from Argyreia nervosa (Burm. f.) Bojer Influencing Human Gut Health †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification
2.2. Preparation of Leaf Extracts
2.3. Preliminary Phytochemical Analysis
2.4. Qualitative Starch-Iodide Assay
2.5. HPTLC Analysis
2.5.1. Preparation of Standard Stock Solutions
2.5.2. Sample Preparation
2.5.3. Instrumentation and Experimental Conditions
2.6. HPLC Analysis
2.7. ESI-MS Analysis
2.8. Quantitative 3,5-Dinitrosalicyclic Acid (DNSA) Assay
2.9. Statistical Data Analysis
2.10. In Silico Docking Studies
2.10.1. Protein Preparation
2.10.2. Ligand Preparation
2.10.3. Docking Studies
3. Results
3.1. Preliminary Phytochemical Analysis
3.2. Qualitative Starch-Iodide Assay
3.3. HPTLC Analysis
3.4. HPLC Analysis
3.5. ESI-MS Analysis
3.6. Quantitative 3,5-Dinitrosalicyclic Acid (DNSA) Assay
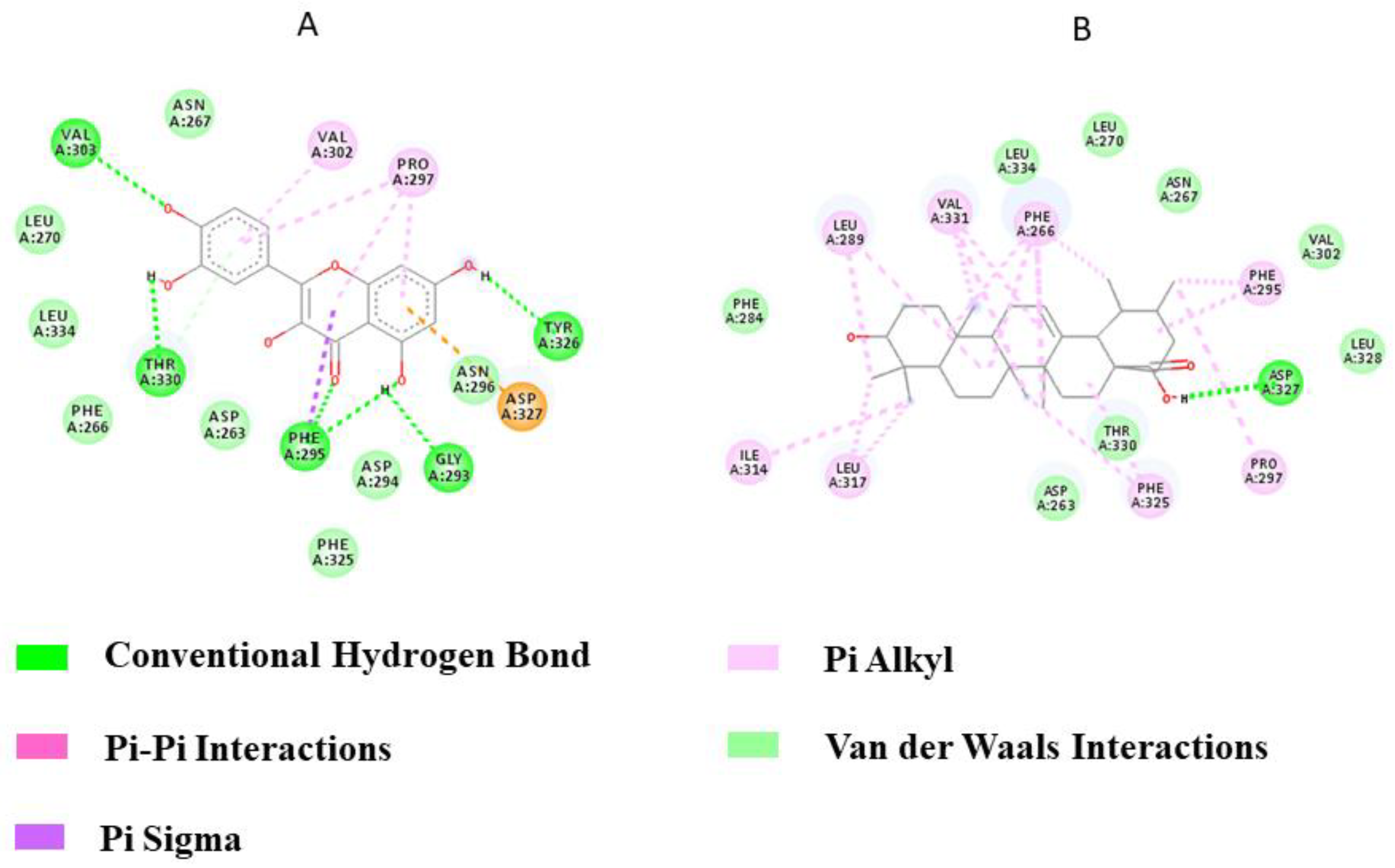
3.7. In Silico Docking Studies
3.7.1. With PPA
3.7.2. With TLR-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J. Epidemiol. Glob. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Tarling, C.A.; Woods, K.; Zhang, R.; Brastianos, H.C.; Brayer, G.D.; Andersen, R.J.; Withers, S.G. The search for novel human pancreatic α-amylase inhibitors: High-throughput screening of terrestrial and marine natural product extracts. ChemBioChem 2008, 9, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Chen, H.; Hu, J.; Fan, S.; Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Luo, Y.; Hu, Z.; Qin, D.; Luo, F. Targeting gut microbiota in type 2 diabetes mellitus: Potential roles of dietary flavonoids. Food Biosci. 2022, 45, 101500. [Google Scholar] [CrossRef]
- Galani, V.J.; Patel, B.G.; Patel, N.B. Argyreia speciosa (Linn. f.) sweet: A comprehensive review. Pharmacogn. Rev. 2010, 4, 172. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Kamble, A.D. Flavonoids from Argyreia nervosa (Burm. f.) Bojer: A ready arsenal against pests as well as Diabetes. Biol. Life Sci. Forum 2020, 4, 56. [Google Scholar] [CrossRef]
- Jialal, I.; Kaur, H. The role of toll-like receptors in diabetes-induced inflammation: Implications for vascular complications. Curr. Diabetes Rep. 2012, 12, 172–179. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 15th ed.; Saunders Publishers: London, UK, 2002; pp. 42–44, 221–229, 246–249, 304–306, 331–332, 391–393. [Google Scholar]
- Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Med. Ther. 2011, 11, 5. [Google Scholar] [CrossRef]
- Rout, K.K.; Singh, R.K.; Barik, D.P.; Mishra, S.K. Thin-Layer chromatographic separation and validated HPTLC method for quantification of Ursolic acid in various Ocimum species. J. Food Drug Anal. 2012, 20, 22. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kamble, R.P.; Ghosh, P.; Kulkarni, A.A. Identification of α-amylase inhibitory compounds from leaves of Careya arborea Roxb. and in silico docking studies. S. Afr. J. Bot. 2022, 151, 493–503. