Abstract
The strong link between coagulation and inflammation has recently been investigated in multiple sclerosis (MS) in the light of coagulant serine proteases as pro-inflammatory mediators in MS animal models. Our work attempted to enlighten the role of IgG antibodies against the coagulant proteases and characterize their effects in MS pathology. Serum samples from 15 seropositive MS patients for IgG against factor(F)VIIa, thrombin, prothrombin, FXa, FXII, plasmin, and protein C were subjected to antibody purification, followed by in vitro stimulation of human astrocytes. Samples from fourteen healthy controls and eight negative MS patients for antibodies were also subjected to the same procedure as negative controls. The expression levels of the thrombin-activated receptor (PAR-1) and activated pro-inflammatory ERK1/2 kinases were analyzed by immunoblot to evaluate intracellular pathways triggered by these antibodies. PAR-1 and ERK1/2 were upregulated up to four-fold and 2.5-fold, respectively, after stimulation with anti-thrombin IgG fraction or fractions with multiple antibodies, compared to untreated cells. Conversely, no substantial alteration was observed when samples from negative patients for IgG and controls were applied. Thus, IgG against coagulation components might be pro-inflammatory molecules useful for prognosis, monitoring, and developing new therapeutic approaches for MS.
1. Introduction
Multiple sclerosis (MS) is a chronic, autoimmune inflammatory disease in nature, affecting the white and gray matter of the central nervous system (CNS) and presenting multifocal plaques as a result of inflammation caused by localized and infiltrated reactive immune cells. The myelin sheath surrounding nerve axons is destroyed, interrupting impulse transmission or causing neuronal axon loss [1,2]. Pro-inflammatory molecules secreted by immune cells in the CNS further promote the neurodegenerative phenotype [3].
Although the etiopathogenesis of MS is still elusive, increasing evidence points to insufficient regulation of the coagulation-inflammation circuit, which can cause prolonged activation of clotting factors, contributing to chronically activated lesions and an inflammatory milieu in the CNS [4]. Comprehensive studies revealed higher levels of coagulant factor (F)Xa and thrombin expression in patients with RRMS, promoting the secretion of IL-6, IL-8, and C-C motif chemokine ligand 2 (CCL2) and CCL3 [5,6]. Activated FXIIa and FXIIIa have also been evident in MS cases, especially in RRMS and SPMS patients [7], modulating the structure of monocytes, remodeling their cytoskeleton, and further enhancing the phagocytosis process. Simultaneously, elevated FXIIa expression levels have been associated with a high risk of relapse [8].
Interestingly, fibrin(ogen) deposition was detected in acute CNS plaques and co-localized with active astrocytes, microglia, invasive macrophages, and T cells in reactive MS lesions [9]. Fibrin(ogen) deposits frequently appear in the deepest cortical layers, V and VI, contributing to neurodegeneration and axonal loss [10]. Recently, much attention has been paid to characterizing the role of thrombin and fibrin(ogen), considering their accumulation in the brain parenchyma of MS animal models prior to neurological manifestations [11]. They appear to attract and regulate the microglia’s mobility in areas where the myelin sheath will eventually be destroyed, while they stimulate pro-inflammatory mediator expression, enhancing inflammation further [11,12].
Recent studies in diseases with overlapping clinical symptoms with MS, such as antiphospholipid syndrome (APS), have shown that the presence of IgG antibodies against coagulation components can interfere with anticoagulant mechanisms and promote thrombosis [13]. Considering that patients with MS have an increased risk of deep vein thrombosis, ischemic stroke, and cardiovascular disorders in the early onset of the disease [4], it is of great importance to identify the common molecular and cellular pathways activated by abnormal signaling mechanisms between coagulation and inflammation and to understand the role of IgG antibodies against coagulation factors in the context of MS pathophysiology. Along this line, our research aimed to shed some light on potential inflammatory signaling pathways that might be induced by IgG stimulation to provide new evidence regarding the nexus of coagulation and inflammation and reveal potential therapeutic targets for developing novel strategies for personalized MS treatment. Preclinical studies were performed to analyze the expression levels of the thrombin-activated receptor (PAR-1) as well as phosphorylated extracellular signal-regulated protein kinases (ERK1/2) that are involved in inflammatory signaling pathways and induce the transcription of various pro-inflammatory molecules.
