Abstract
Primary biliary cholangitis (PBC) is a slowly progressive cholestatic autoimmune liver disease which leads to fibrosis, cirrhosis, and liver failure. Oxidative stress seems to play an important role in the pathogenesis of chronic liver diseases. The serum level of 8-isoprostane is a marker of oxidative stress in vivo. Oxidative stress causes the production of interleukin 8 (IL-8), which belongs to pro-inflammatory cytokines. The aim of the study was to determine whether the degree of lipid peroxidation determined by measuring the serum level of 8-isoprostane and the elevated concentration of IL-8 influences the progression of PBC. In the study, 72 PBC patients, 15 pathological controls (patients with other autoimmune liver diseases), and 15 healthy blood donors were enrolled. Serum levels of IL-8 and 8-isoprostane in PBC patients were significantly higher compared with the control groups: 91.1 ± 20.1 vs. 4.8 ± 0.6 pg/mL, p = 0.0077; 238.9 ± 226.9 pg/mL vs. 12.3 ± 11.9 pg/mL, p < 0.001, respectively. Serum 8-isoprostane values were positively correlated with a higher concentration of IL-8, bilirubin concentration, and severe liver fibrosis. A correlation between the concentration of IL-8, 8-isoprostane, and specific autoantibodies was observed. The results show that IL-8 and 8-isoprostane may be an important factor in liver pathologies in patients with PBC, especially in the development of inflammatory processes. Serum 8-isoprostane might be a promising marker for the prediction of the degree of liver fibrosis.
1. Introduction
Primary biliary cholangitis (PBC) is a progressive cholestatic, autoimmune liver disease characterized by fibrosis and, in the final stages, cirrhosis and liver failure [1,2]. Specific antimitochondrial (AMA) and antinuclear (ANA) antibodies were found in the sera of most patients [3]. Some studies have shown that oxidative stress may play an important role in the pathogenesis of various chronic liver diseases [4,5]. The serum level of 8-isoprostane, a product of lipid peroxidation, is a marker of oxidative stress in vivo [5]. In previous investigations, 8-isoprostane was determined in the plasma of patients with non-alcoholic fatty liver disease and chronic hepatitis C as a biomarker for increased lipid peroxidation [5,6]. Oxidative stress can induce the production of interleukin 8 (IL-8), which belongs to the group of pro-inflammatory cytokines. Some studies proposed that IL-8 pathways might also be implicated in the pathogenesis of chronic liver disease [7,8]. Laboratory diagnostics use various markers useful in the assessment of liver fibrosis, based on which the advancement of the fibrosis process can be assessed [9].
The aim of the present study was to determine whether the degree of lipid peroxidation determined by measuring the serum level of 8-isoprostane influences the progression of PBC. We also evaluated the serum concentration of IL-8 and studied the correlation between the level of IL-8, 8-isoprostane, specific autoantibodies, biochemical parameters, and histological stage.
2. Materials and Methods
Serum samples of PBC were collected from 72 patients (69 women, 3 men), diagnosed at the Centre of Postgraduate Medical Education (Warsaw, Poland). The diagnosis of PBC was established using generally accepted criteria, corresponding to the practice guidelines of the European Association For The Study of Liver Diseases (EASL) for PBC [10,11]. A biopsy was performed on all patients. The pathologic control group consisted of 10 patients (3 females, 7 males) with PSC (primary sclerosing cholangitis) and 5 AIH (autoimmune hepatitis) patients (4 females, 1 male). Serum samples from 15 healthy adult blood donors (11 females, 4 males) were collected at the Warsaw Blood Bank.
The concentration of 8-isoprostane was measured using a commercially available ELISA kit (Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer’s instructions. Intra-assay and inter-assay performances were 7.0% and 9.5%, respectively. 8-isoprostane concentrations >40 pg/mL were considered positive.
The level of IL-8 was evaluated by applying a commercially available ELISA kit (Sanquin, Amsterdam, The Netherlands), according to the manufacturer’s instructions. Intra-assay and inter-assay performances were 3.5% and 6.6%, respectively. IL-8 concentrations > 10 pg/mL were considered positive.
For statistical analysis, we used MedCalc for Windows, version 7.4.1.0 (MedCalc Software, Mariakerke, Belgium) and Statistica 8.0 (Stat-Soft, Kraków, Poland).
