Abstract
The focus of this study is to investigate the gene expression related to the formation of neutrophil extracellular traps (NETs) stimulated by Calloselasma rhodostoma L-amino acid oxidase (Cr-LAAO). LAAOs found in snake venom have been shown to activate human neutrophils, leading to the production of reactive oxygen species (ROS), chemotaxis, phagocytosis, and the release of pro-inflammatory cytokines and lipid mediators. Additionally, it has been found that Cr-LAAO activates NADPH oxidase, which is responsible for the release of ROS. Neutrophils are known to release NETs to combat pathogens, and this process involves the migration of DNA from the nucleus to the cytoplasm, where it merges with the contents of the granules to produce NETs. Initially, the formation of NETs was associated with cell death, and this process was known as NETosis. However, two forms of NETosis have now been identified: classical or suicidal NETosis, which results in cell death, and vital NETosis, in which the cell retains its viability and many of its effector functions. To evaluate the gene expression related to the formation of NETs, a microarray assay was performed on human neutrophils stimulated with Cr-LAAO. The results show that Cr-LAAO stimulates the expression of important genes for the formation of NETs, such as TXNIP, FOXO3, PPARA, ELANE, CXCL8, and PADI4. This is the first report that shows the transcriptome of neutrophils related to Cr-LAAO-stimulated NETosis, which may lead to the development of local inflammatory effects observed in snakebite victims.
1. Introduction
L-amino acid oxidases (LAAOs) can be found in various organisms, including snake venoms [1,2,3,4]. Although the amino acid sequences of LAAOs from different snake venoms are about 80% similar, these proteins follow a similar catalytic mechanism [5]. The process begins with the conversion of FAD to FADH2, which then oxidizes the specific substrate amino acid into an imino acid. This leads to spontaneous hydrolysis that generates an α-keto acid and ammonia. The reoxidation of FADH2 produces hydrogen peroxide (H2O2). Studies suggest that H2O2 is the primary factor associated with the toxicity and cell activation caused by LAAOs [2,3,4].
Research data conducted by Pontes et al. [6,7] have demonstrated that the LAAO from Calloselasma rhodostoma (Cr-LAAO) activates human neutrophils and stimulates the production of reactive oxygen species (ROS) such as superoxide anion and hydrogen peroxide. Later, Paloschi et al. [8] showed that the production of ROS by Cr-LAAO is derived from the activation of the NADPH oxidase complex in human neutrophils. Additionally, Pontes et al. [6] showed that Cr-LAAO induced DNA liberation, along with IL-8, contributing to the formation of Neutrophil Extracellular Traps (NET).
Despite phagocytosis and degranulation being traditionally considered the main defense mechanisms of neutrophils, it is now widely recognized that these cells can also release NETs through a process called NETosis [9]. NET formation is a complex process involving the migration of DNA from the nucleus to the cytoplasm, where it combines with granule contents to form NETs. These NETs play various significant roles in inflammation [10]. NETs consist of loosely packed chromatin that forms DNA structures resembling web-like networks with pores approximately 200 nm in size [11]. These DNA structures are coated with nuclear proteins, including histones, granule proteins, and cytosolic proteins [12].
While profound cellular and molecular rearrangements occur during the initiation of NETosis, the primary signal that triggers this process remains unknown. Considering that Cr-LAAO induces the release of ROS and DNA [6,7,8], the objective of this study is to evaluate the gene expression profile in the process of Cr-LAAO-induced NETosis activation.
2. Methods
2.1. Chemicals and Reagents
Trypan blue, RPMI-1640, L-glutamine, gentamicin, Histopaque 1077, 3,3′,5,5′-tetramethylbenzidine, ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetra acetic acid, HEPES, and ethylenediaminetetraacetic acid disodium salt dihydrate (Na2 EDTA) were purchased from Sigma-Aldrich Chem. Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from Cultilab (São Paulo, Brazil). Pierce™ Chromogenic Endotoxin Quant Kit, GeneChip WT PLUS Reagent Kit, and GeneChip Clariom S Array Human were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Salts and reagents used in this study were obtained from Merck Millipore (Darmstadt, Germany) with low endotoxin or endotoxin-free grades.
