Ecosystem Services Provided by Seaweeds
Abstract
1. Introduction
2. A General Overview of Ecosystem Services Provided by Seaweeds
3. Seaweed Ecosystem Services
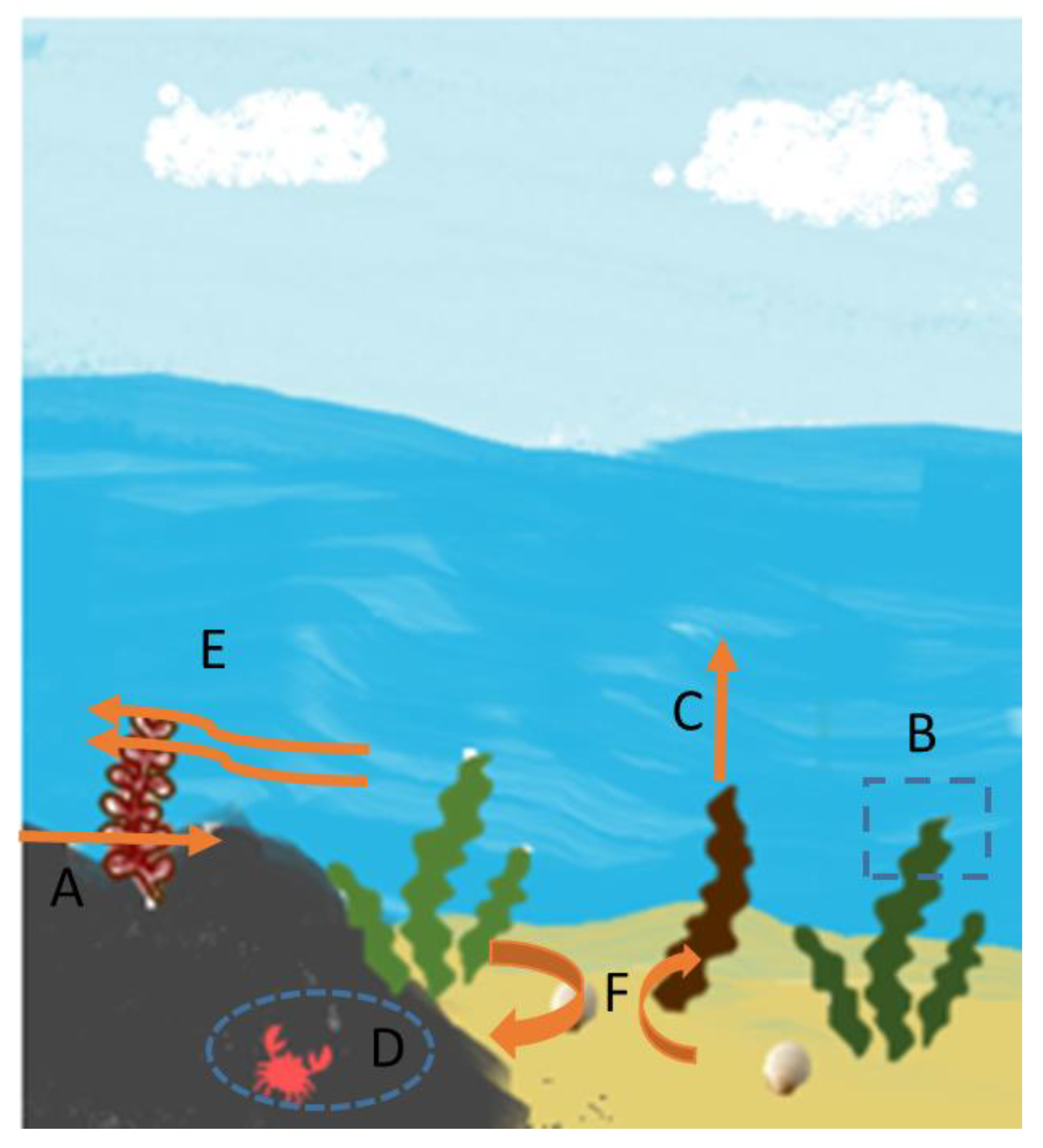
3.1. Seaweed Functions/Supporting Services
3.2. Regulating Services
3.3. Provisioning Services
3.4. Cultural Services
4. Future of Seaweed Ecological Services
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfister, C.A.; Altabet, M.A.; Weigel, B. Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology 2019, 100, e02798. [Google Scholar] [CrossRef] [PubMed]
- García-Poza, S.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Marine macroalgae as a feasible and complete resource to address and promote Sustainable Development Goals (SDGs). Integr. Environ. Assess. Manag. 2022, 18, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Butt, K.R.; Méline, C.; Pérès, G. Marine macroalgae as food for earthworms: Growth and selection experiments across ecotypes. Environ. Sci. Pollut. Res. 2020, 27, 33493–33499. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.; Johnson, C.R.; Ross, J.; Hermand, J.-P. Acoustic investigation of the primary production of an Australian temperate macroalgal (Ecklonia radiata) system. J. Exp. Mar. Biol. Ecol. 2020, 524, 151309. [Google Scholar] [CrossRef]
- Hay, K.H.; Hays, G.; Orth, R. Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar. Ecol. Prog. Ser. 2003, 253, 123–136. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.; Orth, R. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]
- García-Poza, S.; Cotas, J.; Morais, T.; Pacheco, D.; Pereira, L.; Marques, J.C.; Gonçalves, A.M.M. Global Trade of Seaweed Foods. In Sustainable Global Resources of Seaweeds Volume 2; Springer International Publishing: Cham, Switzerland, 2022; pp. 325–337. [Google Scholar]
- Fredericq, S. The Wonderful World of Seaweeds. Available online: https://oceanexplorer.noaa.gov/explorations/03mex/background/seaweeds/seaweeds.html#:~:text=Because%20seaweeds%20are%20photosynthetic%20organisms,1) (accessed on 14 August 2022).