3. Results
3.1. PBC Patients’ Characteristics
The clinical, histological, and laboratory features of PBC patients are presented in Table 1.

Table 1.
The demographic, biochemical, immunological, and histological features of PBC patients.
3.2. Occurrence and Diagnostic Value of Serum 8-Isoprostane
Higher concentrations of 8-isoprostane were found in 62 of the 72 sera of PBC patients (86%). Serum 8-isoprostane was significantly elevated in PBC samples (238.9 ± 226.9 pg/mL), in comparison to healthy controls (12.3 ± 11.9 pg/mL), p < 0.001. PBC patients also had greater 8-isoprostane levels than pathological controls with other autoimmune liver diseases (238.9 ± 226.9 pg/mL vs. 115.4 ± 110.0 ng/mL, p = 0.043).
We found a positive correlation between serum 8-isoprostane concentration and specific AMA M2 levels in PBC patients (r = 0.5, p < 0.001).
A significant association was presented for serum bilirubin level and 8-isoprostane concentration, r = 0.4, p < 0.001.
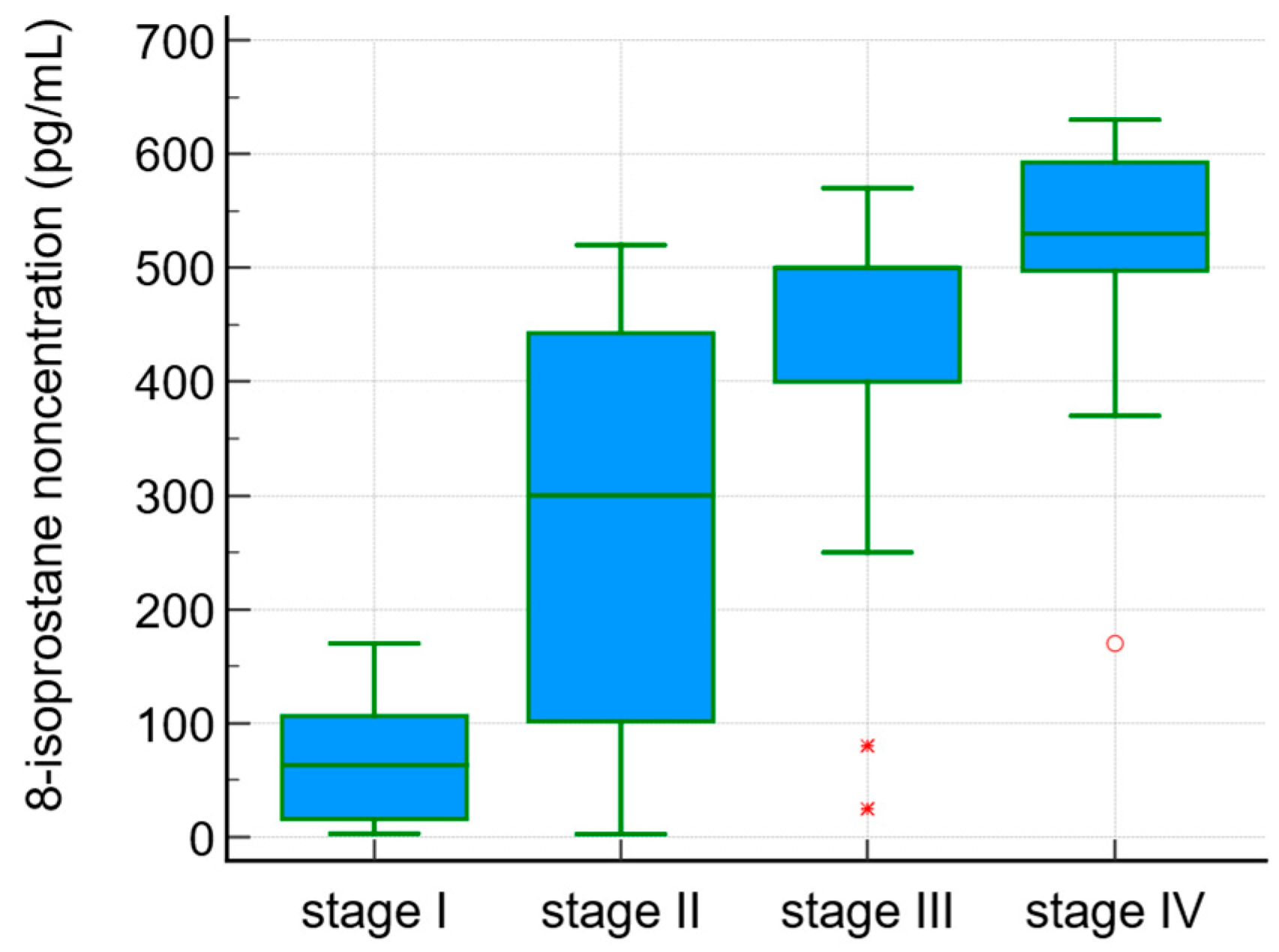
The serum 8-isoprostane concentrations were correlated with the degree of liver fibrosis (r = 0.5, p < 0.0001). 8-isoprostane levels in the sera of PBC patients in the different stages of fibrosis according to Ludwig’s classification are presented in Figure 1.
