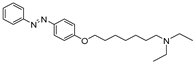
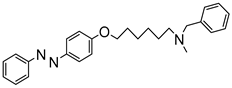
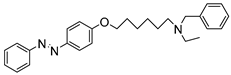
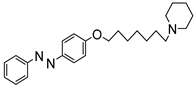
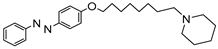
1. Introduction
AChE inhibitors (iAChE) improve AD symptoms by inhibiting AChE and raising the levels of acetylcholine (ACh) in the synaptic cleft, thus constituting a pharmacotherapeutic strategy for reducing AD symptoms [
1,
2]. Resveratrol (3,5,4′-trihydroxystilbene) is a natural polyphenol, which is widely found in foods and medicinal plants. It exerts a variety of promising biological activities, including anti-inflammatory, neuroprotective, anticancer, and antioxidant properties [
3,
4]. However, resveratrol has limited pharmacokinetic parameters [
5]. This limitation has led to the development of multiple strategies aiming to increase its bioavailability and/or solubility through synthesizing resveratrol analogs. In this work, using a simple, rapid, and efficient microwave synthesis, a series of new resveratrol derivatives were synthesized, incorporating secondary amines to increase structural diversity.
2. Materials and Methods
All the chemicals were from Aldrich-Merck and were used without further purification. The AChE (from electric eel, type VI-S), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodide (ATChI), and tacrine were obtained from Sigma Aldrich.
The NMR spectra were recorded on a Bruker Avance ARX-300 spectrophotometer, in CDCl3. A CEM Discover reactor (CEM Corp, Matthews, NC, USA) was employed in the microwave-assisted reactions. The alumina (mm) for the column chromatography was acquired from Merck, Argentina. The TLC detection was carried out using p-anisaldehyde-acetic acid spray reagent (Mallinckrodt, New York, NY, USA) and 254 and 366 nm UV light.
The SH-SY5Y cells were grown in a DMEM/Ham F12 medium (1:1) (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Internegocios, Mercedes, Buenos Aires, Argentina), 100 U/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco) at 37 °C in a 5% CO
2. For biochemical determinations, 2 × 10
5 SH-SY5Y cells were seeded in 24 well culture plates (Gibco) and maintained in the conditions described above [
6].
The cellular damage/death was analyzed through the activity of the cytoplasmic enzyme lactate dehydrogenase (LDH), which is released by cells with damaged plasma membranes. The SH-SY5Y cells, grown as described above for 72 h, were treated with M1–M5 in the growth medium for 24–48h. Then, 100 μL of the medium was collected, and the LDH activity was spectrophotometrically determined using a kinetic assay, according to the manufacturer’s instructions (Wiener LDH-P UV). The LDH release was compared to the 100% LDH release (complete lysis) obtained by treating the cells with Triton X-100 0.1% in phosphate-buffered saline (PBS; 150 mM NaCl, 10 mM Na
2HPO
4, 10 mM, NaH
2PO
4, pH 7.4) [
6,
7].
The AChE activity was determined according to Ellman’s method with minor modifications [
8]. Briefly, the SH-SY5Y cells grown in 24-well culture plates for 72h were washed three times with PBS and pre-incubated for 15 min with M1–M5. Then, the cells were maintained for 30 min in the presence of DTNB (final concentration of 0.31 mM in PBS) and ATChI (final concentration of 0.9 mM in PBS). DMSO was used as a vehicle and was kept below 0.1% in all cases. The control cells were incubated in the presence of the vehicle. After 30 min, 0.9 mL of the solution was collected from each condition, and the absorbance was measured at 412 nm. The results are expressed as a percentage of the control.
All the results are presented as mean ± SEM from at least three independent experiments. The LDH and AChE activity determinations were analyzed using one-way ANOVA followed by a Tukey’s test for multiple comparisons. The differences were considered to be statistically significant when p < 0.05. The analysis of the data was performed using GraphPad Prism 6 software.