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Gonçalves, A.M.M.; Dias, J.M.; Pereira, L. Seaweeds Used in Wastewater Treatment: Steps to Industrial Commercialization. In Phycology-Based Approaches for Wastewater Treatment and Resource Recovery; CRC Press: Boca Raton, FL, USA, 2021; pp. 247–262. [Google Scholar]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates—A General Overview. In Alginates—Recent Uses of This Natural Polymer; IntechOpen: London, UK, 2020. [Google Scholar]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Pacheco, D.; Araújo, G.S.; Cotas, J.; Gaspar, R.; Neto, J.M.; Pereira, L. Invasive Seaweeds in the Iberian Peninsula: A Contribution for Food Supply. Mar. Drugs 2020, 18, 560. [Google Scholar] [CrossRef]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Chiriboga, A.; Cortés, J.; Delbeek, J.C.; DeVantier, L.; et al. One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 2008, 321, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Filbee-Dexter, K.; Wernberg, T. Rise of Turfs: A New Battlefront for Globally Declining Kelp Forests. Bioscience 2018, 68, 64–76. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Unsworth, R.K.F.; Gullström, M.; Cullen-Unsworth, L.C. Global significance of seagrass fishery activity. Fish Fish. 2018, 19, 399–412. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef]
- Hasselström, L.; Visch, W.; Gröndahl, F.; Nylund, G.M.; Pavia, H. The impact of seaweed cultivation on ecosystem services—A case study from the west coast of Sweden. Mar. Pollut. Bull. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Burg, S.V.D.; Termeer, E.; Skirtun, M.; Poelman, M.; Veraart, J.; Selnes, T. Exploring mechanisms to pay for ecosystem services provided by mussels, oysters and seaweeds. Ecosyst. Serv. 2022, 54, 101407. [Google Scholar] [CrossRef]
- Chopin, T.; Tacon, A.G.J. Importance of Seaweeds and Extractive Species in Global Aquaculture Production. Rev. Fish. Sci. Aquac. 2021, 29, 139–148. [Google Scholar] [CrossRef]
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2022, 5, 185–193. [Google Scholar] [CrossRef]
- Watson, R.T.; Rosswall, T.; Steiner, A.; Töpfer, K.; Arico, S.; Bridgewater, P. Ecosystems and Human Well-Being; Island Press: Washington, DC, USA, 2003; ISBN 1559634022. [Google Scholar]
- Carpenter, S.R.; DeFries, R.; Dietz, T.; Mooney, H.A.; Polasky, S.; Reid, W.V.; Scholes, R.J. Millennium Ecosystem Assessment: Research Needs. Science 2006, 314, 257–258. [Google Scholar] [CrossRef]
- Gomes, E.; Inácio, M.; Bogdzevič, K.; Kalinauskas, M.; Karnauskaitė, D.; Pereira, P. Future land-use changes and its impacts on terrestrial ecosystem services: A review. Sci. Total Environ. 2021, 781, 146716. [Google Scholar] [CrossRef]
- Haines-Young, R.; Potschin-Young, M. Revision of the Common International Classification for Ecosystem Services (CICES V5.1): A Policy Brief. One Ecosyst. 2018, 3, e27108. [Google Scholar] [CrossRef]
- Thomaz, S.M. Ecosystem services provided by freshwater macrophytes. Hydrobiologia 2021, 1–21. [Google Scholar] [CrossRef]
- Bennett, S.; Wernberg, T.; Connell, S.D.; Hobday, A.J.; Johnson, C.R.; Poloczanska, E.S. The ‘Great Southern Reef’: Social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshw. Res. 2016, 67, 47. [Google Scholar] [CrossRef]
- Duffy, J.E.; Benedetti-Cecchi, L.; Trinanes, J.; Muller-Karger, F.E.; Ambo-Rappe, R.; Boström, C.; Buschmann, A.H.; Byrnes, J.; Coles, R.G.; Creed, J.; et al. Toward a Coordinated Global Observing System for Seagrasses and Marine Macroalgae. Front. Mar. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Zuñiga, S.; Tala, F.; Piaget, N.; Rodríguez, D.C.; Vega, J.M.A. Economic valuation of kelp forests in northern Chile: Values of goods and services of the ecosystem. J. Appl. Phycol. 2014, 26, 1081–1088. [Google Scholar] [CrossRef]
- Blamey, L.K.; Bolton, J.J. The economic value of South African kelp forests and temperate reefs: Past, present and future. J. Mar. Syst. 2018, 188, 172–181. [Google Scholar] [CrossRef]
