Effect of Pre-Treatment of Quinoa Seeds on Alcalase Hydrolysis and Antiradical Activity of Peptides Fractions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Quinoa Seeds Origin
2.2. Quinoa Seeds Processing
2.3. Protein Concentrate Preparation
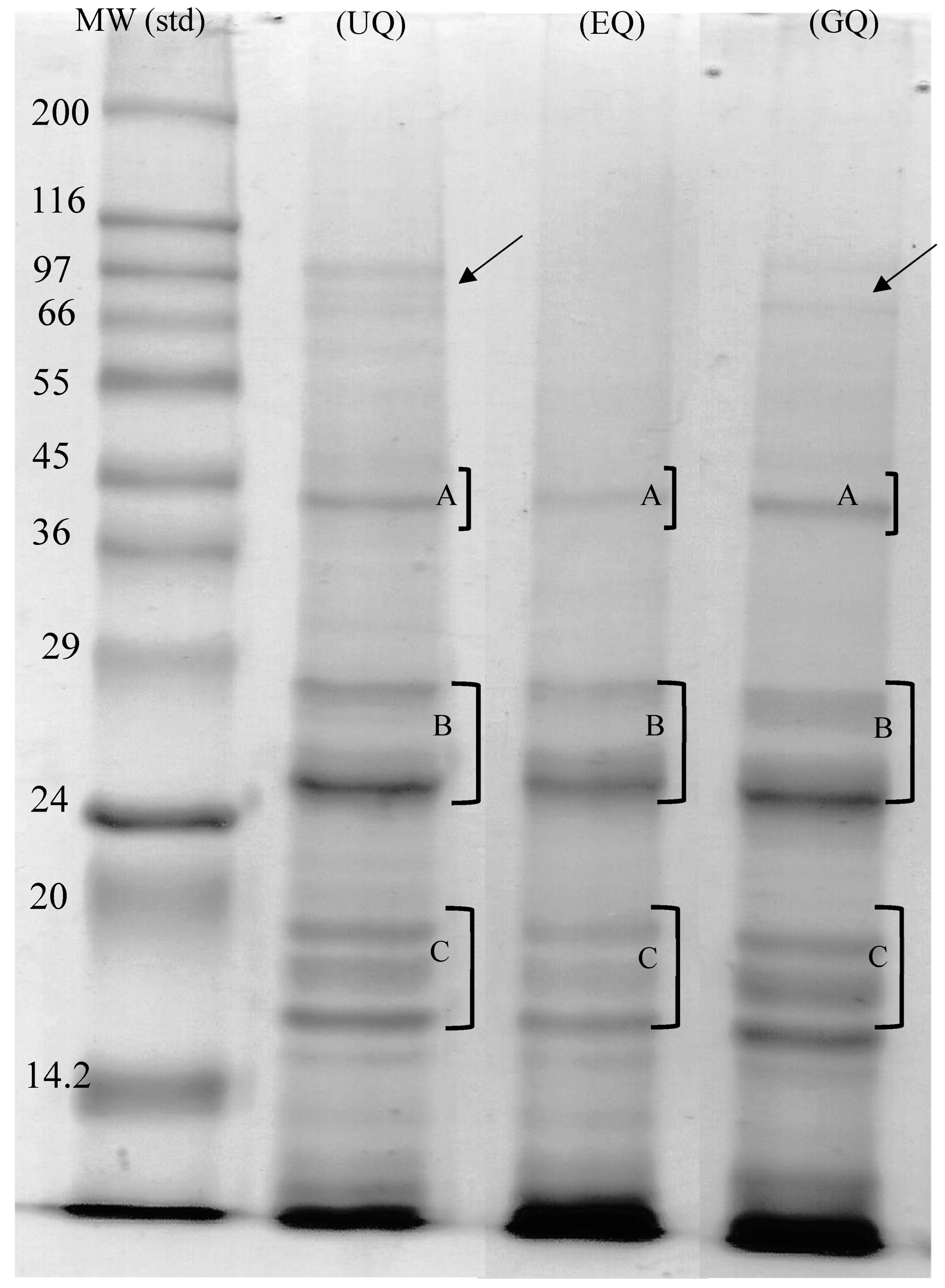
2.4. Electrophoretic Profile by SDS-PAGE
2.5. Protein Hydrolysis, Fractionation and Quantification of Peptides
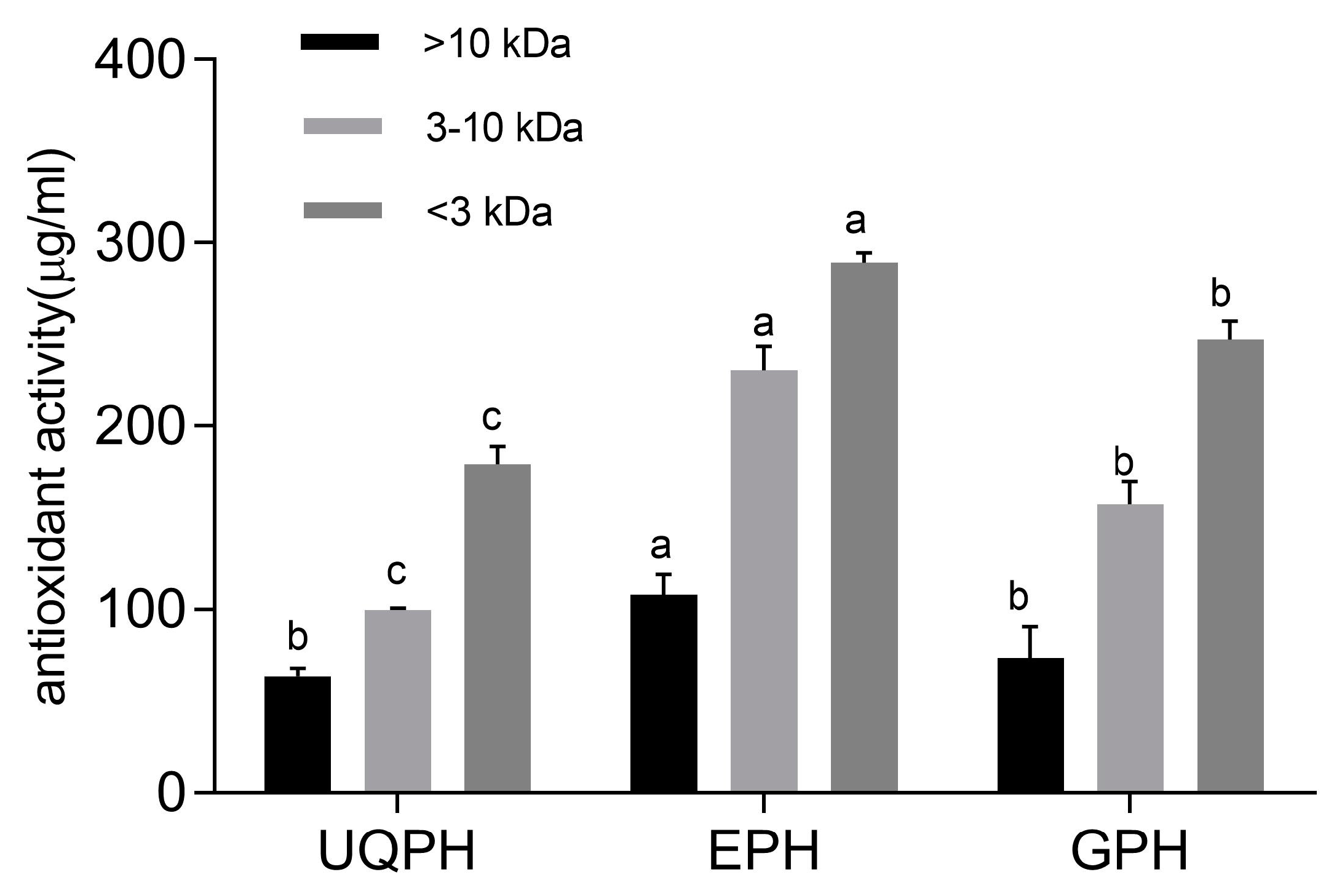
2.6. Antiradical Activity of Peptide Fractions
2.7. Statistics Analysis
3. Results and Discussion
3.1. Electrophoretic Protein Profile of Extruded, Geminated and Control Quinoa Proteins
3.2. Impact of Processing on the Degree of Hydrolysis
3.3. Peptide Quantification in Protein Hydrolysates
3.4. AOC of Peptide Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Pedrouso, M.; Díaz-Reinoso, B.; Lorenzo, J.M.; Cravotto, G.; Barba, F.J.; Moure, A.; Domínguez, H.D.F. Applications of green technologies-based approaches for food safety enhancement: A comprehensive review. Food Sci. Nutr. 2022, 10, 2855–2867. [Google Scholar] [CrossRef]
- Kamau EH Nkhata, S.G.; Ayua, E.O. Extrusion and nixtamalization conditions influence the magnitude of change in the nutrients and bioactive components of cereals and legumes. Food Sci. Nutr. 2020, 8, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Uppal, V.; Bains, K. Effect of germination periods and hydrothermal treatments on in vitro protein and starch digestibility of germinated legumes. J. Food Sci. Technol. 2012, 49, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.C.; Liu, N.; Feng, C.X. Research on the effect of papain co-extrusion on pea protein and enzymolysis antioxidant peptides. J. Food Process. Preserv. 