2. Materials and Methods
2.1. Study Participants
In the current study, 15 patients diagnosed with MS and referred to the Cyprus Institute of Neurology and Genetics between September 2017 and January 2019, were recruited to investigate the role of IgG antibodies against thrombus-related components. The patients were found positive for the presence of IgG directed against factor (F)VIIa, thrombin, prothrombin, FXa, FXII, plasmin, and protein C in our previous work (Table 1) [14]. Samples from age and gender-matched 14 healthy participants and eight MS patients seronegative for the study IgG antibodies were used as negative controls. The participants signed a written consent form approved by the Cyprus National Bioethics Committee (ΕΕΒΚ/ΕΠ/2016/51).

Table 1.
Laboratory findings of MS participants positive in IgG against coagulation factors.
2.2. Preliminary Investigation of IgG Antibodies upon Astrocyte Activation
The serum samples were subjected to the IgG purification procedure by affinity chromatography using the Nab Protein G Spin Columns (ThermoFisher Scientific, Altrincham, United Kingdom). Pierce High Capacity Endotoxin Removal Resin (ThermoFisher Scientific, Altrincham, United Kingdom) was subsequently applied to all purified samples to remove any endotoxin trace, known to stimulate the inflammatory response and activate the innate immune system. All samples were tested negative for endotoxin presence (<0.06 EU/mL) by the Limulus Amoebocyte Lysate assay (Sigma, UK). The presence of IgG tested was confirmed in all purified samples using the ELISA protocols as described in our previous work [14].
A concentration of 100 μg/mL of endotoxin-free purified IgG was used to stimulate the astrocytic U87 line, as well as purified samples derived from negative MS patients and HCs (negative controls), thrombin and TNF-a (positive controls), and untreated cells (reference). The experiment was repeated using human primary astrocytes to validate our results in conditions close to those seen in vivo. Lysis of cells was prepared by radio-immunoprecipitation assay (RIPA).
PAR-1 overexpression and upregulation of activated ERK 1 and 2 kinase levels were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, proteins were transferred into nitrocellulose membrane and were detected and quantified using monoclonal anti-PAR-1, anti-phospho-p44/42 ERK1/2, and anti-p44/42 ERK1/2. Normalization of the protein expression levels was performed with GAPDH housekeeping protein using anti-GAPDH antibodies. ImageJ 1. x was used to quantify protein levels (LOCI, NIH).
2.3. Statistical Analysis
The analysis of the results was carried out using GraphPad Prism V.5.00 for Windows (La Jolla, CA, USA). The non-parametric Mann–Whitney U-test was applied to analyze age-matching data and the Fisher’s exact test was performed to assess the matching of gender among the study groups.
3. Results
3.1. Expression of PAR-1 and ERK1/2 in Activated U87 Astrocytic Cell Line
Three independent experiments were performed to analyze signaling pathways triggered by IgG antibodies against coagulation components upon four hours of U87 astrocyte stimulation, according to Y. Ishida and colleagues [15].
The relative expression levels of PAR-1 and phosphorylated ERK1/2 were defined after density normalization using the GAPDH housekeeping molecule and compared with the unstimulated cell sample, which serves as the reference sample.