- Henriques, B.; Rocha, L.S.; Lopes, C.B.; Figueira, P.; Monteiro, R.J.; Duarte, A.; Pardal, M.; Pereira, E. Study on bioaccumulation and biosorption of mercury by living marine macroalgae: Prospecting for a new remediation biotechnology applied to saline waters. Chem. Eng. J. 2015, 281, 759–770. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Prasad, D.M.R.; Govindarajan, L.; Saravanakumar, K.; Prasad, B.S.N. Green alga-mediated treatment process for removal of zinc from synthetic solution and industrial effluent. Environ. Technol. 2019, 40, 1262–1270. [Google Scholar] [CrossRef]
- Gao, K.; Beardall, J.; Häder, D.-P.; Hall-Spencer, J.M.; Gao, G.; Hutchins, D.A. Effects of Ocean Acidification on Marine Photosynthetic Organisms Under the Concurrent Influences of Warming, UV Radiation, and Deoxygenation. Front. Mar. Sci. 2019, 6, 00322. [Google Scholar] [CrossRef]
- Trowbridge, L. (Ed.) A Better World: Volume 3; Gomer Press Ltd.: Ceredigion, UK, 2014; Volume 168, ISBN 9780995648739. [Google Scholar]
- Macreadie, P.I.; Trevathan-Tackett, S.; Skilbeck, G.; Sanderman, J.; Curlevski, N.; Jacobsen, G.; Seymour, J. Losses and recovery of organic carbon from a seagrass ecosystem following disturbance. Proc. R. Soc. B Boil. Sci. 2015, 282, 20151537. [Google Scholar] [CrossRef]
- Garden, C.J.; Smith, A.M. Voyages of seaweeds: The role of macroalgae in sediment transport. Sediment. Geol. 2015, 318, 1–9. [Google Scholar] [CrossRef]
- Morris, R.L.; Graham, T.D.J.; Kelvin, J.; Ghisalberti, M.; Swearer, S. Kelp beds as coastal protection: Wave attenuation of Ecklonia radiata in a shallow coastal bay. Ann. Bot. 2019, 125, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Smith, S. Climatic Factors—Wild seaweed harvesting: Strategic environmental assessment—Environmental report—Gov.scot. In Wild Seaweed Harvesting: Strategic Environmental Assessment—Environmental Report; Scottish Government: Edinburgh, UK, 2016; ISBN 9781786526229. [Google Scholar]
- Airoldi, L.; Ballesteros, E.; Buonuomo, R.; van Belzen, J.; Bouma, T.; Cebrian, E.; de Clerk, O.; Engelen, A.; Ferrario, F.; Fraschetti, S.; et al. Marine forests at risk: Solutions to halt the loss and promote the recovery of Mediterranean canopy-forming seaweeds. In Proceedings of the 5th Mediterranean Symposium on Marine Vegetation, Portorož, Slovenia, 27–28 October 2014; Labgar, H., Bouafif, C., Ouerghi, A., Eds.; United Nations Environment Programme, Mediterranean Action Plan, Regional Activity Center for Specially Protected Areas: Portoroz, Slovenia, 2014; pp. 27–28. [Google Scholar]
- Field, C.B.; Barros, V.; Stocker, T.F.; Dahe, Q.; Jon Dokken, D.; Ebi, K.L.; Mastrandrea, M.D.; Mach, K.J.; Plattner, G.K.; Allen, S.K.; et al. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Cambridge University Press: Cambridge, UK, 2012; ISBN 9781139177245. [Google Scholar]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Temmerman, S.; Govers, G.; Wartel, S.; Meire, P. Modelling estuarine variations in tidal marsh sedimentation: Response to changing sea level and suspended sediment concentrations. Mar. Geol. 2004, 212, 1–19. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chiscano, C.L. Seagrass biomass and production: A reassessment. Aquat. Bot. 1999, 65, 159–174. [Google Scholar] [CrossRef]
- Smale, D.A.; Pessarrodona, A.; King, N.; Burrows, M.T.; Yunnie, A.; Vance, T.; Moore, P. Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Duarte, C.M. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences 2017, 14, 301–310. [Google Scholar] [CrossRef]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.; Pereira, L.; Gonçalves, A. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, P.A.U.I.; Thiripuranathar, G.; Uzair, B.; Iqbal, H.; Khan, B.A.; Menaa, B. Ecological and Industrial Implications of Dynamic Seaweed-Associated Microbiota Interactions. Mar. Drugs 2020, 18, 641. [Google Scholar] [CrossRef] [PubMed]
- Pessarrodona, A.; Foggo, A.; Smale, D.A. Can ecosystem functioning be maintained despite climate-driven shifts in species composition? Insights from novel marine forests. J. Ecol. 2019, 107, 91–104. [Google Scholar] [CrossRef]
- National Ocean Service How Much Oxygen Comes from the Ocean? Available online: https://oceanservice.noaa.gov/facts/ocean-oxygen.html (accessed on 1 April 2022).