3. Results
3.1. Chemistry
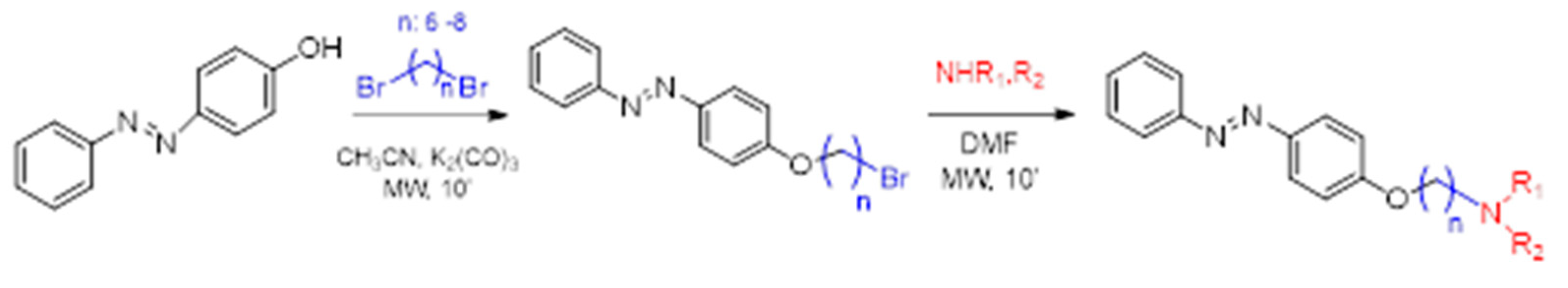
To obtain a series of resveratrol derivatives, azobenzene was first reacted with the corresponding dibromo alkane (6–8 carbon atoms) and then the intermediate was reacted with the secondary amine. Five compounds (M1–M5) were synthesized in moderate yields. These compounds were purified using column chromatography and identified using 1H and 13C NMR. The derivatives were evaluated as iAChE by performing Ellman’s method [
8] (
Figure 1).
3.2. In Vitro AChE-Inhibition Studies
Table 1 shows the obtained IC50 ± SD values of the different resveratrol analogs. All of them were found to be active against AChE. The most active, with an IC50 of 0.27 µM, was the derivative with an 8-carbon-atoms spacer and a piperidine ring, compound M5.
3.3. Cytotoxicity Assay