2.2. Isolation and Biochemical Characterization of Cr-LAAO
The isolation of Cr-LAAO was performed following the protocol described by Pontes et al. [6] and Paloschi et al. [13]. The presence of endotoxin in Cr-LAAO samples was determined using the Quant kit obtained from Pierce™ Chromogenic Endotoxin. The Cr-LAAO preparation exhibited the presence of 0.1 EU/mL of endotoxin, which was within the acceptable threshold of 1 EU/mL [14].
2.3. Isolation and Activation of Neutrophils
Neutrophils were isolated from peripheral blood obtained from healthy donors aged 18 to 40 years. Donors provided informed consent before blood collection, and all procedures were conducted as per applicable regulations. The study was approved by the Ethical Committee of the Center of Tropical Medicine Research (CEPEM, Rondônia, Brazil-approval number CAAE: 77529817.8.0000.0011), and participants provided informed consent before participating in the study according to the method described by Paloschi et al. [14]. Subsequently, the human neutrophils were incubated with RPMI (control) or Cr-LAAO (50 µg/mL) for analyses. The incubation was carried out for 1 h at 37 °C in a humidified atmosphere with 5% CO2.
2.4. Gene Expression Profile
Human neutrophils were isolated and stimulated as described previously. mRNA was extracted from 5 × 106 neutrophils using the PureLink kit (Thermo Fisher Scientific) following the protocol outlined by Paloschi et al. [13,14]. The extracted RNA was used for microarray analysis with the GeneChip WT PLUS Reagent Kit (Applied Biosystems, Thermo Fisher Scientific) using the GeneChip Clariom S Array Human (Applied Biosystems, Thermo Fisher Scientific) according to the manufacturer’s instructions. A total of 83 genes associated with NET formation, inflammasomes, and NADPH oxidase complex were chosen from the microarray assay and subjected to pathway enrichment analysis using Metascape [15]. Process and pathway enrichment analysis were performed for this gene list using various ontology sources, including KEGG Pathway, GO Biological Processes, Reactome Gene Sets, Canonical Pathways, CORUM, WikiPathways, and PANTHER Pathway. The entire set of genes in the genome was used as background for the enrichment analysis. Terms with a p-value < 0.01, a minimum count of 3, and an enrichment factor > 1.5 were identified and clustered based on their association similarities. The p-values were calculated based on the cumulative hypergeometric distribution, and q-values were calculated using the Benjamini–Hochberg procedure to correct for multiple testing. Hierarchical clustering of the enriched terms was performed using Kappa scores as a similarity metric, and sub-trees with a similarity >0.3 were considered a cluster [16,17,18]. Data visualization was carried out using R software to display the enrichment of the KEGG pathway [19,20,21]. Gene fold change bar plots and chord diagrams were generated using the free online platform for data analysis and visualization available in https://www.bioinformatics.com.cn/en (accessed on 10 June 2023).
3. Results and Discussion
Paloschi et al. [13,14] conducted a study to investigate the gene expression of approximately 22,000 genes in human neutrophils that were either stimulated with Cr-LAAO or not. The GeneChip Clariom S Human Transcriptome Array was used for this analysis. From the assay, genes related to the pathways involved in NET formation, inflammasomes, and the NADPH oxidase complex were selected, and the fold change matrix of expression values was examined in tabular text format to identify target genes. Six chips containing Cr-LAAO-stimulated and non-stimulated (RPMI) neutrophil transcripts from three independent donors were utilized in the study. In the resulting heatmap, upregulated genes (shown in red) indicated higher gene expression in Cr-LAAO-stimulated neutrophils compared to non-stimulated (RPMI) human neutrophils. Conversely, downregulated genes (shown in blue) indicated higher gene expression in the negative control (RPMI)-treated neutrophils compared to Cr-LAAO-treated neutrophils. The data exhibited significant positive or negative fold changes, leading to the selection of a total of 83 genes based on their involvement in the processes described here (Figure 1).
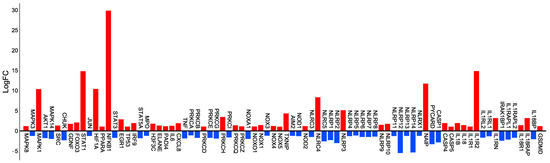
Figure 1.