- Mouritsen, O.G. Seaweeds: Edible, Available and Sustainable; University of Chicago Press: London, UK, 2013; ISBN 978-0-226-04436-1. [Google Scholar]
- Gallagher, J.B. Kelp Won’t Help: Why Seaweed May Not Be a Silver Bullet for Carbon Storage After All. Available online: https://theconversation.com/kelp-wont-help-why-seaweed-may-not-be-a-silver-bullet-for-carbon-storage-after-all-178018#:~:text=At%20present%20it’s%20thought%20seaweed,%20fight%20to%20stop%20climate%20change (accessed on 17 July 2022).
- Mishra, S.; Maiti, A. The efficacy of bacterial species to decolourise reactive azo, anthroquinone and triphenylmethane dyes from wastewater: A review. Environ. Sci. Pollut. Res. 2018, 25, 8286–8314. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A. Integrated Modelling of Eutrophication and Organic Contaminant Fate & Effects in Aquatic Ecosystems. A Review. Water Res. 2001, 35, 3517–3536. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystem, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; Volume 35, ISBN 9780127447605. [Google Scholar]
- Miranda, L.E.; Driscoll, M.P.; Allen, M.S. Transient physicochemical microhabitats facilitate fish survival in inhospitable aquatic plant stands. Freshw. Biol. 2000, 44, 617–628. [Google Scholar] [CrossRef]
- Layton, C.; Coleman, M.A.; Marzinelli, E.; Steinberg, P.D.; Swearer, S.; Verges, A.; Wernberg, T.; Johnson, C.R. Kelp Forest Restoration in Australia. Front. Mar. Sci. 2020, 7, 74. [Google Scholar] [CrossRef]
- Eger, A.; Vergés, A.; Choi, C.G.; Christie, H.; Coleman, M.A.; Fagerli, C.W.; Fujita, D.; Hasegawa, M.; Kim, J.H.; Mayer-Pinto, M.; et al. Financial and Institutional Support Are Important for Large-Scale Kelp Forest Restoration. Front. Mar. Sci. 2020, 7, 535277. [Google Scholar] [CrossRef]
- Resende, L.; Flores, J.; Moreira, C.; Pacheco, D.; Baeta, A.; Garcia, A.C.; Rocha, A.C.S. Effective and Low-Maintenance IMTA System as Effluent Treatment Unit for Promoting Sustainability in Coastal Aquaculture. Appl. Sci. 2021, 12, 398. [Google Scholar] [CrossRef]
- Pacheco, D.; Miranda, G.; Rocha, C.P.; Pato, R.L.; Cotas, J.; Gonçalves, A.M.M.; Santos, S.M.D.; Bahcevandziev, K.; Pereira, L. Portuguese Kelps: Feedstock Assessment for the Food Industry. Appl. Sci. 2021, 11, 10681. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Burkepile, D.E.; Thurber, R.V. A Vicious Circle? Altered Carbon and Nutrient Cycling May Explain the Low Resilience of Caribbean Coral Reefs. Bioscience 2016, 66, 470–476. [Google Scholar] [CrossRef]
- Bak, U.G. Seaweed Cultivation in the Faroe Islands: An Investigation of the Biochemical Composition of Selected Macroalgal Species, Optimised Seeding Technics, and Open-Ocean Cultivation Methods from a Commercial Perspective. Ph.D. Thesis, Technical University of Denmark, Kgs. Lyngby, Denmark, 2019; p. 157. [Google Scholar]
- Theuerkauf, S.J.; Barrett, L.T.; Alleway, H.K.; Costa-Pierce, B.A.; Gelais, A.S.; Jones, R.C. Habitat value of bivalve shellfish and seaweed aquaculture for fish and invertebrates: Pathways, synthesis and next steps. Rev. Aquac. 2022, 14, 54–72. [Google Scholar] [CrossRef]
- Turan, G.; Neori, A. Intensive seaweed aquaculture: A potent solution against global warming. In Seaweeds and Their Role in Globally Changing Environments; Israel, A., Einav, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 359–372. [Google Scholar]
- Chung, I.K.; Oak, J.H.; Lee, J.A.; Shin, J.A.; Kim, J.G.; Park, K.-S. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J. Mar. Sci. 2013, 70, 1038–1044. [Google Scholar] [CrossRef]
- Wernberg, T.; Krumhansl, K.; Filbee-Dexter, K.; Pedersen, M.F. Status and Trends for the World’s Kelp Forests. In World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 57–78. [Google Scholar]
- Forests of Seaweed Can Help Climate Change—Without Risk of Fire. Available online: https://www.nationalgeographic.com/environment/article/forests-of-seaweed-can-help-climate-change-without-fire (accessed on 1 April 2022).