The cytotoxic effect of the resveratrol analogs was evaluated in the human neuroblastoma cell line, SH-SY5Y [
7]. The treatment of the SH-SY5Y cells with a concentration equal to 6 times the IC50 (previously determined from the in vitro enzyme assays) of the tested compounds was not cytotoxic during 24–48h of treatment.
3.3.1. Cellular Assay for the Inhibition of Acetylcholinesterase
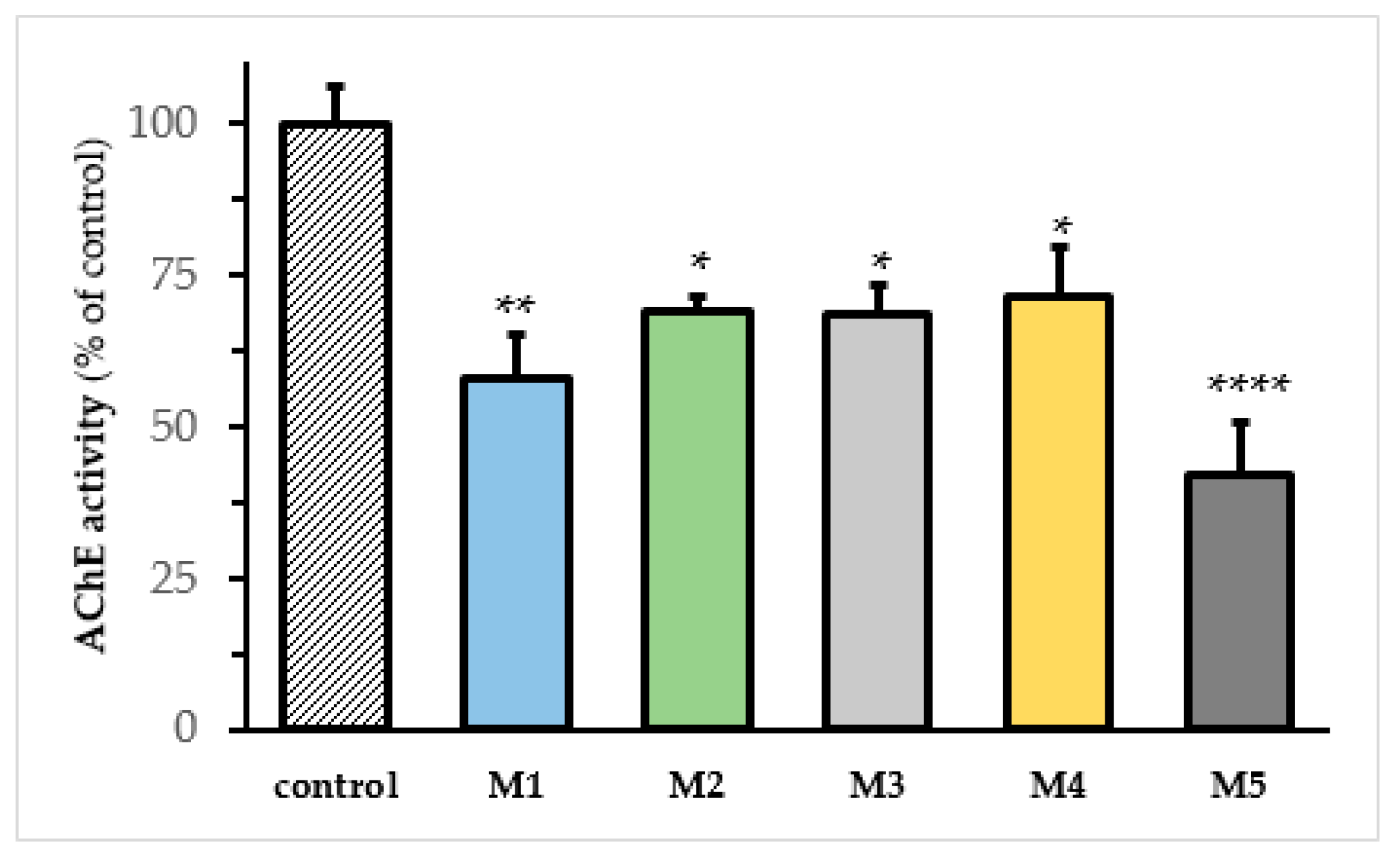
We used SH-SY5Y cells to evaluate the effects of M1–M5 analogs on the AChE activity. For this purpose, the SH-SY5Y cells were treated for 24 h with the aforementioned compounds, and the AChE activity was determined using Elman’s method [
8]. All the tested compounds significantly inhibited the AChE activity in the cell assay (
Figure 2). The drug concentration used in this assay corresponded to the IC50 previously determined in vitro [
6,
7].