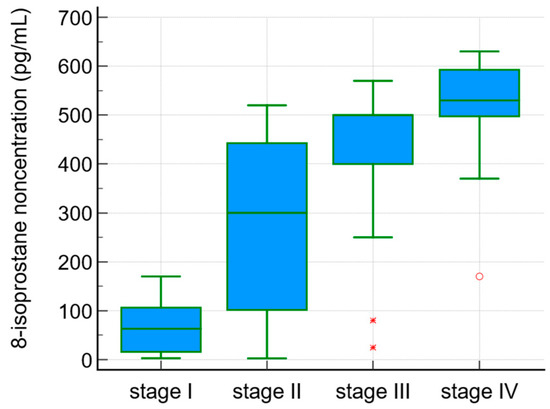
Figure 1.
8-isoprostane concentration in sera of PBC patients and the stage of fibrosis according to Ludwig’s classification.
3.3. Occurrence and Diagnostic Value of Serum IL-8
Elevated levels of IL-8 were measured in 58% of patients with PBC. In the group of patients with positive results of AMA, higher levels of IL-8 were observed in 70% of the subjects. The serum IL-8 concentrations in the PBC group were definitely higher compared with the healthy control group: 91.1 ± 20.1 vs. 4.8 ± 0.6 pg/mL, p = 0.007.
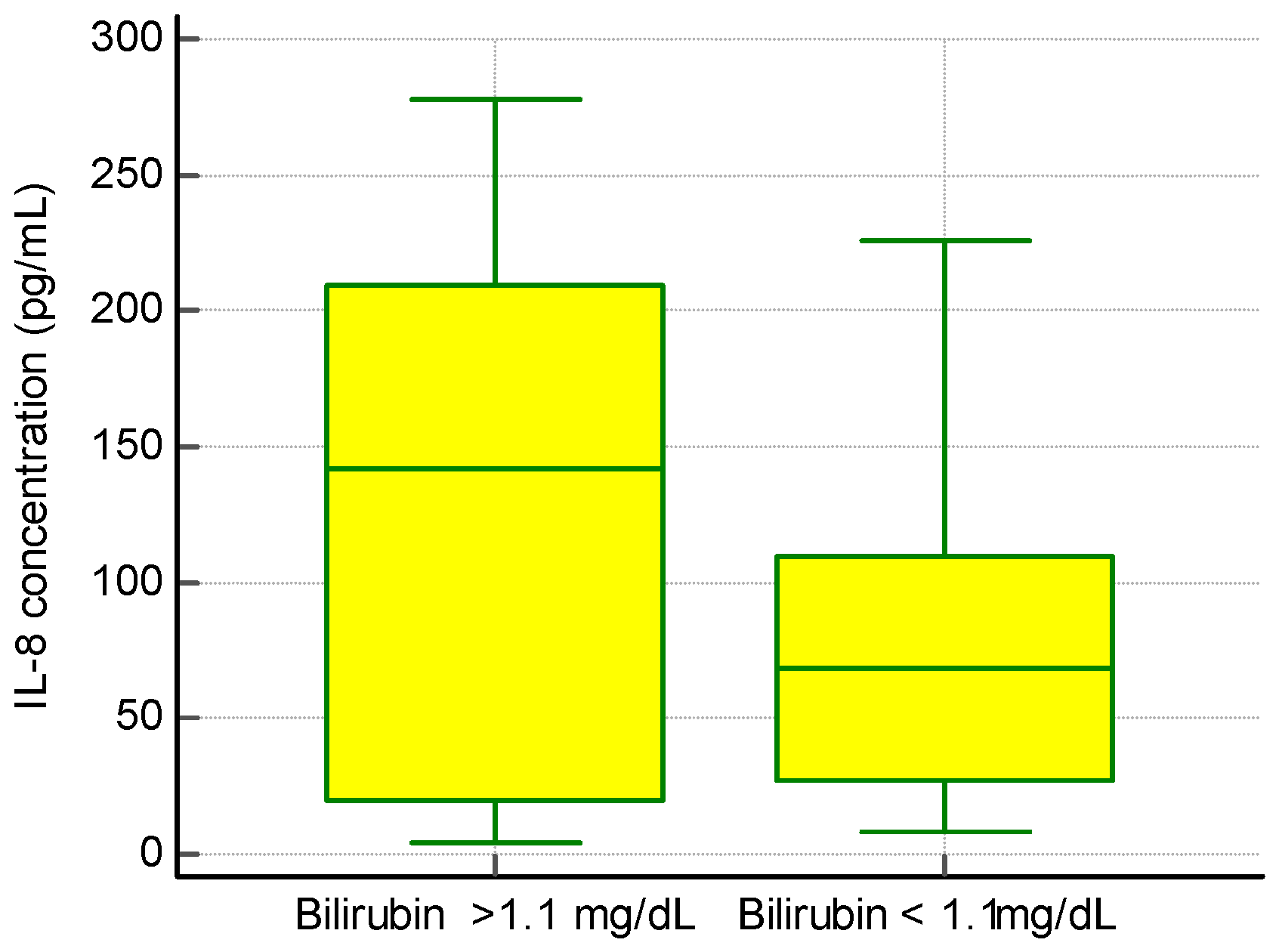