2017, 41, e13301. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Mojica, L.; Johnson, M.H.; Chang, Y.K.; González de Mejía, E. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 2015, 76, 150–159. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; de Mejía, E.G.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-κB signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Rueda, J.; Lobo, M.O.; Sammán, N. Changes in the Antioxidant Activity of Peptides Released during the Hydrolysis of Quinoa (Chenopodium quinoa willd) Protein Concentrate. Proceedings 2020, 53, 12. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Hager, A.S.; Arendt, E.K. Localisation and development of proteolytic activities in quinoa (Chenopodium quinoa) seeds during germination and early seedling growth. J. Cereal Sci. 2014, 60, 484–489. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.S.; Milán-Noris, A.K.; Luna-Vital, D.A.; Milán-Carrillo, J.; Montoya-Rodríguez, A. Anti-inflammatory and antioxidant effects of peptides released from germinated amaranth during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 343, 128394. [Google Scholar] [CrossRef] [PubMed]
- Nor Afizah, M.; Rizvi, S.S.H. Functional properties of whey protein concentrate texturized at acidic pH: Effect of extrusion temperature. Lwt 2014, 57, 290–298. [Google Scholar] [CrossRef]
- Obaroakpo, J.U.; Liu, L.; Zhang, S.; Lu, J.; Pang, X.; Lv, J. α-Glucosidase and ACE dual inhibitory protein hydrolysates and peptide fractions of sprouted quinoa yoghurt beverages inoculated with Lactobacillus casei. Food Chem. 2019, 299, 124985. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Pan, Y.; Li, W.; Wang, C.; Chen, H. Effect of extrusion on physicochemical properties, functional properties and antioxidant activities of shrimp shell wastes protein. Int. J. Biol. Macromol. 2019, 136, 1096–1105. [Google Scholar] [CrossRef] [PubMed]

| Time (min) | UQPH | GPH | EPH |
|---|---|---|---|
| 0 | n.a | n.a | n.a |
| 30 | 28.45 a ± 1.00 | 23.98 b ± 3.32 | 25.04 ab ± 0.83 |
| 60 | 31.27 a ± 1.00 | 27.74 a ± 2.66 | 28.80 a ± 0.83 |
| 120 | 34.56 a ± 1.00 | 27.51 b ± 1.00 | 30.91 ab ± 0.83 |
| 150 | 35.38 a ± 0.83 | 30.09 b ± 3.32 | 31.85 ab ± 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda, J.; Lobo, M.O.; Samman, N.C. Effect of Pre-Treatment of Quinoa Seeds on Alcalase Hydrolysis and Antiradical Activity of Peptides Fractions. Biol. Life Sci. Forum 2023, 25, 8. https://doi.org/10.3390/blsf2023025008
Rueda J, Lobo MO, Samman NC. Effect of Pre-Treatment of Quinoa Seeds on Alcalase Hydrolysis and Antiradical Activity of Peptides Fractions. Biology and Life Sciences Forum. 2023; 25(1):8. https://doi.org/10.3390/blsf2023025008
Chicago/Turabian StyleRueda, Julio, Manuel O. Lobo, and Norma C. Samman. 2023. "Effect of Pre-Treatment of Quinoa Seeds on Alcalase Hydrolysis and Antiradical Activity of Peptides Fractions" Biology and Life Sciences Forum 25, no. 1: 8. https://doi.org/10.3390/blsf2023025008
APA StyleRueda, J., Lobo, M. O., & Samman, N. C. (2023). Effect of Pre-Treatment of Quinoa Seeds on Alcalase Hydrolysis and Antiradical Activity of Peptides Fractions. Biology and Life Sciences Forum, 25(1), 8. https://doi.org/10.3390/blsf2023025008






