Abstract
The contemporary distribution of crenobiontic endemics in central Texas is enigmatic because only some springs are occupied by crenobionts despite other unoccupied springs having seemingly suitable habitats. In the absence of complete paleo-records, a cohesive and widely accepted explanation for this biogeography has eluded researchers for many years. We suggest that data on contemporary species with obligate coevolution, such as parasites with multiple obligate hosts in their life cycles, can help to fill intervening gaps in the paleo-record because the contemporary distribution of such a parasite indicates that its hosts cohabitated without interruption since the arrival of the parasite. To test this conjecture, we studied one such parasite endemic to a select few central Texas springs, Huffmanela huffmani. By studying the distribution of the intermediate host, geologic and paleo-climatic records, performing lab experiments with live animals, and examining archived museum specimens of the definitive hosts from the 1950s, we were able to test multiple predictions about how the distribution of H. huffmani became what it is today. Our results corroborate a narrative suggesting that several severe droughts since the Wisconsin glaciations are responsible for having sculpted the present-day distribution of central Texas crenobionts.
Keywords:
cider refugium; crenobiology; biogeography; pleistocene; holocene; interglacial drought; relict; Hyalella; Huffmanela 1. Introduction
Unexpected Distributional Discontinuities
A transitional boundary zone that Huxley would later name “Wallace’s Line” was first described by Wallace as he explored the Malaysian Archipelago in the 19th Century [1]. Today Wallace’s Line is known to be a transition zone of floristic and faunistic distributions without an obvious and present cause separating the “radical distinctions of the Indo-Malayan and Austro-Malayan regions of the great Malay Archipelago” [2].
Since Wallace’s initial reports, scientists have been fascinated by such unexpected discontinuities in the distributions of taxa across the planet, at scales ranging from intercontinental to small springs that contain communities of clades unique to that region. Eventually, the fields of ecology, evolutionary biology, and biogeography began offering hypothetical causal mechanisms in attempts to develop synthesis narratives [3] that might help explain how some of these unexpected distributional discontinuities may have come about.
The unexpected discontinuities that brought our team into this study are, like Wallace’s Line, fuzzy and usually invisible boundaries; but unlike Wallace’s Line, our systems are on a much smaller geographic scale. Our discontinuities of interest are delineated by the downstream end of spring-dominated conditions that are most easily detected by the downstream distributional limit of endemic crenobiontic clades [4]. The surface area of a typical spring run upstream from such defining boundaries, and to which the crenobionts are restricted, is usually only a few hectares, and sometimes much less.
We will attempt to establish a hypothetical narrative with theoretical cause/effect mechanisms supported by data and experiments to help explain how several of the crenobiontic endemics of central Texas might have become restricted to specific springs [5,6,7,8,9,10], and will narrow down the factors that still limit the distributions of these endemics to the small world upstream from the invisible boundaries that still restrain them. However, before we delve into this fascinating biogeography, we must first address the confusing array of ambiguous terms used throughout the literature in respect to endemism and refugial concepts (see Appendix A for our comprehensive review of the terminological morass and attempts to resolve ambiguities). After considerable deliberation and reflection on the terminological confusion, we have decided to (1) avoid the use of modifiers to the term “endemic” and will simply refer to clades as endemics sensu Darwin’s original glossary entry, “peculiar to a given locality;” (2) avoid use of the term “relict” all together, and (3) use the term “refugia” to refer only to biotopes that have protected biocenoses from extirpation for durations that are sufficiently long to be evolutionarily relevant.
2. Background Review and Proposed Model
In this section, we describe:
- the geographical setting and the geological and chronological scopes of our study,
- the biocenotic communities and their crenal biotopes that led us to develop the proposed model, and
- the functional aspects of our proposed refugial model, the logic underpinning it, and the new name we have proposed for this unique category of evolutionary refugia.
2.1. Our Study
Our study deals with contemporary biocenoses of endemic crenobiontic clades representing diverse higher taxa that are collectively localized in perennial springheads of central Texas. We hypothesize that these biocenoses have persisted, in one form or another, through multiple landscape-scale climate cycles wherein the surrounding terrain apparently varied from lush forest to extreme aridity and back again three or four times over the last several million years. While some biogeographers would probably be comfortable referring to the springheads in our study as “interglacial evolutionary refugia,” this expression alone is inherently ambiguous (see Appendix A) in that it does not distinguish between the functionally and historically different refugia shaped by fundamentally different combinations of forces and constraints that were applied during glacial/interglacial transitions.
2.2. The Setting
The focal setting for the study is the geographic province known as the Edwards Plateau in central Texas (Figure 1) but inferentially extends to the adjacent regions of the southwestern USA.