Visualization of the microarray data depicting the gene expression profile associated with NET formation. The microarray assay was performed using human neutrophils (5 × 106) derived from three different donors (three individuals) stimulated with RPMI (control) or Cr-LAAO (50 μg/mL) for 1 h at 37 °C in an atmosphere containing 5% CO2. Subsequently, RNA was extracted, and treated for cDNA hybridization on a microchip. A total of 22,000 genes were analyzed, and 83 genes that participate in NET formation, inflammasomes, and NADPH oxidase complex were selected. The genes’ relative expression, obtained in the microarray assay by comparing Cr-LAAO-stimulated cells with negative control-stimulated (RPMI) human neutrophils, were represented by the fold change, demonstrating the gene upregulation (red) and downregulation (blue) fold change bar plot.
In order to better visualize the interrelationships between the enriched terms, a subset of terms exhibiting significant enrichment was chosen and presented in a network plot. In this plot, terms with a similarity greater than 0.3 are connected by edges. To construct the network, we selected the terms with the most favorable p-values from each of the 20 clusters. However, we imposed the constraint that each cluster should have no more than 15 terms, and the total number of terms should not exceed 250. The resulting network was visualized using Cytoscape5, where each node represents an enriched term. The nodes are initially colored based on their cluster ID (Figure 2).
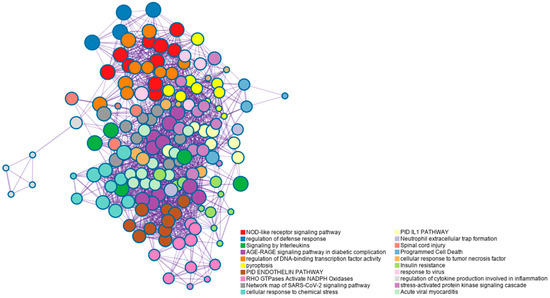
Figure 2.
Network of enriched terms colored by cluster-ID. Based on the data obtained from the microarray assay and gene selection for the study, nodes sharing the same cluster-ID are typically located in close proximity to each other.
From the enrichment analysis, several pathways were identified, including “NOD-like receptor signaling pathway”, “Regulation of defense response Signaling by Interleukins”, “Pyroptosis”, “RHO GTPases Activate NADPH Oxidases”, “Neutrophil extracellular trap formation”, “Programmed Cell Death”, and “Regulation of cytokine production involved in inflammatory response”. These pathways are closely associated with the inflammatory process previously described to be induced by Cr-LAAO. To further investigate the correlation between the genes involved in NET formation and these pathways, we specifically selected these pathways for visualization purposes (Figure 3). By examining the relationship between these pathways and the genes involved in NET formation, we can gain insights into their interconnectedness and potential roles in the inflammatory response mediated by Cr-LAAO (Figure 3).
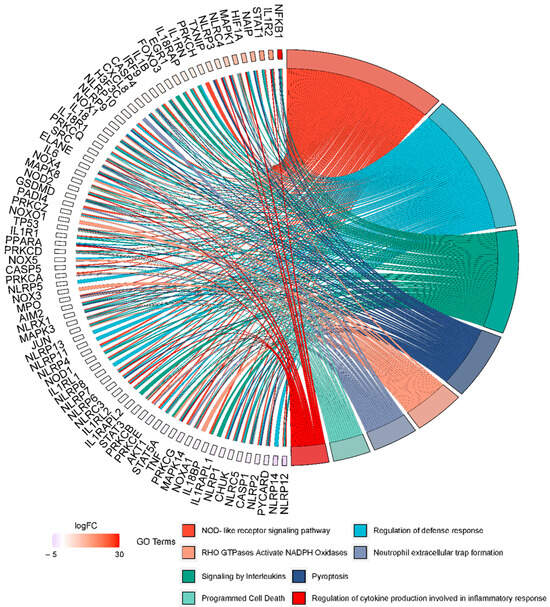
Figure 3.
Correlation of the gene expression profile of NETs with other signaling pathways. Enriched items from pathways related to the formation of NETs, inflammasomes, and the NADPH oxidase complex were compared with pathways related to the inflammatory process and plotted in a chord diagram to visualize the enrichment result.
The results obtained in this study align with previous research, demonstrating the involvement of these pathways in the inflammatory response. The NOD-like receptor signaling pathway and the regulation of cytokine production, for example, have been identified as critical components of the innate immune response and inflammation [22,23]. Likewise, the RHO GTPases Activate NADPH Oxidases pathway is known to regulate the production of ROS, which plays a pivotal role in the inflammatory response [24].