3.3.2. Antioxidant Activity
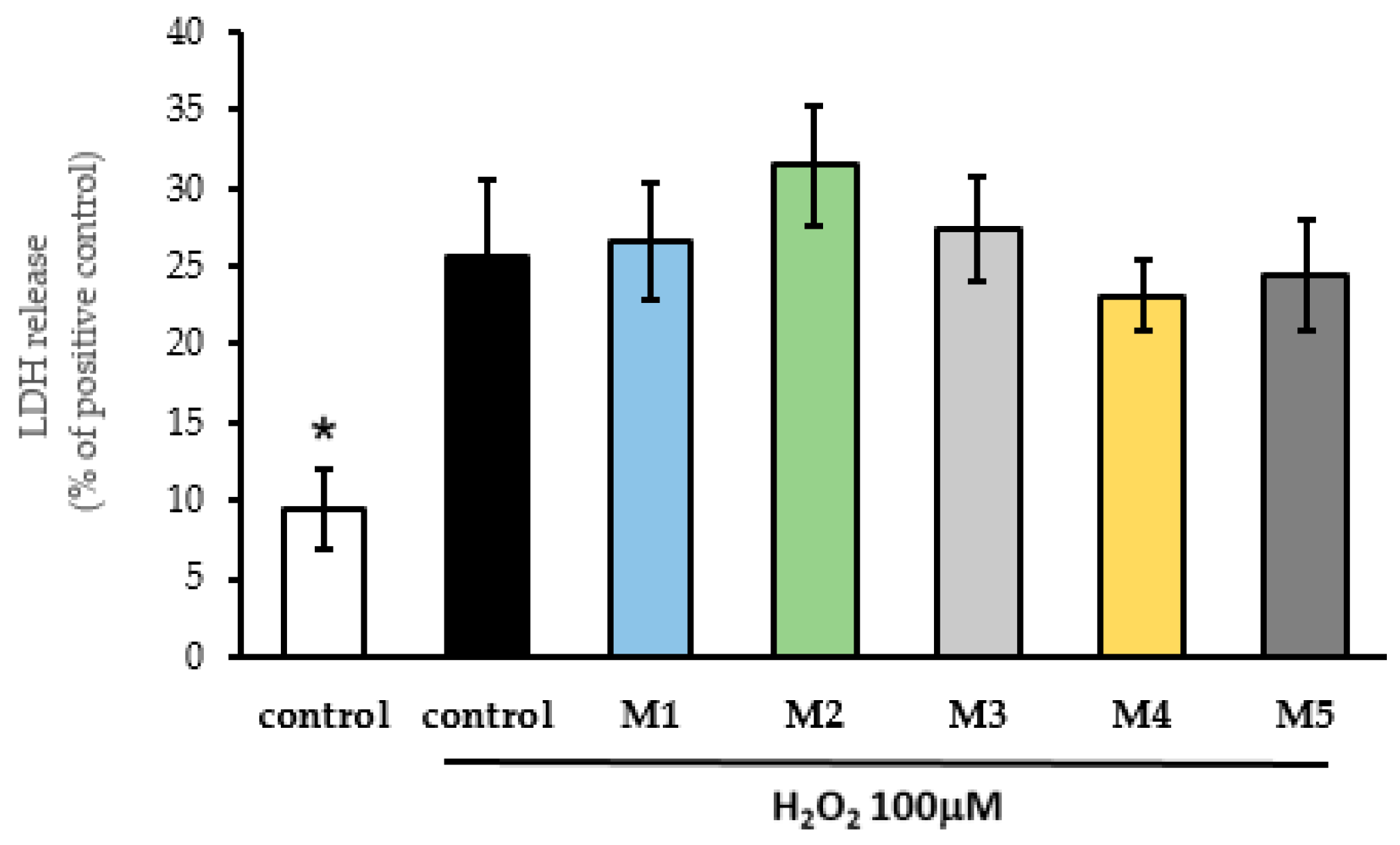
The antioxidant activity of M1, M2, M3, M4, and M5 was evaluated in the SH-SY5Y cells exposed to H
2O
2 100 μM for 24 h. The M1–M5 compounds were administered 24 h before the incubation of the cells with H
2O
2 and maintained throughout the experiment [
8]. The results are shown in
Figure 3. None of the administered compounds showed antioxidant activity when the SH-SY5Y cells were exposed to H
2O
2.
3.3.3. Cellular Protection
We investigated the potential protective activity of M1–M5 against Ca
2+ overload in the SH-SY5Y cells as in Cavallaro et al. [
8]. For this purpose, the SH-SY5Y cells, previously treated with the aforementioned compounds, were subsequently exposed for 24 h to depolarizing medium containing 70 mM KCl, which induced Ca
2+ overload and cell death. The M1–M5 were administered 24 h before the incubation of the cells with high K
+ and maintained throughout the experiment. Subsequently, the LDH release was measured as a marker of the cell damage. As shown in
Figure 4, only compound M1 was shown to exhibit cytoprotective effects against 70 mM KCl treatment.