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Front. Mar. Sci. 2017, 4, 00100. [Google Scholar] [CrossRef]
- Keeling, R.F.; Körtzinger, A.; Gruber, N. Ocean Deoxygenation in a Warming World. Annu. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Alvera-Azcárate, A.; Ferreira, J.; Nunes, J. Modelling eutrophication in mesotidal and macrotidal estuaries. The role of intertidal seaweeds. Estuarine, Coast. Shelf Sci. 2003, 57, 715–724. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine macroalgae in a circular economy context: A comprehensive analysis focused on residual biomass. Biotechnol. Adv. 2022, 60, 107987. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Ortiz-Calderon, C.; Silva, H.C.; Vásquez, D.B. Metal Removal by Seaweed Biomass. In Biomass Volume Estimation and Valorization for Energy; InTech: London, UK, 2017. [Google Scholar]
- Dugeny, E.; Lorgeril, J.; Petton, B.; Toulza, E.; Gueguen, Y.; Pernet, F. Seaweeds influence oyster microbiota and disease susceptibility. J. Anim. Ecol. 2022, 91, 805–818. [Google Scholar] [CrossRef]
- Al-Adilah, H.; Feiters, M.C.; Carpenter, L.J.; Kumari, P.; Carrano, C.J.; Al-Bader, D.; Küpper, F.C. Halogens in Seaweeds: Biological and Environmental Significance. Phycology 2022, 2, 132–171. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Fairclough, A.C.; Hall, A.C.; Paxman, J.R. The potential health benefits of seaweed and seaweed extract. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 119–136. [Google Scholar]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.; Pereira, L.; Gonçalves, A. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Cotas, J.; Pacheco, D.; Gonçalves, A.M.M.; Silva, P.; Carvalho, L.G.; Pereira, L. Seaweeds’ nutraceutical and biomedical potential in cancer therapy: A concise review. J. Cancer Metastasis Treat. 2021, 7, 13. [Google Scholar] [CrossRef]
- Laudadio, V.; Lorusso, V.; Lastella, N.M.B.; Dhama, K.; Karthik, K.; Tiwari, R.; Alam, G.M.; Tufarelli, V. Enhancement of Nutraceutical Value of Table Eggs Through Poultry Feeding Strategies. Int. J. Pharmacol. 2015, 11, 201–212. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds, Chapter 2. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 15–47. ISBN 9781614708780. [Google Scholar]
- Vilà, B. Improvement of biologic and nutritional value of eggs. CIHEAM -Opt. Méditerr. 2008, 37, 390. [Google Scholar]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Cotas, J. Historical Use of Seaweed as an Agricultural Fertilizer in the European Atlantic Area. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed-Based Polymers from Sustainable Aquaculture to “Greener” Plastic Products. In Sustainable Global Resources of Seaweeds Volume 1; Springer International Publishing: Cham, Switzerland, 2022; pp. 591–602. [Google Scholar]
- Coxworth, B. Wool and Seaweed Makes Bricks Stronger. Available online: https://newatlas.com/bricks-made-with-wool-and-seaweed/16580/ (accessed on 13 February 2022).
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strat. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from Marine Sources. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. ISBN 978-3-642-53971-8. [Google Scholar]
- Nepper-Davidsen, J.; Magnusson, M.; Glasson, C.R.K.; Ross, P.M.; Lawton, R.J. Implications of Genetic Structure for Aquaculture and Cultivar Translocation of the Kelp Ecklonia radiata in Northern New Zealand. Front. Mar. Sci. 2021, 8, 749154. [Google Scholar] [CrossRef]
- Charrier, B.; Rolland, E.; Gupta, V.; Reddy, C.R.K. Production of genetically and developmentally modified seaweeds: Exploiting the potential of artificial selection techniques. Front. Plant Sci. 2015, 6, 127. [Google Scholar] [CrossRef]
- Jarald, E.; Joshi, S.B.; Jain, D.C. Diabetes and Herbal Medicines. Iran. J. Pharmacol. Ther. 2008, 7, 97–106. [Google Scholar]
- Freile-Pelegrín, Y.; Tasdemir, D. Seaweeds to the rescue of forgotten diseases: A review. Bot. Mar. 2019, 62, 211–226. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Dhargalkar, V.; Verlecar, X. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Pereira, L. Therapeutic and Nutritional Uses of Algae; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781498755382. [Google Scholar] [CrossRef]
- Mayer, A.; Guerrero, A.; Rodríguez, A.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2016–2017: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2021, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Neuroprotective Effects of Marine Algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Somova, L.M.; Kuznetsova, T.A. Review: Prospects for the Use of Extracts and Polysaccharides from Marine Algae to Prevent and Treat the Diseases Caused by Helicobacter pylori. Helicobacter 2015, 20, 89–97. [Google Scholar] [CrossRef]
- Cotas, J.; Pacheco, D.; Araujo, G.; Valado, A.; Critchley, A.; Pereira, L. On the Health Benefits vs. Risks of Seaweeds and Their Constituents: The Curious Case of the Polymer Paradigm. Mar. Drugs 2021, 19, 164. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Hauter, D.; Hauter, S. Cultivating Macroalgae in Your Saltwater Aquarium. Available online: https://www.thesprucepets.com/cultivating-macroalgae-in-your-aquarium-2924568 (accessed on 3 February 2022).