Additionally, this study sheds light on the potential contribution of these pathways to NET formation, a process in which neutrophils release DNA fibers to ensnare and eliminate invading microorganisms. The analysis has revealed differential expression of several genes associated with NET formation upon Cr-LAAO stimulation, suggesting that these pathways may play a crucial role in governing this process. These findings are consistent with previous studies that have demonstrated the involvement of these pathways in the regulation of NET formation [25,26].
In conclusion, the present study offers valuable insights into the alterations in gene expression induced by Cr-LAAO stimulation in human neutrophils, as described in previous studies by Pontes et al. [6,7] and Paloschi et al. [8,13,14]. It highlights the potential significance of several pathways in controlling the inflammatory response and NET formation. These findings are in line with earlier investigations and provide a foundation for further exploration of the mechanisms underlying the inflammatory response triggered by Cr-LAAO.
Author Contributions
Conceptualization, M.V.P. and J.P.Z.; methodology, M.V.P., S.N.S., M.D.S.S. D.G.C., A.V.E.U., C.N.B., J.A.L., C.M.A.R., H.M.S., Y.J.I., B.J.C.F., K.P.F., M.d.M.C.E., L.F.C., J.G.d.S.M., S.d.S.S. and J.P.Z.; software, M.V.P.; formal analysis, M.V.P.; investigation, M.V.P., S.N.S., M.D.S.S., D.G.C., A.V.E.U., C.N.B., J.A.L., C.M.A.R., H.M.S., Y.J.I., C.P.d.S., B.J.C.F., K.P.F., M.d.M.C.E., L.F.C., J.G.d.S.M., S.d.S.S. and J.P.Z.; data curation, M.V.P. and J.P.Z.; writing—original draft preparation, M.V.P.; writing—review and editing, J.P.Z.; visualization, A.M.S. and J.P.Z.; supervision, J.P.Z.; project administration, M.V.P. and J.P.Z.; funding acquisition, A.M.S. and J.P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), FINEP convênio 0418010400 (0654/16), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Rondônia (FAPERO) for the financial support (Universal 01.1331.00032.009/2015) and Instituto Nacional de Epidemiologia na Amazônia Oci-dental-INCT-EpiAmO. This study was supported by grants (479316-2013-6 and 428774/2016-4) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Programa de Ex-celência em Pesquisa–PROEP-FIOCRUZ Rondonia. Juliana Pavan Zuliani was a recipient of productivity grant 306672/2014-6 and 311696/2021-0 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This study was financed in part by the Coordenação de Aper-feiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001, and Mauro V. Paloschi was the beneficiary of CNPq post- doctoral fellowship (151253/2022-6).
Institutional Review Board Statement
The study was conducted in accordance with the project approved by the Ethics Committee of the Brazilian IRB (Institutional Review Board) of the Center of Tropical Medicine Research (CEPEM, Rondônia, Brazil—Approval Number 1.739.023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to confidentiality reasons as they are stored in the Fiocruz Rondônia archives.
Acknowledgments
Mauro V. Paloschi thanks his family and friends for their support and encouragement. The authors express their gratitude to the Andreimar M. Soares for providing the Cr-LAAO from Calloselasma rhodostoma venom from LABIOPROT/FIOCRUZ-RO, and the team of the Laboratório de Imunologia Celular Aplicada à Saúde da Fiocruz-RO who collaborated on this project. Figures were created with the aid of Universo Da Vinci startup.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du, X.-Y.; Clemetson, K.J. Snake Venom L-Amino Acid Oxidases. Toxicon 2002, 40, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Izidoro, L.F.M.; Sobrinho, J.C.; Mendes, M.M.; Costa, T.R.; Grabner, A.N.; Rodrigues, V.M.; da Silva, S.L.; Zanchi, F.B.; Zuliani, J.P.; Fernandes, C.F.C.; et al. Snake Venom L-Amino Acid Oxidases: Trends in Pharmacology and Biochemistry. Biomed Res. Int. 2014, 2014, 196754. [Google Scholar] [CrossRef]