We found no significant correlation between IL-8 levels and ALT and AST activity or total serum γ-globulins, but we observed an association between the presence of IL-8 and a higher concentration of bilirubin (Figure 2).
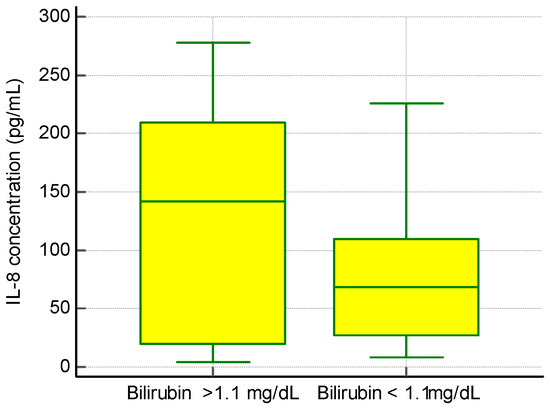
Figure 2.
The concentration of IL-8 and bilirubin levels in PBC patients.
In a group of patients with an elevated level of IL-8 bilirubin, the concentration was 2.2 ± 1.9 mg/dL, while in a group of patients with a normal level of Il-8 bilirubin the concentration was 1.0 ± 1.2 mg/dL, p < 0.01.
We noticed a relationship between elevated IL-8 and the liver fibrosis stage. The concentration of IL-8 was also significantly higher in the group of patients with advanced fibrosis (III/IV stage): 200.8 ± 70.8 pg/mL vs. 69.3 ± 20.9 ng/mL, p < 0.01.