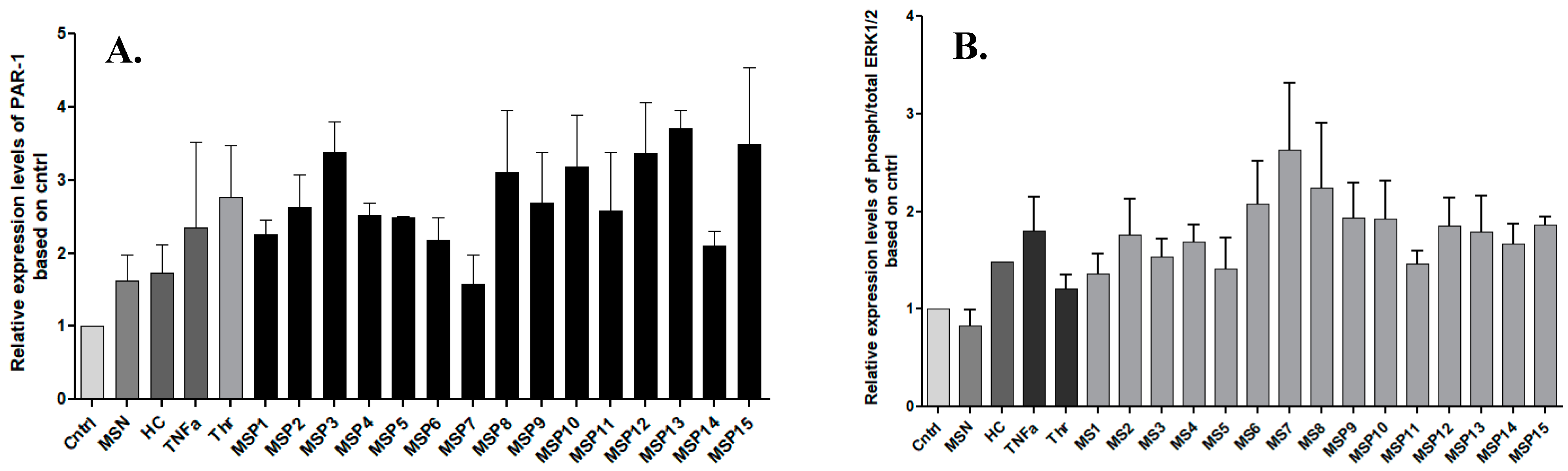
Figure 1 shows up to a four-fold increase in the relative expression levels of PAR-1 and up to 2.5-fold in ERK1/2 levels, respectively in cells stimulated with IgG fractions from positive MS patients. In particular, stimulation with anti-thrombin IgG and anti-plasmin IgG antibodies showed the highest activation of PAR-1 as indicated by the samples MSP-13 and MSP-3, respectively.
Figure 1.
Fold changes in the expression of PAR-1 (A) and ERK1/2 (B) upon stimulation of U87 cells with IgG against coagulation components. Graphs show the relative expression levels based on the control expression (Cntrl) and the bars represent the average of each value from three independent experiments. All the samples were normalized using GAPDH. The highest expression of PAR-1 was observed upon the activation with IgG antibodies against thrombin (MSP-13), while sample with cross-reactivity (MSP-7) showed upregulation of activated ERK1/2. MSN: negative MS patients; HC: healthy controls; MSP: positive MS patients for IgG studied.
Exposure to MS-derived IgG fractions with cross-reactivities showed higher expression levels of activated ERK1/2 than the samples with only one purified antibody. Namely, MSP-6, MSP-7, and MSP-8 demonstrated elevated ERK1/2 expression, while the MSP7 showed the highest relative expression levels, noticing that this sample was seropositive among others for antibodies against thrombin.
In addition, our results showed that stimulation of astrocytes with pooled samples from negative MS patients resulted in low expression levels of PAR, and there was no increase in ERK1/2 phosphorylation. This finding further supports the activation of intracellular signaling pathways in astrocytes mediated by IgG antibodies against coagulation components and the action of such antibodies to trigger both procoagulant and pro-inflammatory signaling pathways.
3.2. Activation of PAR-1 and ERK1/2 upon Stimulation of Human Primary Astrocytes
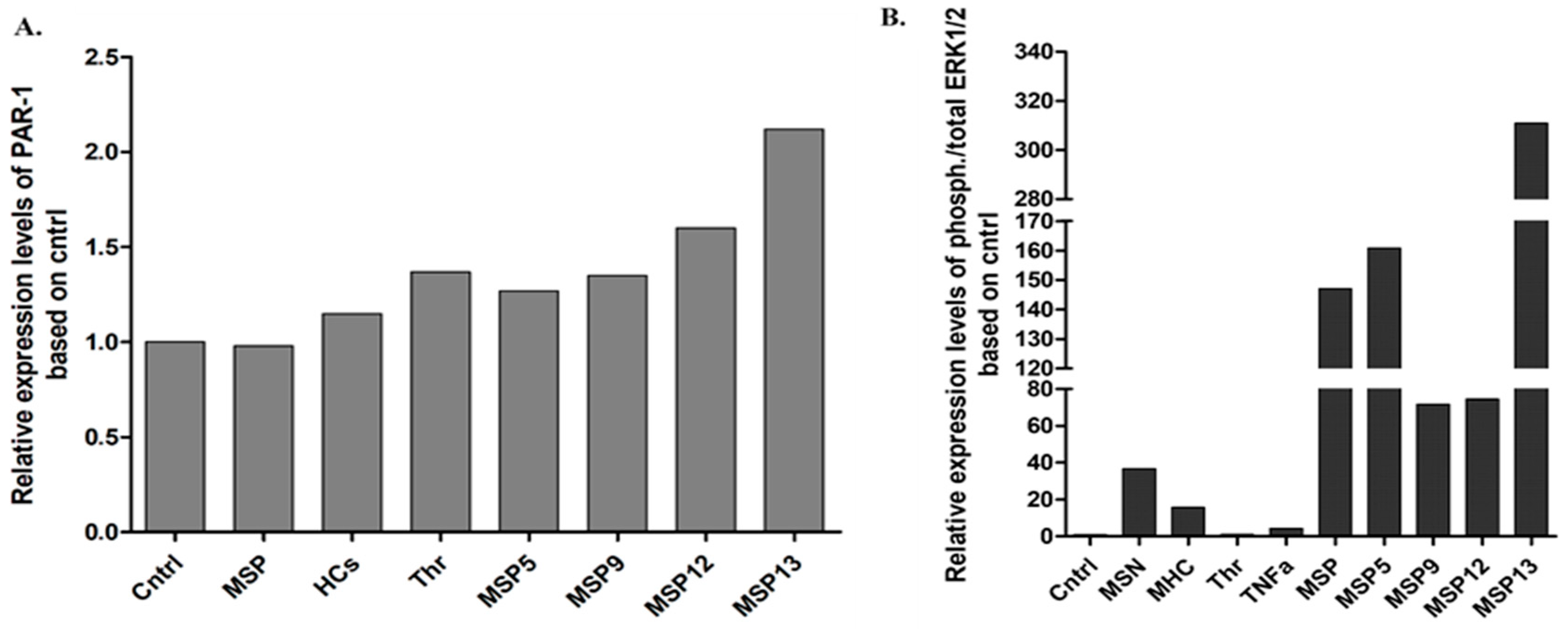
Following four hours of stimulation, the expression of PAR-1 levels and activated ERK1/2 (Figure 2) were similar to those observed in vitro. Specifically, the IgG fraction of the MSP-13 patient, who showed thrombin activity, had the highest levels of PAR-1 expression, confirming the results of U87 cell activation. Moreover, as Figure 2 shows, exposure to purified samples derived from patients with seropositivity for more than one antibody (MSP-6, MSP-7, MSP-8) induced increased relative expression levels of ERK1/2 kinases, with the highest levels being detected when astrocytes were treated with the sample that provided, among others, activity against thrombin (MSP-7).