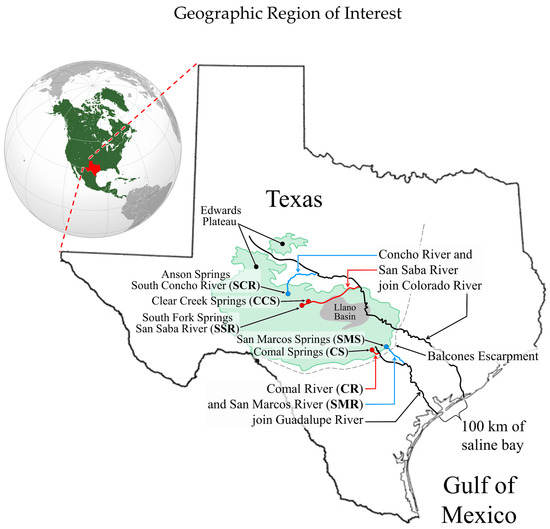
Figure 1.
Map of Texas showing a rough outline of the Edwards Plateau with the springs of interest and their connecting streams. Map of NA from Wikimedia Commons.
The refugial ecosystems of focus in our study are the perennial rheocrenic springs that emerge along the southern and eastern boundaries of the Edwards Plateau. All of the springs of interest are fed by numerous interconnected pools of the Edwards/Trinity Aquifer System [5,6,7,8,9,10].
2.3. Pre-Pleistocene Geological History of the Region
Much of western North America was formerly inundated by an inland sea throughout most of the Mesozoic until around the time of the K-Pg (Cretaceous-Paleogene) boundary [11]. Subsequent to the K-Pg boundary, the seas inundating Central Texas began to regress [12], partially due to a decrease in eustatic sea level, and partially due to regional uplifting associated with the Laramide Orogeny [13]. The Texas component of the uplifting is known as the Llano Uplift and is thought to have raised the strata of Central Texas several thousand meters [14,15]. This uplifting provided forces contributing to the Balcones faulting during the mid-Cenozoic [14,15,16]. The faulting resulted in a vertical displacement of strata by as much as 260 m [17]. The eroded karstic remnant of the footwall scarp now survives as the Balcones Escarpment, and the now eroded plateau formed by the footwall block is the Edwards Plateau.
Today, the only remaining marine deposits exposed in Central Texas are Cretaceous or early Cenozoic in age [18,19]. There is some evidence that, on several occasions subsequent to the Cretaceous, eustatic sea level rose high enough to have submerged the terrain east and south of the Edwards Plateau, perhaps as far inland as the Balcones Escarpment [20,21,22,23,24], but we could find no report of a stratigraphic record of marine transgressions over the Plateau since the Cretaceous.
One of the Cretaceous formations (Edwards Limestone) in the footwall block still exposed along the eastern and southern slopes of the Balcones Escarpment is riddled with caverns, the youngest of which are below the fault trace and support permanent subterranean streams of the Edwards Aquifer [14], with estimates of total water capacity ranging upwards to over 200 km3 [17]. At progressively higher strata of Edwards in the now vadose portion of the formation above the trace there are progressively older caverns which formerly carried aquifer water but now harbor terrestrial endemics, all this indicating a long Cenozoic history of coastward hydrological evolution in the Edwards Plateau [16]. It is in the perennial rheocrenic springs that have been issuing from this series of caverns for millions of years that we focus our attention.
2.3.1. The Wisconsin and Holocene Climates
To set the stage for our discussion, we must establish the presumptive history of a series of very wet and then very dry periods following in relatively quick succession in the region since about 22 kya. During most of the Cenozoic Era, the mean annual temperature of Earth apparently experienced a gradual cooling trend [21] and then dipped dramatically to near 0 °C during the four glacial maxima of the Pleistocene.
The climate of the SW USA during the latter stages of the Wisconsin Glaciation and the beginning of the Holocene is thought to have been much wetter than the present. Cooke et al. [25] provided convincing isotopic evidence that soil thickness over the Edwards Plateau during late Pleistocene averaged nearly 2 m thick, but starting at about 21 kya, soils eroded during the wet period at a rate of 11 cm/ky until about 5 kya. This rapid precipitation/erosion narrative is corroborated by various findings, including a fossil record indicating the presence, then decline, and then disappearance of burrowing mammals that require deep soils [26]. Within that erosional interval, there apparently was also a period of intense channelization of regional streams between about 15 and 11 kya [27,28,29] and another episode at 9.6–7.4 kya [29]. The soil erosion estimates of [25] suggest that most of the precipitation was probably in the winter, with droughts in the summers.