- An Bollenessor. Seaweeds in the Aquarium Part I. Available online: https://anbollenessor.com/2013/11/13/seaweeds-in-the-aquarium-part-i/ (accessed on 1 April 2022).
- Neto, J.M.; Gaspar, R.; Pereira, L.; Marques, J.C. Marine Macroalgae Assessment Tool (MarMAT) for intertidal rocky shores. Quality assessment under the scope of the European Water Framework Directive. Ecol. Indic. 2012, 19, 39–47. [Google Scholar] [CrossRef]
- Wallenstein, F.M.; Neto, A.I.; Patarra, R.F.; Prestes, A.C.L.; Álvaro, N.V.; Rodrigues, A.S.; Wilkinson, M. Indices to monitor coastal ecological quality of rocky shores based on seaweed communities: Simplification for wide geographical use. Rev. Gestão Costeira Integr. 2013, 13, 15–25. [Google Scholar] [CrossRef]
- Laboratory of Biology of Aquatic Organisms and Ecosystems. Bioindicators of Marine Environment: Using Seaweeds as a Tool for Biomonitoring the Quality of Coastal Waters. Response to a Problem of Seaweed Beachings. Available online: https://borea.mnhn.fr/en/bioindicators-marine-environment-using-seaweeds-tool-biomonitoring-quality-coastal-waters-response (accessed on 1 April 2022).
- Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T.; Pérez-Lloréns, J.L. Saved by seaweeds: Phyconomic contributions in times of crises. J. Appl. Phycol. 2021, 33, 443–458. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L.; Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T. Seaweeds in mythology, folklore, poetry, and life. J. Appl. Phycol. 2020, 32, 3157–3182. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & amp Mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar] [CrossRef]
- O’Connor, K. Seaweed: A Global History (Edible); Reaktion Books, Limited: London, UK, 2017; ISBN 9781780237534. [Google Scholar]
- Pérez Lloréns, J.L.; Hernández Carrero, I.; Vergara Oñate, J.J.; Brun Murillo, F.G.; León González, A. Those Curious and Delicious Seaweeds: A Fascinating Voyage from Biology to Gastronomy; Pérez Lloréns, J.L., Hernández Carrero, I., Vergara Oñate, J.J., Brun Murillo, F.G., León González, A., de Harland, A.B., Eds.; Editorial UCA: Cadiz, Spain, 2018; ISBN 978-84-9828-666-3. [Google Scholar]
- Seaweed Bungalows Repaired as Cultural Heritage in E China—China.org.cn. Available online: http://www.china.org.cn/arts/2015-08/26/content_36418615.htm (accessed on 30 March 2022).
- Seaweed in the Azores: Biodiversity for Food and Nutrition. Available online: http://www.b4fn.org/case-studies/case-studies/seaweed-in-the-azores/ (accessed on 30 March 2022).
- Westmoreland, P.L. Ancient Greek Beliefs; Lee And Vance Publishing Co.: San Ysidro, CA, USA, 2007; ISBN 0979324815. [Google Scholar]
- Turner, N.J. Food Plants of Coastal First Peoples; Royal BC Museum: Victoria, BC, Canada, 2006; ISBN 0772656274. [Google Scholar]
- Khalilieh, H.S.; Boulos, A. A glimpse on the uses of seaweeds in islamic science and daily life during the classical period. Arab. Sci. Philos. 2006, 16, 91–101. [Google Scholar] [CrossRef]
- Rimmer, M.A.; Larson, S.; Lapong, I.; Purnomo, A.H.; Pong-Masak, P.R.; Swanepoel, L.; Paul, N.A. Seaweed Aquaculture in Indonesia Contributes to Social and Economic Aspects of Livelihoods and Community Wellbeing. Sustainability 2021, 13, 10946. [Google Scholar] [CrossRef]
- Msuya, F.E.; Hurtado, A.Q. The role of women in seaweed aquaculture in the Western Indian Ocean and South-East Asia. Eur. J. Phycol. 2017, 52, 482–494. [Google Scholar] [CrossRef]
- Hussin, R.; Yasir, S.; Kunjuraman, V. Potential of Homestay Tourism Based on Seaweed Cultivation from the Views of Seaweed Cultivators in District of Semporna Sabah, East Malaysia. SHS Web Conf. 2014, 12, 01005. [Google Scholar] [CrossRef]
- VisitLæsø. Seaweed Roofs on Læsø. Available online: https://www.visit-laesoe.com/tourist/experiences/seaweed-roofs-laeso (accessed on 30 March 2022).