Figure 2.
Relative expression of PAR-1 (A) and ERK1/2 (B) following activation of primary human astrocytes. Stimulation of primary human astrocytes with pooled IgG fractions derived from the positive MS patients (MSP), and HCs as well as thrombin and IgG from four positive MS patients for 4 h. All the samples were first normalized using housekeeping b-tubulin.
4. Discussion
In our previous study, we detected the presence of IgG antibodies against thrombus-related proteases in a significantly increased proportion of patients diagnosed with MS (43%) compared to healthy participants (20%, p < 0.001), demonstrating that antibodies against plasmin and thrombin were associated with advanced disability and disease worsening, respectively [14].
In the current study, substantial evidence provided the effects of IgG antibodies against coagulant serine proteases in MS pathology, supporting that such antibodies can provoke pro-inflammatory signaling pathways contributing to MS underlying mechanisms. In particular, IgG isolated from MS patients induced upregulation of the thrombin-activated PAR-1 receptor, responsible for the thrombin-mediated inflammatory responses, and the activated pro-inflammatory ERK1/2 kinases in treated astrocytes compared to untreated cells. A four-fold increase in PAR-1 expression levels was observed in the cells stimulated with antibodies against thrombin.
Noteworthy, our findings confirmed the implication of the coagulation-inflammation interplay in chronic inflammatory diseases, and specifically, we revealed for the first time that IgG antibodies against coagulation components have a role in neuroinflammation and neurodegeneration.
Interestingly, in the context of chronic neurodegenerative diseases, few studies have assessed the role of activated ERK1/2 kinases in terms of demyelination and neuroinflammation following astrocyte activation. Astrogliosis and ERK phosphorylation have both been observed in the early stages of neurodegenerative diseases, such as Alzheimer’s disease (AD), while it has also been reported when considering cell survival after ischemia. Z. Jiang et al. demonstrated an immediate increase in ERK1/2 phosphorylation in astrocytes after 1 h of exposure to ischemic conditions, with peak phosphorylation levels being reached four hours later [16].