Following the transition between the wet Pleistocene and the present, a period of “exceptionally rapid warming” occurred [30,31] that is thought to have been accompanied by several periods of extensive and severe droughts [32]. Speleothem (cave formations consisting of minerals deposited from percolating water) studies from the southwestern U.S.A. indicate that stalactites grew rapidly immediately following the last glacial maximum, but then abruptly stopped dripping completely at about 10 kya and ceased to grow at all until about 2.7 kya when the dripping started again but at a slower rate [33]. This speleothem record, which is corroborated by parallel records of lakes in the western and southwestern U.S.A. becoming desiccated [34,35], is consistent with an extremely arid period 10 kya–2.7 kya. While the dates of onset and durations of the early Holocene wet period and Mid-Holocene droughts vary with locality, there is widespread consensus that the Pleistocene/Holocene transition was much wetter than present, and that the Mid-Holocene droughts were much drier than present, with the most extreme of the Holocene droughts primarily occurring between 6 and 2 kya [30,32,36,37,38]. The effective outcome of these droughts is that the volume of freshwater exposed at the surface in some parts of the western U.S.A. may have been reduced by 90% relative to contemporary surface water [39] and by 75% within the Edwards Plateau region [40,41].
Climatological Effects on Local Aquatic Communities
Given this condensed narrative of the Holocene climatic history in Texas, and that the highest concentrations of endemic aquatic biocenoses in the study area are found in the eucrenal reaches of rheocrenic spring runs, it seems reasonable to surmise that the Holocene droughts caused a dramatic reduction of available aquatic habitat exposed at the surface in Central Texas [40,41] which forced surviving aquatic taxa into isolated refugial patches of groundwater-sourced surface water, and some were even forced into the aquifer itself. We are obviously not the first to propose such a narrative for the Edwards Plateau, but intend to add several lines of clarifying information to the narrative that had not been reported before. In so doing, we will use a proxy in the form of a crenobiontic nematode parasite that has two obligate contemporary hosts in its life cycle, both of which are common and cosmopolitan in most Texas streams.
The fragmentation and rarefication of surface waters during the mid-Holocene droughts are thought to have lasted for long durations relative to the generation time of most aquatic invertebrates. This would have halted the gene flow that was previously occurring between the populations of the permanently aquatic clades via fluvial conduits that were formally perennially interconnected. The genetic bottlenecks caused by reductions in local abundance, and the restriction of gene flow caused by fluvial fragmentation, are thought to have provided isolated natural laboratories for extreme adaptation to the stable conditions of groundwater-sourced surface water. Over time, genes that had, for probably since the Precambrian, provided complex systems of cellular damage control and repair necessary for ectotherms to survive in physicochemically variable waters were either lost or repurposed. Once these springs were later reconnected to fluvial surface waters, the crenobionts would be unable to re-disperse downstream into thermally ambient surface streams. Thus, unexpectedly abrupt distributional discontinuities are now common just downstream from some of the perennial spring sources flowing from the Edwards Aquifer. Another factor that enriches the drama playing out in these abrupt distributional discontinuities is that the returning fluvial relatives of the endemics taper in abundance upstream from the discontinuities because they cannot outcompete the endemics in their native biotope.
2.3.2. The Chronology of Our Model
The chronology of our study begins sometime after the last marine regression, which permanently exposed the Edwards Plateau of Central Texas and which probably occurred no earlier than the late Cretaceous, and no later than the mid-Pliocene [42]. Although there have been four or five interglacial warming periods during the Pleistocene that have probably contributed to the aquatic endemism of the Edwards Plateau, the main period of our investigative focus starts about 20 kya just before the transition from the Wisconsin glaciation to the interglacial warming of the Holocene.
2.4. The Model System
2.4.1. The Clades of Focus
There are numerous permanently aquatic clades in this landscape that form very local biocenotic communities of low-dispersion invertebrates having narrowly restricted and sharply defined distributions. Some of the clades are constrained to a single spring [12,43] despite many of the springs being permanently interconnected via underground conduits and perennial surface streams throughout recorded local history.
The mosaic of endemism observed across diverse higher taxa within this region is difficult to explain comprehensively—how could so many species, both related and unrelated, share such similar distributional patterns, and why are there so many abrupt distributional discontinuities associated with the Edwards Plateau? The most parsimonious explanation seems to be that these extant biocenoses share a common evolutionary history while cohabitating in their isolated spring biotopes, and that the highly restricted contemporary distributions resulted from some series of events that acted simultaneously in sequence across the entire region.
The water of the Edwards Aquifer, which supports >100 stygobiontic clades [10], breaks through the surface at the bottom of the exposed Balcones Escarpment and feeds large perennial and thermally stable rheocrenic springs such as San Marcos Springs (SMS) and Comal Springs (CS; Figure 1) in Hays and Comal counties, TX, USA. The SMS supports diverse communities of aquatic organisms that include many endemic crenobiontic clades and is the headsprings of the San Marcos River (SMR).