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Shakil, S.; Haneef, M. A simple click by click protocol to perform docking: AutoDock 4.2 made easy for non-bioinformaticians. EXCLI J. 2013, 12, 831. [Google Scholar] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Path. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Yan, Y.; Liu, D.; Wang, C.; Wang, H. Inhibition of glycosidase by ursolic acid: In vitro, in vivo and in silico study. J. Sci. Food Agric. 2020, 100, 986–994. [Google Scholar] [CrossRef]
- Lolok, N.; Sumiwi, S.A.; Muhtadi, A.; Susilawati, Y.; Hendriani, R.; Ramadhan, D.S.F.; Levita, J.; Sahidin, I. Molecular docking and molecular dynamics studies of bioactive compounds contained in noni fruit (Morinda citrifolia L.) against human pancreatic α-amylase. J. Biomol. Struct. Dyn. 2022, 40, 7091–7098. [Google Scholar] [CrossRef]
- Guo, P.; Wu, C.M. Gut microbiota brings a novel way to illuminate mechanisms of natural products in vivo. Chin. Herb. Med. 2017, 9, 301–306. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Z.; Liu, C.; Huang, C.K.; Luo, F.Y.; Zhu, X. Ursolic acid improves intestinal damage and bacterial dysbiosis in liver fibrosis mice. Front. Pharmacol. 2019, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Kohan, F. Toll-like receptor 2 and type 2 diabetes. Cell. Mol. Biol. Lett. 2016, 21, 2. [Google Scholar] [CrossRef] [PubMed]




| Sr. No. | Compound Name | Binding Energy Kcal/mol | Interaction | Amino Acid Residues |
|---|---|---|---|---|
| 1 | Quercetin | −9.89 | 3 H-Bonds | Asp197, Tyr62, Gln63 |
| 2 | Ursolic acid | −8.96 | 1 H-Bond | Asp300 |
| Van der Waal | Glu233, Arg195, Asp197, His299, Trp58, His305, Gly164, Gln 63 | |||
| 3 | Acarbose | −12.48 | 9 H-Bonds | His305, His101, Lys200, Tyr151, His201, Glu233, Asp300, His299, Trp59 |
| Van der Waal | Val163, Ile235, Leu162, Gly306, Ala198, Arg195, Trp58, Asp197, Leu165, Gln63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamble, A.D.; Kumbhar, A.A.; Kulkarni, R.P.; Kulkarni, A.A. Potential Hypoglycemic Secondary Metabolites from Argyreia nervosa (Burm. f.) Bojer Influencing Human Gut Health. Med. Sci. Forum 2023, 21, 42. https://doi.org/10.3390/ECB2023-14090
Kamble AD, Kumbhar AA, Kulkarni RP, Kulkarni AA. Potential Hypoglycemic Secondary Metabolites from Argyreia nervosa (Burm. f.) Bojer Influencing Human Gut Health. Medical Sciences Forum. 2023; 21(1):42. https://doi.org/10.3390/ECB2023-14090
Chicago/Turabian StyleKamble, Anuja D., Anupa A. Kumbhar, Rashmi P. Kulkarni, and Anjali A. Kulkarni. 2023. "Potential Hypoglycemic Secondary Metabolites from Argyreia nervosa (Burm. f.) Bojer Influencing Human Gut Health" Medical Sciences Forum 21, no. 1: 42. https://doi.org/10.3390/ECB2023-14090
APA StyleKamble, A. D., Kumbhar, A. A., Kulkarni, R. P., & Kulkarni, A. A. (2023). Potential Hypoglycemic Secondary Metabolites from Argyreia nervosa (Burm. f.) Bojer Influencing Human Gut Health. Medical Sciences Forum, 21(1), 42. https://doi.org/10.3390/ECB2023-14090