Similar findings were observed in primary astrocytes from rat animal models when subjected to oxidative or glutamate stress. Overexpression and sustained activation of ERK1/2 kinases have been indicated when astrocytes were exposed to excessive glutamate, inducing cell apoptosis, demonstrating that activated ERK signaling may contribute to the glutamate-dependent death of astrocytes [17]. On the other hand, using ERK inhibitors such as the neuroprotective drug FK506 the activation of ER1/2 is suppressed, preventing cell death in the brain [17]. According to recent studies, ERK1/2 activation in astrocytes triggers the release of CXCL-1, CXCL-2, CCL2, and CCL5 [18].
In the current study, we found that the highest expression of phosphorylated ERK1/2 kinases was observed in the purified fraction with reactivity to thrombin, FXa, and plasmin. Other samples showing reactivity to plasmin and FXa, but not to thrombin did not exhibit similarly high levels of activated ERK1/2 expression. Antibodies to thrombin, therefore, appear to upregulate pro-inflammatory kinases and activate the signaling pathways that are involved. Indeed, earlier studies provided evidence that thrombin stimulation induced the ERK1/2 phosphorylation through different pathways [19]. Thrombin-mediated EGFR phosphorylation could promote activation of ERK1/2 with the recruitment of increased intracellular calcium concentration. Conversely, the Src kinase, located upstream of the EGFR-ERK1/2 signaling pathway, can be activated by thrombin-mediated phosphorylation and subsequently activate the ERK1/2 kinases without the influx of intracellular calcium [19]. Therefore, further investigation of the ERK1/2 activation by thrombin could broaden the horizons of evaluating and developing new therapies concerning the pathways implicated.
An important limitation of this study was the time-consuming protocol followed and the expensive reagents used. We only collected a small number of purified fractions for our preclinical experiments. A large number of samples should therefore be analyzed in future studies and include IgG fractions against various study antigens and fractions with more than one reactivity to make more valid comparisons.
5. Conclusions
Assessing the role of IgG antibodies against coagulation components, we found compelling evidence that these antibodies may act as potential effectors in the coagulation-inflammation interplay in neuroinflammatory diseases such as MS. Moreover, our research could eventually benefit novel therapeutic approaches since such antibodies can serve as targets for new treatments.
Author Contributions
A.L. and M.S.H. conceived and designed the study. M.P. recruited the patients. M.S.H. performed the main experimental work and analyzed the data. M.S.H. and A.L. interpreted the data and drafted the manuscript. M.S.H. prepared the figures and edited the manuscript. A.L., G.K. and C.C. revised the manuscript. All authors provided substantial input throughout the process. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Cyprus Institute of Neurology and Genetics.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Cyprus National Bioethics Committee (EEBK/EΠ/2016/51 obtained in 2016).
Informed Consent Statement
All procedures were carried out according to the ethical standards of the Cyprus National Bioethics Committee. Informed consent was obtained from all subjects involved in the study (EEBK/EΠ/2016/51).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors have no potential conflict of interest to report.