One such clade is an innominate hyalellid amphipod we will call the SMS Hyalella; a mucronate (possessing dorsal spines) and crenobiontic amphipod restricted to the eucrenal waters of the first 3 km of the Upper SMR. This amphipod coexists with, and is reproductively isolated from [44] the cosmopolitan Hyalella cf. azteca. Both hyalellids also serve as functional intermediate hosts [45] in the life cycle of a locally endemic histoparasitic nematode of centrarchid fishes, Huffmanela huffmani [46] (Nematoda: Trichosomoididae: Huffmanelinae). H. huffmani is the only reported freshwater species of the subfamily and its distribution in the SMR is also restricted to the upper 3 km of the spring run, where prevalence of H. huffmani is often 100% in several centrarchids species [47]. Recently, two other endemic freshwater populations of Huffmanela were reported from Texas headsprings some 230 km NW; one from Clear Creek Springs (CCS) on the San Saba River (SSR) [48] and the other from the headsprings of the South Concho River (SCR) [49]. No adults have been recovered from these new populations, but their eggs cannot be reliably distinguished from those of H. huffmani in the SMR. Another mucronate and crenobiontic hyalellid amphipod clade distinct from the SMS Hyalella occurs at CCS (H. texana [50]). A fourth, but now extinct population of Huffmanela was discovered to have been infecting centrarchids in the nearby Comal Springs until the drought of 1956 [45]. Recently, adults and eggs that are morphometrically and genetically undisguisable from SMR H. huffmani were discovered infecting freshwater poeciliids from ornamental fish ponds in Ruskin, Florida [51]. The 21 other nominate species and about 15 additional innominate but distinctive populations of Huffmanela have all been reported exclusively from marine or estuarine fishes, and H. huffmani remains the only reported freshwater species. Interestingly, and perhaps pertinent to our focus, many of the other endemic invertebrates of the Edwards Aquifer and its springs appear to be marine-derived freshwater representatives of higher taxa that are mostly or exclusively marine [10,52].
The extremely narrow endemism of H. huffmani and its SMS Hyalella host in freshwaters of Central Texas provides an opportunity for a novel line of proxy reasoning to enhance the biogeographic inferences that underpin the existing narrative regarding the region’s geologic and climatic history.
2.4.2. Overview of Corroborating Experiments
We performed a series of stepwise, mostly experimental investigations using this nematode/amphipod/fish host–parasite system as a model to explore some of the mysteries of the biogeographic history of these springs in central Texas and their diverse biocenoses of narrowly endemic crenobionts. Firstly, we tested the possibility that the contemporary distributions of these crenobiontic clades could have been sculpted out of previously widespread distributions by the severe droughts of the Pleistocene interglacials. We focused on the most recent Holocene droughts, for which we have a relatively high resolution chronology, as an example. Secondly, we explored the possibility that these nonaerial aquatic invertebrates were forced by desertification into the stable physicochemistry of rheocrenic springs for tens or hundreds or thousands of generations resulting in genetic losses that are currently preventing those endemics from colonizing the now-reconnected surface streams. Thirdly, we investigated evidence that the evolution of the SMS Hyalella and its Huffmanela parasite were affected by the same large-scale environmental events.
2.5. Our Research Hypothesis
Our overarching hypothesis that we are investigating through a series of specific and testable hypotheses is that the Hyalella/Huffmanela host parasite system can shed light on the dynamical paleohistory of these functionally related biotopes populated richly with other endemic clades from diverse higher taxa, and that the descriptive name we have chosen to label the type of karstic rheocrene refugia we are dealing with in Central Texas will be strengthened by skeptical challenges and serve as an example for uniquely labeling several other types of refugia that have traditionally been cast into the functionally meaningless categories of glacial or interglacial refugia.
2.5.1. “Crenal Interglacial-drought Evolutionary Refugia” (CIdER)
Our study focuses on several biocenoses of contemporary, narrowly endemic, crenobiontic clades of central Texas that are hypothesized to have once been widespread in the interconnected crenic and lotic freshwaters of the region. The refugia to which our endemics are currently restricted are karstic-spring microrefugia sourced by deep, expansive, largely interconnected subterranean reservoirs; thus, spring discharge is largely uncoupled from local precipitation/drought/temperature transients and many of the springs are perennial rheocrenes. We will start with brief descriptions of stages in the chronology of events that are hypothesized to have led to the endemism we find there today, and will apply a new term to help clarify the distinctions between this type of evolutionary refugium vs. other evolutionary refugia associated with glacial/interglacial cycles.
Following the general admonition of Ashcroft [53] (Appendix A.3.4), we will be using the new term and acronym “Crenal Interglacial-drought Evolutionary Refugia (CIdER).” While the name is longer than most category names, the modifiers capture the biotope and biocenosis, the climate, the dynamics, and the duration (long enough to be evolutionarily relevant). Although details in composition, timing and location will vary from site to site and region to region, the term will uniquely represent a cohesive type of refugium with a specific set of contemporary and historical factors that must be understood in order to fine-tune conservation efforts and potentially, to protect the many biotopes that are examples of this type of refugium and their delicate biocenoses. It also provides an example of how to address some of the issues revealed by workers who have published extensively about the terminological morass we must all wade through when reviewing the literature on the subject of biodiversity hot spots [4,53,54,55,56,57,58,59] (see Appendix A).
