PLGA Nanoparticles Loaded with Cinnamon Extract and Coated with PVA/Poloxamer188 †
Abstract
:1. Introduction
2. Materials and Methods
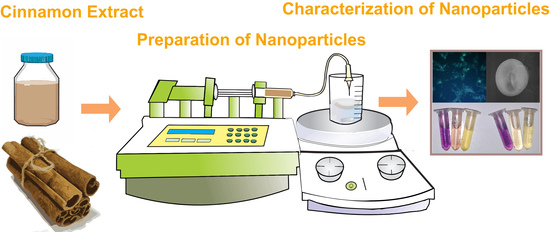
2.1. Preparation of Nanoparticles
2.2. Characterization of NPs
2.2.1. Size Distribution and Zeta Potential
2.2.2. Drug Loading (DL) % and Encapsulation Efficiency (EE) %
2.2.3. In Vitro Drug Release
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Antioxidant Activity
2.4. Blood Compatibility
2.5. Cellular Uptake of Nanoparticles
2.6. Cytotoxicity of Nanoparticles
3. Results
3.1. Physiochemical Characterization of Cin/PLGA NPs
3.2. Antioxidant Activity
3.3. Blood Compatibility
3.4. Cellular Uptake of NPs
3.5. Cytotoxicity of NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haghi-Aminjan, H.; Asghari, M.H.; Farhood, B.; Rahimifard, M.; Hashemi Goradel, N.; Abdollahi, M. The role of melatonin on chemotherapy-induced reproductive toxicity. J. Pharm. Pharmacol. 2018, 70, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, A.; Robertson, G.P. Realizing the Clinical Potential of Cancer Nanotechnology by Minimizing Toxicologic and Targeted Delivery ConcernsLimitations and Approaches of Cancer Nanotechnology. Cancer Res. 2012, 72, 5663–5668. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, N.; Rosiak, A.; Kałużna-Czaplińska, J. The potential role of cinnamon in human health. Forests 2021, 12, 648. [Google Scholar] [CrossRef]
- Hemalatha, N.; Kumar, K.R. Synthesis and characterization of PEG-cinnamon essential oil nanoparticles and their application as an insecticidal agent. J. Adv. Sci. Res. 2021, 12, 142–148. [Google Scholar] [CrossRef]
- Ahmed, J.; Altun, E.; Aydogdu, M.O.; Gunduz, O.; Kerai, L.; Ren, G.; Edirisinghe, M. Anti-fungal bandages containing cinnamon extract. Int. Wound J. 2019, 16, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, T.; Peter, A.; Schulz, N.; Drescher, A.; Bergheim, I.; Machann, J.; Schick, F.; Siegel-Axel, D.; Schürmann, A.; Weigert, C. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS ONE 2014, 9, e92358. [Google Scholar] [CrossRef] [PubMed]
- Frydman-Marom, A.; Levin, A.; Farfara, D.; Benromano, T.; Scherzer-Attali, R.; Peled, S.; Vassar, R.; Segal, D.; Gazit, E.; Frenkel, D. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS ONE 2011, 6, e16564. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Lin, H.; Fang, X.; Wang, Y.; Jiang, Z.; Qu, Y.; Xiang, M.; Shen, Z.; Xin, L.; Lu, Y. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021, 12, 12194–12220. [Google Scholar] [CrossRef] [PubMed]
- Assadollahi, V.; Parivar, K.; Roudbari, N.H.; Khalatbary, A.R.; Motamedi, M.; Ezatpour, B.; Dashti, G.R. The effect of aqueous cinnamon extract on the apoptotic process in acute myeloid leukemia HL-60 cells. Adv. Biomed. Res. 2013, 2, 25. [Google Scholar] [PubMed]
- Gopalakrishnan, S.; Ediga, H.H.; Reddy, S.S.; Reddy, G.B.; Ismail, A. Procyanidin-B2 enriched fraction of cinnamon acts as a proteasome inhibitor and anti-proliferative agent in human prostate cancer cells. IUBMB Life 2018, 70, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; An, T.; Wan, D.; Yu, B.; Fan, Y.; Pei, X. Targets and mechanism used by cinnamaldehyde, the main active ingredient in cinnamon, in the treatment of breast cancer. Front. Pharmacol. 2020, 11, 582719. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Esnaashari, S.S.; Webster, T.J.; Khosravani, M.; Adabi, M. Polymeric nanoparticles for drug delivery in glioblastoma: State of the art and future perspectives. J. Control. Release 2022, 349, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Tayfeh-Ebrahimi, R.; Amniattalab, A.; Mohammadi, R. Evaluation of Effect of Biologically Synthesized Ethanolic Extract of Propolis-Loaded Poly (-Lactic-co-Glycolic Acid) Nanoparticles on Wound Healing in Diabetic Rats. Int. J. Low. Extrem. Wounds, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Madani, F.; Esnaashari, S.S.; Bergonzi, M.C.; Webster, T.J.; Younes, H.M.; Khosravani, M.; Adabi, M. Paclitaxel/methotrexate co-loaded PLGA nanoparticles in glioblastoma treatment: Formulation development and in vitro antitumor activity evaluation. Life Sci. 2020, 256, 117943. [Google Scholar] [CrossRef] [PubMed]
- Husni, P.; Ramadhania, Z.M. Plant extract loaded nanoparticles. Indones. J. Pharm. 2021, 3, 38–49. [Google Scholar] [CrossRef]
- Gursu, B.Y.; Dag, İ.; Dikmen, G. Antifungal and antibiofilm efficacy of cinnamaldehyde-loaded poly (DL-lactide-co-glycolide)(PLGA) nanoparticles against Candida albicans. Int. Microbiol. 2022, 25, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Diab, A.M.; Elzahy, A.F.; Mazrou, K.E.; Tayel, A.A.; Moussa, S.H. Green biosynthesized selenium nanoparticles by cinnamon extract and their antimicrobial activity and application as edible coatings with nano-chitosan. J. Food Qual. 2021, 2021, 6670709. [Google Scholar] [CrossRef]
- Shetty, V.; Jakhade, A.; Shinde, K.; Chikate, R.; Kaul-Ghanekar, R. Folate mediated targeted delivery of cinnamaldehyde loaded and FITC functionalized magnetic nanoparticles in breast cancer: In vitro, in vivo and pharmacokinetic studies. New J. Chem. 2021, 45, 1500–1515. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madani, F.; Khosravani, M.; Adabi, M. PLGA Nanoparticles Loaded with Cinnamon Extract and Coated with PVA/Poloxamer188. Med. Sci. Forum 2023, 20, 8. https://doi.org/10.3390/IECC2023-14262
Madani F, Khosravani M, Adabi M. PLGA Nanoparticles Loaded with Cinnamon Extract and Coated with PVA/Poloxamer188. Medical Sciences Forum. 2023; 20(1):8. https://doi.org/10.3390/IECC2023-14262
Chicago/Turabian StyleMadani, Fatemeh, Masood Khosravani, and Mahdi Adabi. 2023. "PLGA Nanoparticles Loaded with Cinnamon Extract and Coated with PVA/Poloxamer188" Medical Sciences Forum 20, no. 1: 8. https://doi.org/10.3390/IECC2023-14262
APA StyleMadani, F., Khosravani, M., & Adabi, M. (2023). PLGA Nanoparticles Loaded with Cinnamon Extract and Coated with PVA/Poloxamer188. Medical Sciences Forum, 20(1), 8. https://doi.org/10.3390/IECC2023-14262