References
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [PubMed]
- Maghzi, A.H.; Borazanci, A.; McGee, J.; Steven Alexander, J.; Gonzalez-Toledo, E.; Minagar, A. Multiple Sclerosis: Pathophysiology, Clinical Features, Diagnosis, and Management. In Neuroinflammation; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123849137. [Google Scholar]
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2002, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Koudriavtseva, T. Thrombotic Processes in Multiple Sclerosis as Manifestation of Innate Immune Activation. Front. Neurol. 2014, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Kraft, P.; Pankratz, S.; Gross, C.C.; Korsukewitz, C.; Kwiecien, R.; Mesters, R.; Kehrel, B.E.; Wiendl, H.; Kleinschnitz, C.; et al. Prothrombin and Factor X Are Elevated in Multiple Sclerosis Patients. Ann. Neurol. 2016, 80, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.E.; O’Connell, K.; Allen, S.; Egan, K.; Szklanna, P.B.; McGuigan, C.; Ní Áinle, F.; Maguire, P.B. Thrombin Generation Correlates with Disease Duration in Multiple Sclerosis (MS): Novel Insights into the MS-Associated Prothrombotic State. Mult. Scler. J.—Exp. Transl. Clin. 2017, 3, 2055217317747624. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Pankratz, S.; Asaridou, C.M.; Herrmann, A.M.; Bittner, S.; Merker, M.; Ruck, T.; Glumm, S.; Langhauser, F.; Kraft, P.; et al. Blood Coagulation Factor XII Drives Adaptive Immunity during Neuroinflammation via CD87-Mediated Modulation of Dendritic Cells. Nat. Commun. 2016, 7, 11626. [Google Scholar] [CrossRef] [PubMed]
- Sárváry, A.; Szucs, S.; Balogh, I.; Becsky, Á.; Bárdos, H.; Kávai, M.; Seligsohn, U.; Egbring, R.; Lopaciuk, S.; Muszbek, L.; et al. Possible Role of Factor XIII Subunit A in Fcγ and Complement Receptor-Mediated Phagocytosis. Cell. Immunol. 2004, 228, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ziliotto, N.; Bernardi, F.; Jakimovski, D.; Zivadinov, R. Coagulation Pathways in Neurological Diseases: Multiple Sclerosis. Front. Neurol. 2019, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Yates, R.L.; Esiri, M.M.; Palace, J.; Jacobs, B.; Perera, R.; DeLuca, G.C. Fibrin(Ogen) and Neurodegeneration in the Progressive Multiple Sclerosis Cortex. Ann. Neurol. 2017, 82, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Baeten, K.M.; Whitney, M.A.; Mullins, E.S.; Friedman, B.; Olson, E.S.; Ryu, J.K.; Smirnoff, D.S.; Petersen, M.A.; Bedard, C.; et al. Early Detection of Thrombin Activity in Neuroinflammatory Disease. Ann. Neurol. 2014, 82, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Beilin, O.; Karussis, D.M.; Korczyn, A.D.; Gurwitz, D.; Aronovich, R.; Hantai, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Chapman, J. Increased Thrombin Inhibition in Experimental Autoimmune Encephalomyelitis. J. Neurosci. Res. 2005, 79, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Artim-Esen, B.; Pericleous, C.; Mackie, I.; Ripoll, V.M.; Latchman, D.; Isenberg, D.; Rahman, A.; Ioannou, Y.; Giles, I. Anti-Factor Xa Antibodies in Patients with Antiphospholipid Syndrome and Their Effects upon Coagulation Assays. Arthritis Res. Ther. 2015, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Hadjiagapiou, M.S.; Krashias, G.; Deeba, E.; Christodoulou, C.; Pantzaris, M.; Lambrianides, A. Antibodies to Blood Coagulation Components Are Implicated in Patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 62, 103775. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nagai, A.; Kobayashi, S.; Kim, S.U. Upregulation of Protease-Activated Receptor-1 in Astrocytes in Parkinson Disease: Astrocyte-Mediated Neuroprotection through Increased Levels of Glutathione Peroxidase. J. Neuropathol. Exp. Neurol. 2006, 65, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, Y.; Chen, X.; Lam, P.Y.; Yang, H.; Xu, Q.; Yu, A.C.H. Activation of Erk1/2 and Akt in Astrocytes under Ischemia. Biochem. Biophys. Res. Commun. 2002, 294, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska, K.; Gozdz, A.; Dabrowski, M.; Zawadzka, M.; Kaminska, B. Prolonged Activation of ERK Triggers Glutamate-Induced Apoptosis of Astrocytes: Neuroprotective Effect of FK506. J. Neurochem. 2010, 113, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzi, M.; Patrone, M.; Passalacqua, M.; Ranzato, E.; Colamassaro, D.; Sparatore, B.; Pontremoli, S.; Melloni, E. Selective Proinflammatory Activation of Astrocytes by High-Mobility Group Box 1 Protein Signaling. J. Immunol. 2007, 179, 8525–8532. [Google Scholar] [CrossRef] [PubMed]
- Bobe, R.; Yin, X.; Roussanne, M.C.; Stepien, O.; Polidano, E.; Faverdin, C.; Marche, P. Evidence for ERK1/2 Activation by Thrombin That Is Independent of EGFR Transactivation. Am. J. Physiol.—Heart Circ. Physiol. 2003, 285, H745–H754. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).