At this writing, we know of no other named refugial type or classification of endemics that encapsulates the salient and peculiar combination of features, driving forces, and evolutionary consequences just described above for the karstic springs of the Edwards Plateau. Yet, investigators dealing with glacial/interglacial refugia and related endemism often refer collectively to systems that have widely differing functional dynamics using a single ambiguous terminological scheme. For instance, endemics in terrestrial refugia will have arrived there having been driven by dramatically different forces with different consequences compared to aquatic endemics in crenal refugia in the same general region. The two communities may be experiencing the same climatic changes at the same time, but they cannot all be meaningfully referred to as simply “interglacial refugia.”
Note that since we are limiting our definition to groundwater-sourced aquatic refugia, the main factor defining a CIdER refugium is hydrological dynamics driven by advancing aridification followed by a return to precipitation sufficient to maintain perennial fluvial conduits, rather than long-term changes in average annual temperature, although temperature usually occurs as a covariate with precipitation.
If we could show that the SMS is indeed a CIdER refugium, then the occurrence of Huffmanela in the other springs of the Edwards Aquifer would suggest, by inference, that those springs also represent CIdER refugia that had earlier presented groundwater at the surface uninterrupted through the Holocene droughts, and perhaps much longer (potentially as far back as just after the last marine regression). Furthermore, if we could provide corroborative evidence that both the parasite and its obligatory amphipod host probably coevolved and shared the same environment through the period of forced isolation, this finding would provide additional corroboration for the CIdER refugium hypothesis.
2.5.2. Hypothetical Stages in the Development of a CIdER Refugium
Stage 1: Abundant precipitation (Figure 2A). In order to explain how all of these once cosmopolitan clades from diverse higher taxa became so narrowly endemic in the same springs, we hypothesize that, at some time prior to the mid-Holocene Droughts, the spring runs of these rheocrenic springs were interconnected via surface-fed perennial streams.
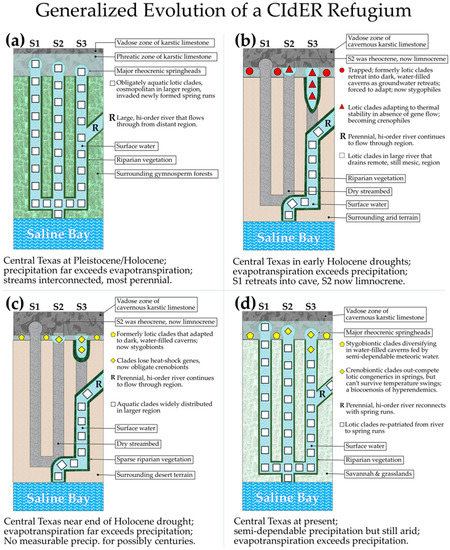
Figure 2.
Schematic of the hypothesized sequence of events leading to the development of a CIdER refugium, using Central Texas as an example. S1–S3 represent three springs interconnected underground by conduits fed by the same aquifer pool, with S1 opening at the highest elevation and S3 at the lowest elevation of the three. (a) Conditions during late Wisconsin Glaciation and early Holocene (ca 20–9 kya) with abundant precipitation well in excess of evapotranspiration, and continuous discharge from all springs flowing into progressively higher order streams and then to coastal bays. (b) Holocene droughts started ca. 9 kya and most aquatic biota were locally extirpated but some biota adapting: S1 withdrew underground with some vertebrates and invertebrates adapting to stygobiosis; S2 stopped flowing out but maintained groundwater-sourced limnocrenic pool at surface; S3 is still flowing out into short spring run that disappears into air and ground with no connections to other surface water. (c) Near end of most severe of Holocene droughts ca. 4.5–2.7 kya with all springs still isolated and most surviving clades now stygobiontic or crenobiontic; (d) Abundant moisture returns and cosmopolitan fluvially adapted congeners are repatriated to interconnected spring runs and are mixing with the now-endemic crenobionts.
Stage 2: Aridification (Figure 2B). Reduced precipitation at the beginning of the mid-Holocene droughts (about 9 kya) eventually reduced discharge from the springs, interrupted surface connections with remaining streams, and isolated the aquatic biota of each spring into microrefugial crenal biotopes [60]. The bulk of the regional aquatic species were either extirpated, retreated into subterranean habitats where they transitioned into stygobionts, or retreated into major rivers that continued to drain remote regions. Hypothetical spring S1 (Figure 2B) stopped flowing and retreated mostly into a cave, S2 maintained a pool of water receiving sunlight at the surface as a limnocrene, and S3 continued to flow into a short unconnected spring run that disappeared due to evaporative and hyporheic losses and lack of phreatic input. The aquatic fauna of S2 and S3 became endemic remnants of clades once widespread in the region, but many of the clades had relatives that we will call sister populations that managed to survive the aridification in major rivers near the region.
