Use of By-Products of Selection Process of Bean (Phaseolus vulgaris L.): Extraction of Protein and Starch †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemical Composition
2.3. Macromolecules Extraction
2.3.1. Starch Extraction
2.3.2. Protein Extraction
2.3.3. Extraction Yields
2.4. Functional Properties
2.5. Protein Solubility
2.6. Infrared Spectroscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Bean Flour (BF)
3.2. Functional Properties of Starch
3.3. Bean Protein Concentrate (BPC) Solubility
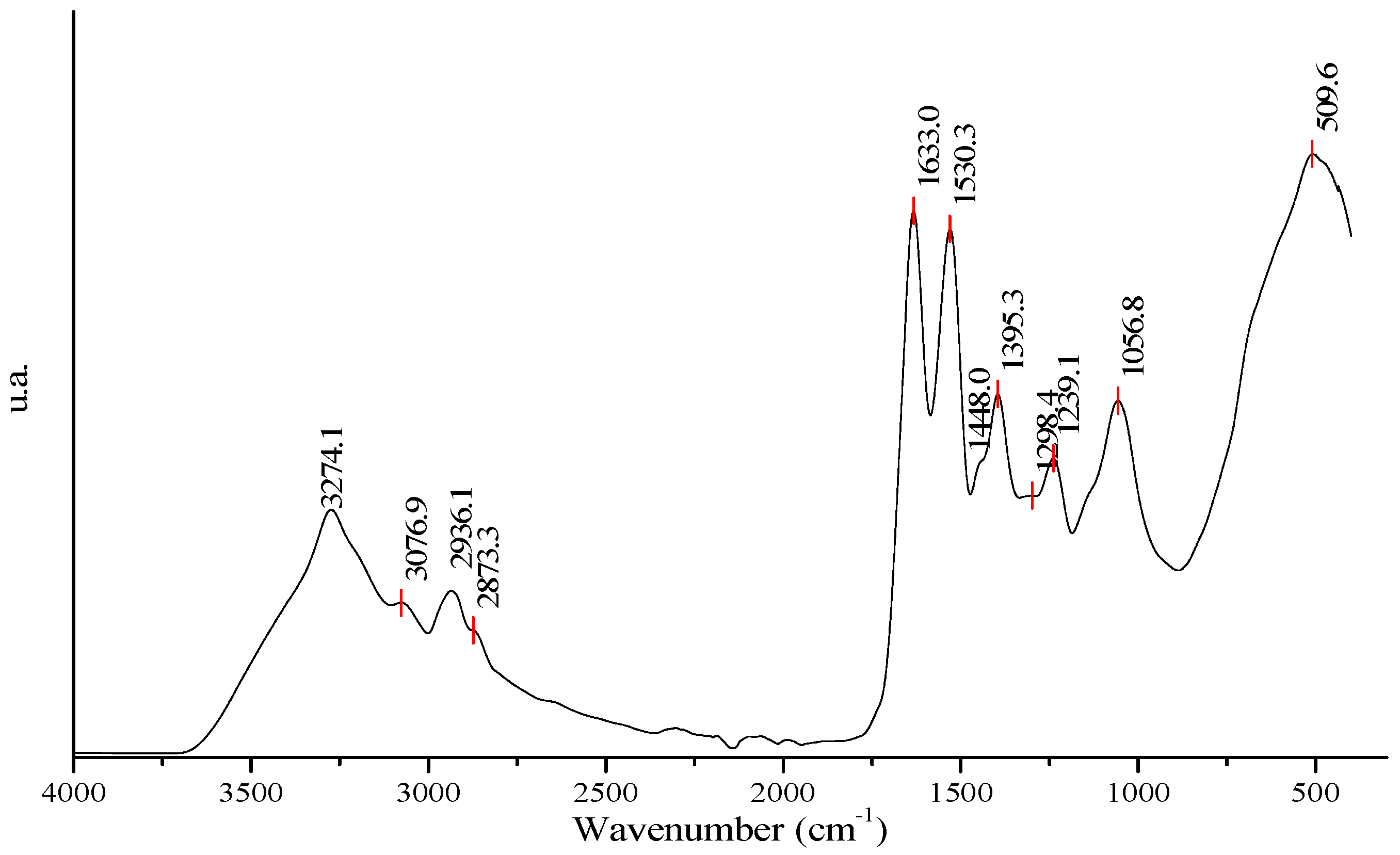
3.4. Profile of FTIR Spectrum of BPC
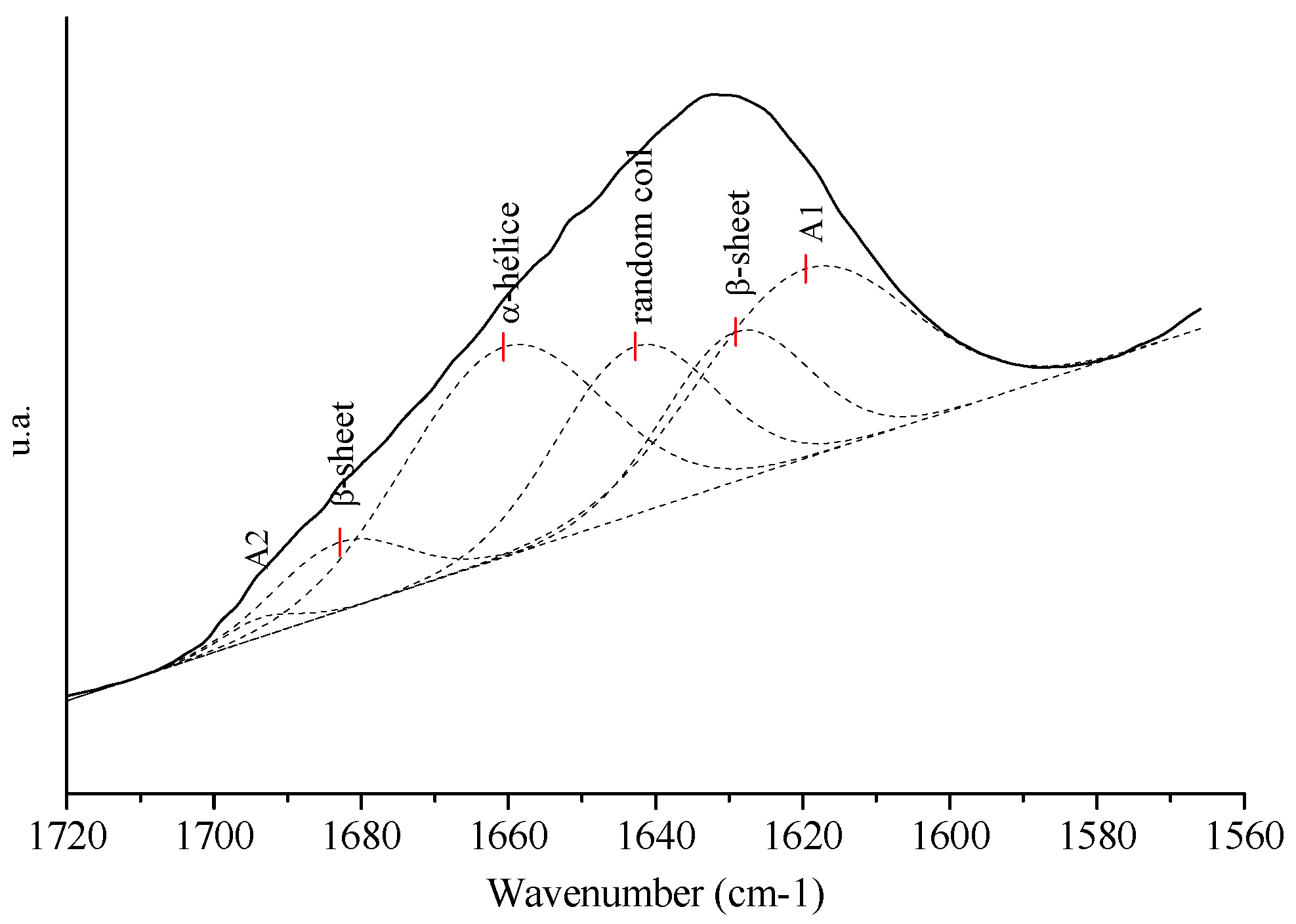
3.5. Proportions of Secondary Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Washington, DC, USA, 2016. [Google Scholar]
- Jan, K.N.; Panesar, P.S.; Rana, J.C.; Singh, S. Structural, Thermal and rheological Properties of Starches Isolated from Indian Quinoa Varieties. Int. J. Biol. Macromol. 2017, 102, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, Physicochemical Characteristics and Functional Properties of Mung Bean Protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Calliope, S.; Wagner, J.; Samman, N. Physicochemical and Functional Characterization of Potato Starch (Solanum Tuberosum Ssp. Andigenum) from the Quebrada De Humahuaca, Argentina. Starch-Stärke 2020, 72, 1900069. [Google Scholar] [CrossRef]
- Przetaczek-Rożnowska, I.; Fortuna, T. Effect of Conditions of Modification on Thermal and Rheological Properties of Phosphorylated Pumpkin Starch. Int. J. Biol. Macromol. 2017, 104, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Ancona, D.; López-Luna, J.; Chel-Guerrero, L. Comparison of the Chemical Composition and Functional Properties of Phaseolus Lunatus Prime and Tailing Starches. Food Chem. 2003, 82, 217–225. [Google Scholar] [CrossRef]
- Sarmento, T.R. Impacto Del Procesamiento Sobre La Pared Celular y Las Propiedades Hipoglucémicas y Tecnofuncionales de Leguminosas, Universidad Autónoma de Madrid, Madrid. 2012. Available online: http://hdl.handle.net/10486/12817 (accessed on 31 July 2023).