3.3.4. Resveratrol Analogs on Intracellular ROS Production
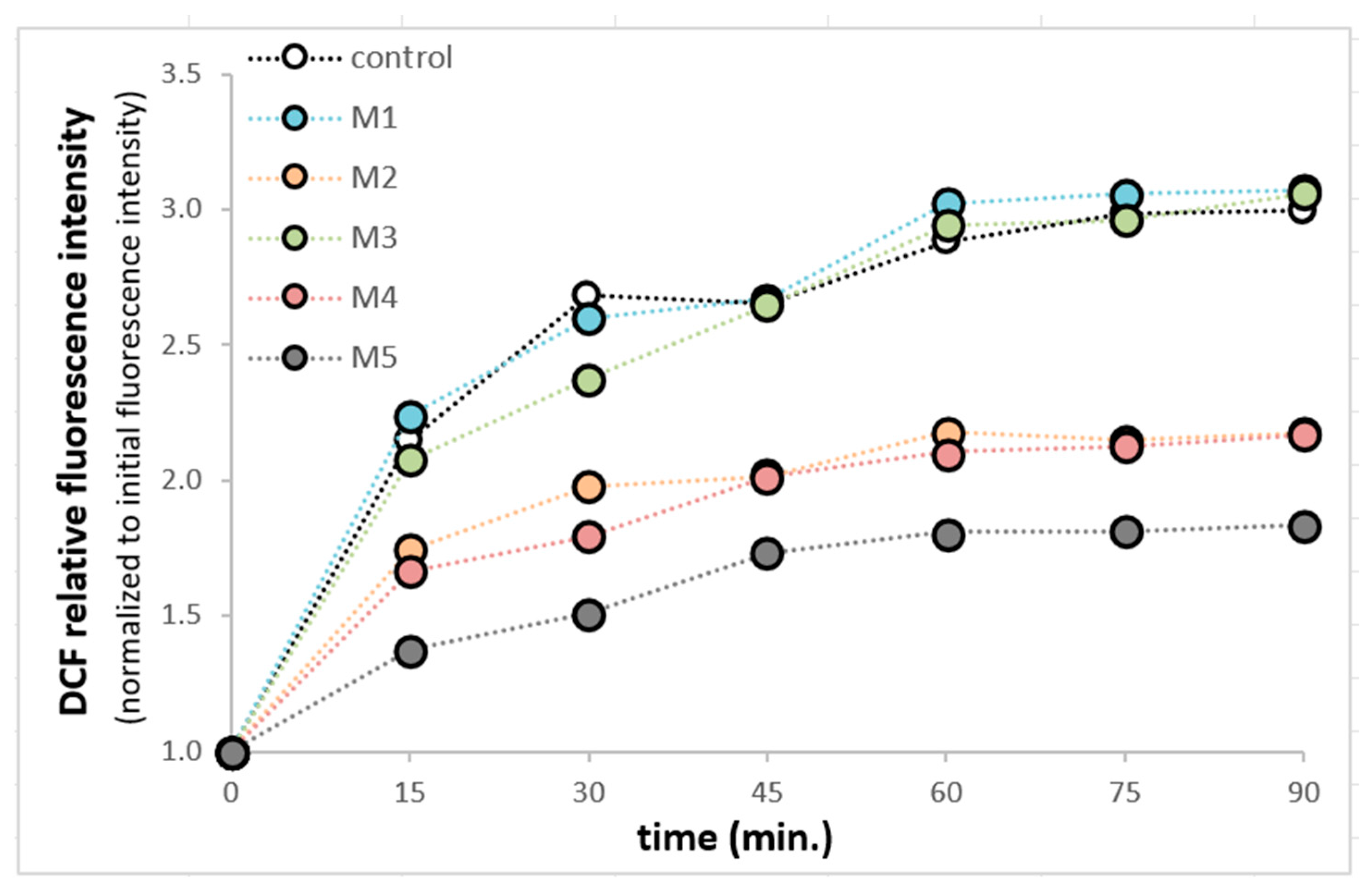
The influence of M1–M5 was analyzed on the intracellular production of ROS in SH-SY5Y cells. This study was performed using fluorogenic 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) dye. After the passive diffusion of the H2DCFDA into the SH-SY5Y cells, it was deacetylated by intracellular esterases into a non-fluorescent compound. In the presence of cellular ROS, it was oxidized into highly fluorescent 2′,7′-dichlorofluorescein (DCF) [
9]. The compounds M2, M4, and M5 can reduce ROS production in SH-SY5Y cells. (
Figure 5).