Stage 3: Continued isolation (Figure 2C): Several thousand generations later toward the end of the mid-Holocene droughts, the spring-bound endemics had lost many alleles formerly used to produce enzymes adapted to diel and seasonal temperature swings [61], and for repairing damage to molecular infrastructure and metabolic systems following exposure to temperature extremes [62]. Such alleles could be lost from the refugial population without fitness penalties due to the direct connections to deep aquifer conduits restraining the thermal variability of the springs to within ±1 to 2 °C. Indeed, Somero [62] showed that congeneric species of intertidal organisms replace each other along a subtidal/intertidal gradient, suggesting that the fitness costs of evolving competitive advantage at a given temperature are actually less than the costs of maintaining the metabolic wherewithal to operate competitively at a range of temperatures. At the same time, the refugial endemics had become much more efficient at exploiting resources from the physicochemically stable refugium. Meanwhile, the sister populations of the crenobiontic clades had adapted to surface water in the remote regions to which their population had retreated as the drought progressed.
Stage 4: Abundant precipitation returns (Figure 2D). Upon the return of precipitation beginning at probably around 4.5 kya, reconnection of the isolated refugia to perennial surface-fed streams began to allow contact between the long-isolated endemic clades and the now-repatriated, but still stream-adapted, congeners from remote regions where surface-fed streams had continued to flow. By this time, genetic divergence between the crenobiontic population and the displaced sister population may have progressed to the degree that even if the sister population is subsequently repatriated into sympatry with their crenobiontic congeners, reproductive incompatibilities may have accumulated to the extent that successful backcrosses between the two populations are now unlikely or impossible. However, if they are reproductive compatible, it is likely that the crenobiontic congener will soon be introgressed out of existence by the much richer allelic gene pool of the repatriated sister group.
At Stage 4 above, our research team steps onto the stage and makes several observations listed below that ultimately engender an array of questions.
- Members of the spring-endemic population are now crenobionts because they are unable to disperse into the connected ambient stream.
- The cosmopolitan congener (repatriated sister group) does not introgressively swamp the endemic population because of apparent reproductive incompatibilities.
- The widespread congener is apparently unable to displace the endemic population from the spring by out-competing it.
- Both populations remain in graduated sympatry [63] in the eucrenal and/or the spring-influenced upper reach of the spring run.
- Our team is unable to determine by mere inspection whether the observed endemism of the crenobionts had originated through sympatric or allopatric processes.
- Our team is also unable to determine by inspection what factors are limiting the down-stream dispersal of the crenobionts.
- Thus, if an endemic species occurs in a patch of habitat that is far from other such patches, that alone is insufficient evidence that the endemism developed in allopatry; likewise, when an endemic species occurs sympatrically with a closely related congener, that observation is not necessarily evidence that the endemism had developed in sympatry. Therefore, while speculation might be useful for developing testable hypotheses, the development of a definitive and supportable narrative that parsimoniously explains the cause of contemporary endemism should be based on multiple lines of reasoning that are corroborated by pertinent data and, where possible, experimental findings.
2.6. The Need for Rigor
We are not the first to have proposed that the springs of the Edwards Plateau were once perennially isolated refugia. While conjecture and speculation abound regarding potential mechanisms that forced the hypothesized range contractions and caused the endemism among the aquatic fauna of the Edwards Plateau, there have also been some rigorous attempts to test such hypotheses.
Adams et al. [64] provided an interesting account of the presumed evolutionary history of saline and freshwater gammarid amphipods in previously isolated springs of the Chihuahuan Desert which borders the Edwards Plateau on the west and has experienced many of the same major climatic and geologic events. Krejca [65] used evolutionary patterns of stygobionts to aid in interpreting the hydrogeologic history of the central Texas karst aquifers and adjacent aquifers of northern Mexico. White et al. [16] studied a troglobiontic spider clade from vadose caverns of the Edwards karst and established links among phylogeography, geomorphology, and hydrogeology. They also developed a robustly supportable interpretation of the Pleistocene evolutionary history of the clade indicating that the spiders had apparently been epigean during successive glacial maxima and then retreated into caves that had been left at progressively lower elevations in the vadose zone during the three interglacial warming periods of the Pleistocene as the aquifer evolved coastward [66]. Craig et al. [8] studied distributions of fishes noted for their preference for springs of the Edwards Plateau and compared these with distributions of related river fishes in connecting streams. They reported findings consistent with one or more episodes of lengthy isolation during the Pleistocene interglacials, each followed by reconnection.