- Paloschi, M.V.; Pontes, A.S.; Soares, A.M.; Zuliani, J.P. An Update on Potential Molecular Mechanisms Underlying the Actions of Snake Venom L-Amino Acid Oxidases (LAAOs). Curr. Med. Chem. 2018, 25, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, J.P.; Paloschi, M.V.; Pontes, A.S.; Boeno, C.N.; Lopes, J.A.; Setubal, S.S.; Zanchi, F.B.; Soares, A.M. Reptile Venom L-Amino Acid Oxidases—Structure and Function. Handb. Venoms Toxins Reptil. 2021, 2, 413–430. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; da Silva, J.F.; Boldrini França, J.; Fonseca, F.P.P.; Otaviano, A.R.; Henrique Silva, F.; Hamaguchi, A.; Magro, A.J.; Braz, A.S.K.; dos Santos, J.I.; et al. Structural and Functional Properties of Bp-LAAO, a New L-Amino Acid Oxidase Isolated from Bothrops Pauloensis Snake Venom. Biochimie 2009, 91, 490–501. [Google Scholar] [CrossRef]
- Pontes, A.S.; Setúbal, S.d.S.; Xavier, C.V.; Lacouth-Silva, F.; Kayano, A.M.; Pires, W.L.; Nery, N.M.; Boeri de Castro, O.; da Silva, S.D.; Calderon, L.A.; et al. Effect of L-Amino Acid Oxidase from Calloselasma Rhodosthoma Snake Venom on Human Neutrophils. Toxicon 2014, 80, 27–37. [Google Scholar] [CrossRef]
- Pontes, A.S.; Setúbal, S.d.S.; Nery, N.M.; da Silva, F.S.; da Silva, S.D.; Fernandes, C.F.C.; Stábeli, R.G.; Soares, A.M.; Zuliani, J.P. P38 MAPK Is Involved in Human Neutrophil Chemotaxis Induced by L-Amino Acid Oxidase from Calloselasma Rhodosthoma. Toxicon 2016, 119, 106–116. [Google Scholar] [CrossRef]
- Paloschi, M.V.; Boeno, C.N.; Lopes, J.A.; Eduardo dos Santos da Rosa, A.; Pires, W.L.; Pontes, A.S.; da Silva Setúbal, S.; Soares, A.M.; Zuliani, J.P. Role of L-Amino Acid Oxidase Isolated from Calloselasma Rhodostoma Venom on Neutrophil NADPH Oxidase Complex Activation. Toxicon 2018, 145, 48–55. [Google Scholar] [CrossRef]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, N.; Singh, M.; Kaur, M.; Singh, G.; Narang, A.; Kanwal, A.; Sharma, K.; Singh, B.; Napoli, M.D.; et al. Neutrophil Extracellular Traps and NLRP3 Inflammasome: A Disturbing Duo in Atherosclerosis, Inflammation and Atherothrombosis. Vaccines 2023, 11, 261. [Google Scholar] [CrossRef]
- Pires, R.H.; Felix, S.B.; Delcea, M. The Architecture of Neutrophil Extracellular Traps Investigated by Atomic Force Microscopy. Nanoscale 2016, 8, 14193–14202. [Google Scholar] [CrossRef] [PubMed]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Paloschi, M.V.; Lopes, J.A.; Boeno, C.N.; Silva, M.D.S.; Evangelista, J.R.; Pontes, A.S.; da Silva Setúbal, S.; Rego, C.M.A.; Néry, N.M.; Ferreira e Ferreira, A.A.; et al. Cytosolic Phospholipase A2-α Participates in Lipid Body Formation and PGE2 Release in Human Neutrophils Stimulated with an l-Amino Acid Oxidase from Calloselasma Rhodostoma Venom. Sci. Rep. 2020, 10, 10976. [Google Scholar] [CrossRef] [PubMed]
- Paloschi, M.V.; Boeno, C.N.; Lopes, J.A.; Rego, C.M.A.; Silva, M.D.S.; Santana, H.M.; Serrath, S.N.; Ikenohuchi, Y.J.; Farias, B.J.C.; Felipin, K.P.; et al. Reactive Oxygen Species-Dependent-NLRP3 Inflammasome Activation in Human Neutrophils Induced by l-Amino Acid Oxidase Derived from Calloselasma Rhodostoma Venom. Life Sci. 2022, 308, 120962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 1999. [Google Scholar]
- Hochberg, Y.; Benjamini, Y. More Powerful Procedures for Multiple Significance Testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Luo, W.; Friedman, M.S.; Shedden, K.; Hankenson, K.D.; Woolf, P.J. GAGE: Generally Applicable Gene Set Enrichment for Pathway Analysis. BMC Bioinform. 2009, 10, 161. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor Package for Pathway-Based Data Integration and Visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard, S.G.; Brouwer, C. Pathview Web: User Friendly Pathway Visualization and Data Integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. Elife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus Aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).