- Merkel, A.; Säwe, F.; Fredriksson, C. The seaweed experience: Exploring the potential and value of a marine resource. Scand. J. Hosp. Tour. 2021, 21, 391–406. [Google Scholar] [CrossRef]
- Gundersen, H.; Bryan, T.; Chen, W.; Moy, F.E. Ecosystem Services: In the Coastal Zone of the Nordic Countries; Nordic Council of Ministers: Copenhagen, Denmark, 2017. [Google Scholar]
- Hasler, B.; Ahtiainen, H.; Hasselström, L.; Heiskanen, A.-S. Marine Ecosystem Services: Marine Ecosystem Services in Nordic Marine Waters and the Baltic Sea, Possibilities for Valuation; Nordisk Ministerrad: Copenhagen, Denmark, 2016. [Google Scholar]
- Johnson, A.F.; Gonzales, C.; Townsel, A.; Cisneros-Montemayor, A.M. Marine ecotourism in the Gulf of California and the Baja California Peninsula: Research trends and information gaps. Sci. Mar. 2019, 83, 177. [Google Scholar] [CrossRef]
- Saavedra, J.R. Lapu-Lapu City to Promote Olango Islands’ Seaweed Ecotourism; Philippine News Agency: Manila, Philippines, 2020. [Google Scholar]
- del Rosario, K.M. Fishing and Seaweed Ecotourism to Benefit Some 10,000 Families in Olango Islands–Getaway.PH. Available online: https://getaway.ph/blog/travel/fishing-and-seaweed-ecotourism-to-benefit-some-10000-families-in-olango-islands/ (accessed on 30 March 2022).
- Subijanto, J.; Djohani, S.W.H.; Welly, M. Ecotourism at Nusa Penida MPA, Bali: A pilot for community based approaches to support the sustainable marine resources management. In Proceedings of the International Conference on Climate Change and Coral Reef Conservation, Okinawa, Japan, 29–30 June 2013. [Google Scholar]
- Intertidal Itineraries—Instituto de Educação. Available online: http://www.ie.ulisboa.pt/en/projetos/intertidal-itineraries (accessed on 30 March 2022).
- Faria, C.; Pacheco, D. Education 4.0: The case study of the app roteiro entre-marés. In Proceedings of the INTED 2022, Valencia, Spain, 7–9 March 2022; p. 3992. [Google Scholar]
- Seaweed Commons—Seaweed Knowledge for Coastal Communities. Available online: https://seaweedcommons.org/ (accessed on 30 March 2022).
- Biró, M.; Molnar, Z.; Babai, D.; Dénes, A.; Fehér, A.; Barta, S.; Sáfián, L.; Szabados, K.; Kiš, A.; Demeter, L.; et al. Reviewing historical traditional knowledge for innovative conservation management: A re-evaluation of wetland grazing. Sci. Total Environ. 2019, 666, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Cotas, J.; Bahcevandziev, K.; Pereira, L. Effects of “sargaço” extraction residues on seed germination. Millenium -J. Educ. Technol. Health 2020, 2, 29–37. [Google Scholar] [CrossRef]
- Sousa, T.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Revisitação científica aos métodos tradicionais de fertilização com macroalgas em Portugal. Rev. APH Assoc. Port. Hortic. 2020, 136, 38–41. [Google Scholar]
- Agostini, S.; Harvey, B.P.; Milazzo, M.; Wada, S.; Kon, K.; Floc’H, N.; Komatsu, K.; Kuroyama, M.; Hall-Spencer, J.M. Simplification, not “tropicalization”, of temperate marine ecosystems under ocean warming and acidification. Glob. Chang. Biol. 2021, 27, 4771–4784. [Google Scholar] [CrossRef] [PubMed]
- Klinger, T. Optimizing seaweed futures under climate change. Bot. Mar. 2021, 64, 439–443. [Google Scholar] [CrossRef]
- Sterley, A. The Feasibility of Using Macroalgae from Anaerobic Digestion as Fertilizer in Grenada. Bachelor’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2020. [Google Scholar]
- Stead, S.M. Rethinking marine resource governance for the United Nations Sustainable Development Goals. Curr. Opin. Environ. Sustain. 2018, 34, 54–61. [Google Scholar] [CrossRef]
- Rinde, E.; Christie, H.; Bekkby, T. Økologiske Effekter av Taretråling. Analyser Basert på GIS-Modellering Og Empiriske Data; Norsk Institutt for Vannforskning: Oslo, Norway, 2006; ISBN 8257748633. [Google Scholar]
- Pechsiri, J.S.; Thomas, J.-B.E.; Risén, E.; Ribeiro, M.S.; Malmström, M.E.; Nylund, G.M.; Jansson, A.; Welander, U.; Pavia, H.; Gröndahl, F. Energy performance and greenhouse gas emissions of kelp cultivation for biogas and fertilizer recovery in Sweden. Sci. Total Environ. 2016, 573, 347–355. [Google Scholar] [CrossRef]