While host/parasite systems are rarely used to test proposed causes of paleogeographic endemism, there have been some productive attempts. Mejía-Madrid et al. [67] investigated the phylogeography of Rhabdochona lichtenfelsi, an intestinal nematode parasite specific to endemic goodeid fishes in Mexico, and provided new information regarding the biogeographical history of fragmentation and recent evolution of the Mesa Central drainages. Martínez-Aquino et al. [68] used molecular dating of cladogenetic events of the intestinal trematode Margotrema spp. and found good correspondence with inferred phylogeographic history of their hosts (goodeine fishes of the western slope of central Mexico). Lumme et al. [69] investigated the historical zoogeography of a monogenetic trematode of a small fish in Europe, and discovered findings consistent with several sequential episodes of isolation bottlenecks and re-expansions in numerous allopatric refugia during the Pleistocene.
Our main goal in this study was to investigate the Crenal Interglacial-drought Evolutionary Refugium (CIdER) Hypothesis as a possible mechanism to explain the extreme endemism of the diverse freshwater clades that are now restricted to tiny distributions of a few hectares in the Edwards Plateau and surrounding regions. A secondary goal was to explain how these same organisms might have continued to remain constrained entirely to the spring-influenced portion of their ancient habitats, despite the return of interconnectivity thousands of years ago between the springhead habitats and surface-fed streams. Therefore, our report will utilize the present biogeography of an extant endemic host/parasite system to make inference into ancient abiotic factors possibly contributing to the original isolation of these populations. In particular, a host/parasite system consisting of a narrowly endemic nematode parasite with two obligately aquatic but ubiquitous hosts (centrarchid fishes and hyalellid amphipods) was used as a proxy for palaeoecological inference. This three-species host/parasite system serves as a more useful proxy than would three free-living crenobiontic endemics, because the occurrence of this narrowly endemic parasite in a CIdER refugium today indicates that not just the parasite, but also both of its obligate host species (or their ancestral clades), were continuously present in the habitat from the hypothesized contraction of the parasite’s formerly broad range to its current very limited range of about 20 hectares of spring-influenced surface water. By using this parasite as the test species, we hope to improve understanding of the likely distributional history and co-evolution of not only the parasite, but also the codivergence of it its crenobiontic amphipod intermediate host, and by broader inference, some of the other endemics of the region.
Our study involves findings from many different types of investigations, from purely descriptive to rigorously experimental. In order to facilitate assimilation and application of the findings, we will accumulate all the issues directly associated with each finding, including purpose, methods, results, and discussion together in narrative form in the section related to each finding.
3. Methods, Results and Discussion
We have organized this section into a question/investigations/answers format to facilitate assimilation of data consistent with our research hypothesis.
3.1. Q1: Is There Evidence That SMS Has Continuously Maintained Groundwater at the Surface since the Last Glacial Maximum?
A major component of the CIdER refugium hypothesis is that the SMS is a permanent spring that has maintained a supply of flowing groundwater at the surface continuously and without interruption through the Holocene and probably much earlier. It has not stopped flowing in recorded history, but the CIdER refugium hypothesis requires that it has not stopped presenting at least a springbrook at the surface for at least the last 14 ky. Interestingly, support for the SMS being a CIdER refugium was obtained indirectly from neighboring Comal Springs (CS).
3.1.1. An isolated Huffmanela Clade Was Extirpated from CS by a Historic 4.5-Month Cessation of Spring Discharge
Comal Springs (Figure 1), the headsprings of the Comal River, has the highest average discharge of any naturally occurring freshwater spring in the southwestern USA [70], is located <30 air km southwest of the SMS (Figure 1), derives its flow from the same pool of the Edwards Aquifer, and is confluent with the same river as the San Marcos River. Despite the close proximity of the Comal and San Marcos springs and the remarkable similarity between their aquatic communities and physicochemistry [71], Huffmanela had never been detected in centrarchids from the CR, despite many attempts (Table 1). Additionally, both hosts of Huffmanela huffmani are known to occur abundantly and continuously throughout the streams connecting the two springs. Thus, the absence of Huffmanela in CS has remained an intriguing enigma since the discovery of Huffmanela in the SMS in 1978, and represented a challenge to the CIdER hypothesis in its early stages of development.