4. Conclusions
The synthesis of five new resveratrol analogs, with different spacer lengths and terminal amine, was performed. All the analogs were found to be active against the AChE enzyme (in vitro) in the micromolar range.
These results prompted us to perform several biological assays in the SH SY5Y cells. The treatments of the SH-SY5Y cells did not affect the cell viability. All the tested compounds significantly inhibited the AChE activity in the cellular assays. In addition to acting as an AChE inhibitor, compound M1 exhibited neuroprotective effects against KCl-induced Ca2+ overload. Furthermore, we observed that compounds M2, M4, and M5 have the ability to decrease ROS production in the SH-SY5Y cells. These findings indicate that these new resveratrol derivatives could be considered as interesting entities with potential therapeutic applications for AD treatment.
Author Contributions
Conceptualization, C.J.B. and A.P.M.; methodology, M.D., V.C. and C.J.B.; formal analysis, M.D. and C.J.B.; investigation, C.J.B. and A.P.M.; resources, C.J.B. and A.P.M.; data curation, C.J.B. and A.P.M.; writing—original draft preparation, M.D.; writing—review and editing, M.D., C.J.B. and A.P.M.; visualization, M.D. and C.J.B.; supervision, C.J.B. and A.P.M.; project administration, C.J.B. and A.P.M.; funding acquisition, C.J.B. and A.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Secretaría General de Ciencia y Tecnología of Universidad Nacional del Sur PGI 24/B278 (CJB), PGI 24/B297 (CJB), CONICET Grant PIP 11220200102660CO (CJB), PGI 24/Q105 (APM), CONICET Grant PIP 11220200100834CO (APM), FONCYT Grant PICT-2017-1443 N (APM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- León, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Preventing Alzheimer’s disease. Science 2012, 337, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, M.; Fraiz, N.; Cano, E.; Orallo, F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem. Biophys. Res. Commun. 2006, 344, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Javed, S.; Javed, S.; Tariq, A.; Šamec, D.; Tejada, S.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017, 54, 2622–2635. [Google Scholar] [CrossRef] [PubMed]
- Alza, N.P.; Richmond, V.; Baier, C.J.; Freire, E.; Baggio, R.; Murray, A.P. Synthesis and cholinesterase inhibition of cativic acid derivatives. Bioorg. Med. Chem. 2014, 22, 3838–3849. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, V.; Baier, C.J.; Murray, M.G.; Estévez-Braun, A.; Murray, A.P. Neuroprotective effects of Flaveria bidentis and Lippia salsa extracts on SH-SY5Y cells. S. Afr. J. Bot. 2018, 119, 318–324. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tetz, L.M.; Kamau, P.W.; Cheng, A.A.; Meeker, J.D.; Loch-Caruso, R. Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of 3 toxicant-stimulated cellular production of reactive oxidant species. J. Pharmacol. Toxicol. Methods 2013, 67, 56–60. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Synthesis of derivatives M1–M5.
Figure 1.
Synthesis of derivatives M1–M5.
Figure 2.
AChE activity in SH-SY5Y cells treated with resveratrol derivatives. AChE activity in SH-SY5Y cells treated for 24 h with vehicle (DMSO, control) M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM), respectively. AChE activity was determined according to Ellman’s method in live cells. The activities of the enzyme are expressed as % of control (AChE activity) ± SEM of at least seven independent experiments. One-way ANOVA followed by Turkey’s test; (*), (**), and (****) denote p < 0.05, p < 0.001, and p < 0.0001, respectively.
Figure 2.
AChE activity in SH-SY5Y cells treated with resveratrol derivatives. AChE activity in SH-SY5Y cells treated for 24 h with vehicle (DMSO, control) M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM), respectively. AChE activity was determined according to Ellman’s method in live cells. The activities of the enzyme are expressed as % of control (AChE activity) ± SEM of at least seven independent experiments. One-way ANOVA followed by Turkey’s test; (*), (**), and (****) denote p < 0.05, p < 0.001, and p < 0.0001, respectively.