- Franca-Oliveira, G.; Fornari, T.; Hernández-Ledesma, B. A Review on the Extraction and Processing of Natural Source-Derived Proteins through Eco-Innovative Approaches. Processes 2021, 9, 1626. [Google Scholar] [CrossRef]
- Miranda-Villa, P.P.; Marrugo-Ligardo, Y.A.; Montero-Castillo, P.M. Caracterización Funcional de Almidón de Frijol Zaragoza (Phaseolus Lunutus) y Cuantificación de su Almidón Resistente. In Tecnológicas; Instituto Tecnológico Metropolitano: Medellín, Colombia, 2013; pp. 17–32. Available online: https://www.redalyc.org/pdf/3442/344234332002.pdf (accessed on 15 August 2023).
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of Low Moisture Extrusion on a Pea Protein Isolate’s Expansion, Solubility, Molecular Weight Distribution and Secondary Structure as Determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
- Alancay, M.M.; Lobo, M.O.; Samman, N.C. Physicochemical and Structural Characterization of Whey Protein Concentrate–Tomato Pectin Conjugates. J. Sci. Food Agric. 2023, 103, 5242–5252. [Google Scholar] [CrossRef] [PubMed]


| Component (g/100 g) | BFA | BS | BPC |
|---|---|---|---|
| Humidity | 11.4 ± 0.1 | 12.6 ± 0.1 | 7.7 ± 0.2 |
| Protein | 20.4 ± 1.2 | 0.5 ± 0.05 | 72.4 ± 0.4 |
| Lipid | 1.1 ± 0.01 | 0.1 ± 0.01 | 0.6 ± 0.03 |
| Ash | 4.7 ± 0.02 | 0.3 ± 0.01 | 8.4 ± 0.1 |
| Carbohydrates * | 62.5 | nd | 10.9 |
| Amilose (g/100 g) | - | 27.9 ± 0.4 | - |
| Amilopectin (g/100 g) | - | 72.1 ± 0.4 | - |
| Phosphorus (mg/100 g BS) | - | 95.9 ± 2.6 | - |
| Yield (%) | - | 50.3 | 13.0 |
| Raw Material | WAI ** | WSI * | SP * |
|---|---|---|---|
| Bean | 3.50 ± 0.54 | 1.67 ± 1.63 | 3.56 ± 0.52 |
| Potato [4] | 2.31–4.84 | 1.16–9.54 | 2.56–4.89 |
| Zaragoza Bean [9] | 3.33–4.43 | 5.70–8.30 | 3.23–4.43 |
| Area (%) | ||||
|---|---|---|---|---|
| Secondary Structures | Minority Fractions | |||
| β-sheet (1625–1640 cm−1) | Random coil (1680–1690 cm−1) | α-helix (1637–1645 cm−1) | A2 * (1690–1695 cm−1) | A1 * (1610–1625 cm−1) |
| 22.0 | 18.8 | 29.7 | 1.0 | 28.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alancay, M.M.; Calliope, S.R.; Miranda, R.M.; Samman, N.C. Use of By-Products of Selection Process of Bean (Phaseolus vulgaris L.): Extraction of Protein and Starch. Biol. Life Sci. Forum 2023, 25, 10. https://doi.org/10.3390/blsf2023025010
Alancay MM, Calliope SR, Miranda RM, Samman NC. Use of By-Products of Selection Process of Bean (Phaseolus vulgaris L.): Extraction of Protein and Starch. Biology and Life Sciences Forum. 2023; 25(1):10. https://doi.org/10.3390/blsf2023025010
Chicago/Turabian StyleAlancay, Matias M., Sonia R. Calliope, Rita M. Miranda, and Norma C. Samman. 2023. "Use of By-Products of Selection Process of Bean (Phaseolus vulgaris L.): Extraction of Protein and Starch" Biology and Life Sciences Forum 25, no. 1: 10. https://doi.org/10.3390/blsf2023025010
APA StyleAlancay, M. M., Calliope, S. R., Miranda, R. M., & Samman, N. C. (2023). Use of By-Products of Selection Process of Bean (Phaseolus vulgaris L.): Extraction of Protein and Starch. Biology and Life Sciences Forum, 25(1), 10. https://doi.org/10.3390/blsf2023025010