- Bohlin, S. Applying the SDG Framework to Emerging Industries; Degree Project in Technology and Sustainable Development; KTH Royal Institute of Technology: Stockholm, Sweden, 2019. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Jackson, E.L.; Nakaoka, M.; Samper-Villarreal, J.; Beca-Carretero, P.; Creed, J.C. Seagrass ecosystem services—What’s next? Mar. Pollut. Bull. 2018, 134, 145–151. [Google Scholar] [CrossRef]
- Gopal, B. Should ‘wetlands’ cover all aquatic ecosystems and do macrophytes make a difference to their ecosystem services? Folia Geobot. 2016, 51, 209–226. [Google Scholar] [CrossRef]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]





| Ecosystem Service Category | Ecosystem Services Provided by Seaweeds | Examples of Benefits for Humans |
|---|---|---|
| Functions (CICES)/Supporting services (MEA) | Soil formation (sediment formation) | Increase environment quality for aquatic plants, increase in Seaweed primary productivity |
| Photosynthesis | Production of oxygen and biomass | |
| Primary production | Biomass for higher trophic levels | |
| Production of oxygen | Providing of suitable habitat for fish and other organisms used by humans | |
| Nutrient cycling | Preservation of ecosystem functioning; indirect benefits to food webs and water purification | |
| Water cycling | Influence seawater balance | |
| Provisioning of habitat | Conservation of biodiversity and biomass production of higher trophic levels (e.g., fish) |
| Ecosystem Service Category | Ecosystem Services Provided by Seaweeds | Examples of Benefits for Humans |
|---|---|---|
| Regulating services | Climate regulation | Ameliorate the global climatic change |
| Erosion regulation | Coastal and shore protection and improvement of water quality | |
| Water purification and waste treatment—both in nature as well as in treatment plants | Enhancement of water quality by reducing nutrients and pollutants | |
| Genetic resources | Conveyance of varieties that help aquaculture | |
| Disease | Improvement of water quality by decreasing pathogens | |
| Environmental monitoring | Indication of water quality, pollution and community integrity | |
| Production of atmospheric oxygen | Seaweeds captures the CO2 (dissolved in water) to produce carbon-based molecules and atmospheric O2 | |
| Provisioning services | Fiber | Conveyance of a number of types of products |
| Food | Delivery of sea crops and wild plant products | |
| Biochemicals, natural medicines, and pharmaceuticals | Conveyance of important compounds for human welfare | |
| Cultural services | Educational value | Benefits human development and critical thinking |
| Esthetic values | Aids human introspective development | |
| Recreation and ecotourism | Economic and other benefits for society and for local populations | |
| Cultural heritage values | Aids human introspective development | |
| Inspiration | Aids human introspective development | |
| Local knowledge system | Benefits social welfare | |
| Spiritual and religious services | Nonphysical benefits |
| Region | Ecosystem Service | Economic Value (US Dollars per Kilometer per Year) | Reference |
|---|---|---|---|
| Pacific | Provisioning services: 91%; Others ecosystem services: 9% | 811,000 | Vásquez et al. [30] |
| Great Southern Reef | Cultural services: 90 %; Provisioning services: 10% | 914,000 | Bennett et al. [28] |
| South Atlantic Ocean | Provisioning services: 45%; Cultural services: 30 %; Others ecosystem services: 25% | 520,000 | Blamey and Bolton [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotas, J.; Gomes, L.; Pacheco, D.; Pereira, L. Ecosystem Services Provided by Seaweeds. Hydrobiology 2023, 2, 75-96. https://doi.org/10.3390/hydrobiology2010006
Cotas J, Gomes L, Pacheco D, Pereira L. Ecosystem Services Provided by Seaweeds. Hydrobiology. 2023; 2(1):75-96. https://doi.org/10.3390/hydrobiology2010006
Chicago/Turabian StyleCotas, João, Louisa Gomes, Diana Pacheco, and Leonel Pereira. 2023. "Ecosystem Services Provided by Seaweeds" Hydrobiology 2, no. 1: 75-96. https://doi.org/10.3390/hydrobiology2010006
APA StyleCotas, J., Gomes, L., Pacheco, D., & Pereira, L. (2023). Ecosystem Services Provided by Seaweeds. Hydrobiology, 2(1), 75-96. https://doi.org/10.3390/hydrobiology2010006