Figure 3.
Evaluation of antioxidant properties of resveratrol derivatives against exposure of SH-SY5Y cells to 100 μM H2O2. SH-SY5Y cells were pretreated M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM) for 24 h before the oxidative insult. Afterward, 100 μM H2O2 was added to the cell culture and incubated for 24h, in the presence of resveratrol derivatives. Cellular damage was analyzed using an LDH release assay. Data are expressed as a percentage of LDH released compared with the positive control. Data are expressed as the means ± SEM of at least four independent experiments. (*) denote p < 0.05 concerning control H2O2.
Figure 3.
Evaluation of antioxidant properties of resveratrol derivatives against exposure of SH-SY5Y cells to 100 μM H2O2. SH-SY5Y cells were pretreated M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM) for 24 h before the oxidative insult. Afterward, 100 μM H2O2 was added to the cell culture and incubated for 24h, in the presence of resveratrol derivatives. Cellular damage was analyzed using an LDH release assay. Data are expressed as a percentage of LDH released compared with the positive control. Data are expressed as the means ± SEM of at least four independent experiments. (*) denote p < 0.05 concerning control H2O2.
Figure 4.
Evaluation of cellular protective effects of resveratrol derivatives against depolarizing concentration of KCl (70 mM) in SH-SY5Y cells. SH-SY5Y cells were pretreated with M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM) for 24 h before the 70 mM KCl insult. Afterward, a depolarizing concentration of KCl (70 mM) was added to the culture medium and the cells were incubated for 24 additional h, in the presence of resveratrol derivatives. Cellular damage was analyzed using an LDH release assay. Data are expressed as the means ± SEM of at least four independent experiments. (*) and (**) denote p < 0.05 and p < 0.01 respect of control KCl.
Figure 4.
Evaluation of cellular protective effects of resveratrol derivatives against depolarizing concentration of KCl (70 mM) in SH-SY5Y cells. SH-SY5Y cells were pretreated with M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM) for 24 h before the 70 mM KCl insult. Afterward, a depolarizing concentration of KCl (70 mM) was added to the culture medium and the cells were incubated for 24 additional h, in the presence of resveratrol derivatives. Cellular damage was analyzed using an LDH release assay. Data are expressed as the means ± SEM of at least four independent experiments. (*) and (**) denote p < 0.05 and p < 0.01 respect of control KCl.
Figure 5.
Resveratrol derivatives effects on DCF fluorescence in SH-SY5Y cells. SH-SY5Y cells were treated with M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM), for 48 h. Then, the cell culture medium was removed via aspiration. After rinsing twice with a warm RPMI medium, the cells were incubated in DCFD 100 μM in RPMI at 37 °C for 1 h. Following the incubation period, the DCFDA-containing medium was replaced with RPMI containing either DMSO or resveratrol derivatives. Fluorescence readings (492 nm excitation and 515 nm emission) were taken at 15 min intervals for 1 h 30 min at 37 °C. Fluorescence at each point was normalized to the initial fluorescence intensity for each experimental condition.
Figure 5.
Resveratrol derivatives effects on DCF fluorescence in SH-SY5Y cells. SH-SY5Y cells were treated with M1 (1.1 μM), M2 (1.4 μM), M3 (0.9 μM), M4 (0.96 μM), and M5 (0.27 μM), for 48 h. Then, the cell culture medium was removed via aspiration. After rinsing twice with a warm RPMI medium, the cells were incubated in DCFD 100 μM in RPMI at 37 °C for 1 h. Following the incubation period, the DCFDA-containing medium was replaced with RPMI containing either DMSO or resveratrol derivatives. Fluorescence readings (492 nm excitation and 515 nm emission) were taken at 15 min intervals for 1 h 30 min at 37 °C. Fluorescence at each point was normalized to the initial fluorescence intensity for each experimental condition.
Table 1.
AChE inhibitory activity values for compounds M1–M5 expressed in IC50 ± SD (µM).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).