3.4. Correlation between Serum Concentration of 8-Isoprostane and the Level of IL-8
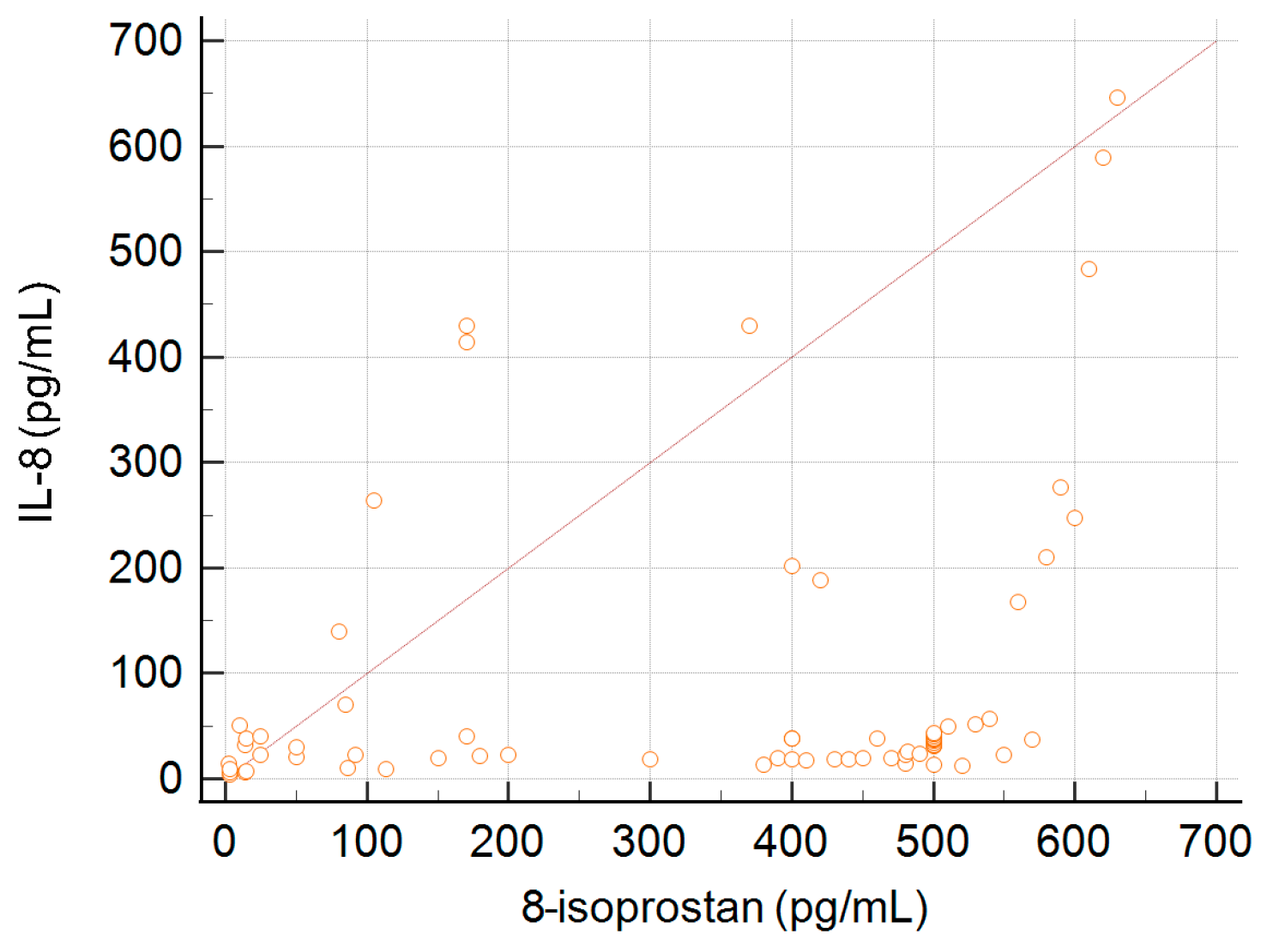
A correlation between the concentration of IL-8 and 8-isoprostane was observed. This association is presented in Figure 3. The coefficient of rank correlation was 0.42, p < 0.001.
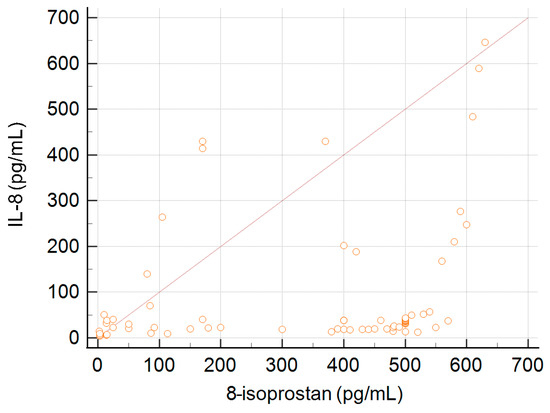
Figure 3.
Correlation between serum concentration of 8-isoprostane and the level of IL-8.
4. Conclusions
The results show that IL-8 may be an important factor in liver pathologies in patients with PBC, especially in the development of inflammatory processes.
In patients with cirrhosis, serum IL-8 levels were significantly higher than those of patients without cirrhosis.
The determination of high concentrations of 8-isoprostane suggests that oxidative stress due to increased lipid peroxidation is involved in the pathogenesis of PBC.
A correlation found between the concentration of IL-8 and 8-isoprostane may confirm the role of 8-isoprostane not only as a marker of oxidative stress, but also as a mediator of inflammation with the participation of cytokine-related mechanisms. Remarkably, the obtained results indicate that serum 8-isoprostane might be a promising marker for the prediction of the degree of liver fibrosis.
Author Contributions
Conceptualization, A.B.; methodology, A.B.; software, A.B; validation, A.B.; formal analysis, A.B.; investigation, A.B.; resources, A.B. and A.H.; data curation, A.H.; writing—original draft preparation, A.B.; writing—review and editing, A.B.; visualization, A.B.; supervision, A.B.; project administration, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by grant: 501-1-025-01-22 from the Centre of Postgraduate Medical Education, Warsaw, Poland.
Institutional Review Board Statement
The studies involving human participants were reviewed and approved by Ethical Committee of the Centre of Postgraduate Medical Education, Warsaw (approval number 71/PB/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article can be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.; Guan, Y.; Han, C.; Zhang, Y.; Liu, Q.; Wei, W.; Ma, Y. The pathogenesis, models and therapeutic advances of primary biliary cholangitis. Biomed. Pharmacother. 2021, 140, 111754. [Google Scholar] [CrossRef]
- Carey, E.J.; Ali, A.H.; Lindor, K.D. Primary biliary cirrhosis. Lancet 2015, 386, 1565–1575. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Wu, S.; Duan, W.; Chen, S.; Ou, X.; You, H.; Kong, Y.; Jia, J. Meta-Analysis of Antinuclear Antibodies in the Diagnosis of Antimitochondrial Antibody-Negative Primary Biliary Cholangitis. Gastroenterol. Res. Pract. 2019, 2019, 8959103. [Google Scholar] [CrossRef]
- de la Rosa, L.C.; Goicoechea, L.; Torres, S.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Role of Oxidative Stress in Liver Disorders. Livers 2022, 2, 283–314. [Google Scholar] [CrossRef]
- Konishi, M.; Iwasa, M.; Araki, J.; Kobayashi, Y.; Katsuki, A.; Sumida, Y.; Nakagawa, N.; Kojima, Y.; Watanabe, S.; Adachi, Y.; et al. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-isoprostane. J. Gastroenterol. Hepatol. 2006, 21, 1821–1825. [Google Scholar] [CrossRef]
- Ciapaite, J.; Bakker, S.J.; Van Eikenhorst, G.; Wagner, M.J.; Teerlink, T.; Schalkwijk, C.G.; Fodor, M.; Ouwens, D.M.; Diamant, M.; Heine, R.J.; et al. Functioning of oxidative phosphorylation in liver mitochondria of high-fat diet fed rats. Biochim. Biophys. Acta 2007, 1772, 307–316. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Seidler, S.; Gassler, N.; Nattermann, J.; Luedde, T.; Trautwein, C.; Tacke, F. Interleukin-8 Is Activated in Patients with Chronic Liver Diseases and Associated with Hepatic Macrophage Accumulation in Human Liver Fibrosis. PLoS ONE 2011, 6, e21381. [Google Scholar] [CrossRef]
- Glass, O.; Henao, R.; Patel, K.; Guy, C.D.; Gruss, H.J.; Syn, W.; Moylan, C.A.; Streilein, R.; Hall, R.; Diehl, A.M.; et al. Serum Interleukin-8, Osteopontin, and Monocyte Chemoattractant Protein 1 Are Associated With Hepatic Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1344–1355. [Google Scholar] [CrossRef]
- Joseph, J. Serum Marker Panels for Predicting Liver Fibrosis—An Update. Clin. Biochem. Rev. 2020, 41, 67–73. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. European Association for the Stud EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The Diagnosis and Management of Patients with Primary Biliary Cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).