One evening while discussing frustration over our failure to find Huffmanela in the centrarchids of the Comal Springs, one of us (MLDW) recalled that the CS had ceased flowing (due to over-pumpage) for >4 months during the drought of record in 1956 [70]. This drought is known to have caused the local extirpation [72] of the fountain darter, Etheostoma fonticola [73], which was formerly endemic to both the CS and the San Marcos Springs, and MLDW conjectured that it might possibly have also caused the extirpation of an ancient clade of Huffmanela there as well. During this drought, the SMS, which issues from the same aquifer, also experienced reduced discharge, but because the elevation of its spring openings is about 14 m lower than that of CS, the SMS had flowed continuously throughout the drought with a minimum discharge of ≥1.3 m3 s−1 [70], and thus spared the SMS populations of the fountain darter and Huffmanela from extirpation there. If we could find evidence consistent with our conjecture that Huffmanela actually had existed in the CS prior to the cessation of flow in 1955, this would be consistent with CS having continuously presented groundwater at the surface throughout the Holocene until over pumpage during the drought of the 50′s caused the spring to stop flowing for the very first time (perhaps since its formation) and brought about the extirpation of not only the fountain darter, but perhaps also an ancient clade of Huffmanela and probably other unreported crenobiontics. It would also mean that the first and only time the CS went dry was in 1955, and that it otherwise had not stopped presenting groundwater at the surface since the Wisconsin glaciation, and probably much earlier.

Table 1.
Texas springs searched for the presence of mucronate Hyalella spp. or Huffmanela spp. prior to this study.
Table 1.
Texas springs searched for the presence of mucronate Hyalella spp. or Huffmanela spp. prior to this study.
| Spring Name | References | Coords. (Deg W, Deg N) | Crenobiontic Huffmanela spp. | Crenobiontic Hyalella spp. |
|---|---|---|---|---|
| Barton Springs | [47] | 30.263759, −97.770876 | N | ? |
| Blue Springs | [48] | 29.893691, −100.994661 | N | N |
| Caroline Spring | [48] | 30.469016, −101.803561 | N | N |
| Clear Creek Springs | [48] | 30.907044, −99.960929 | Y | Y |
| Comal Springs (CS) | [45] (fish from [5]) | 29.714441, −98.135296 | Y 1 | N 2 |
| Comal Springs (CS) | [47,48,74,75,76] | 29.714441, −98.135296 | N 3 | N |
| Fessenden Springs | [47,48] | 30.166927, −99.342635 | N | N |
| Finnegan Springs | [48] | 29.901371, −100.999576 | N | N |
| Hueco Springs | [47] | 29.759169, −98.140878 | N | ? |
| Las Moras Springs | [48] | 29.309747, −100.420961 | N | N |
| San Felipe Springs | [48] | 29.373565, −100.885139 | N | N |
| San Marcos Springs (SMS) | [48,75,76,77] | 29.893931, −97.930088 | Y | Y |
| San Saba Springs | [48] | 30.825901, −100.119022 | Y | Y |
| South Concho Springs | [49] | 31.135639, −100.493499 | Y | N |
1 Fish were collected in 1951 and deposited in museum; examined in 2015. 2 Arrangements only allowed us to examine swim bladders. 3 Local Huffmanela clade apparently went extinct when flow stopped during drought of 1956–7.
To test the hypothesis, we searched museum records for centrarchid collections from anywhere in the Comal River prior to 1956 and found a collection by the Texas ichthyologist Clark Hubbs [5] in the Ichthyology Collection at the University of Texas Natural History Museum. These fish had been collected in December of 1951, well before the drought had become serious enough to stop surface flow. We acquired access to seven centrarchids in the collection. Preserved fish were temporarily removed from jars and examined on site. We excised the retia mirabilia from the swimbladders of these fish (jar catalog # TNHC2331, TNHC2332, TNHC2335, TNHC2338, and TNHC2353) which were returned to their jars less the rete. The retia were transported to our lab and examined for evidence of infection with Huffmanela and we found that 3 of 3 Lepomis miniatus, 1 of 2 L. microlophus, and 0 of 2 L. macrochirus were positive for eggs that were morphometrically indistinguishable from eggs of H. huffmani. Interestingly, these host-specific prevalences mimicked prevalences we had been seeing in the SMS for decades. So, an extinct clade of a crenobiontic endemic parasite of fish from the headsprings of the Comal River had thus been discovered.
To also confidently confirm that Huffmanela is, indeed, still currently absent from CS, we collected 60 more centrarchids of various species from Landa Lake (which impounds Comal Springs) in 2014 and inspected them for the presence of Huffmanela. The effort was repeated in 2015 for a total of 120 centrarchids. We also extensively searched the literature from the years following the drought of 1956 for any suggestion that Huffmanela (or any nematode of centrarchid swimbladders) may have been reported from Comal Springs. The literature search and all 120 fish and were negative, corroborating that Huffmanela had likely been extirpated from the CS sometime between 1951 and 1978, and also suggesting that the eggs of H. huffmani, which had been shown to survive for years at 22 °C in sealed vials with no detectable decrease in infectivity [45], are unable to survive desiccation lasting less than 5 months in 1955–1956.
The discovery of an extinct clade of Huffmanela in Comal Springs seemed to be exciting circumstantial corroboration of the CIdER refugium hypothesis. However, if we could experimentally demonstrate that Huffmanela eggs are, indeed, sensitive to desiccation, the corroboration would be much stronger. Therefore, we sought to determine how long the eggs could survive a drying experience similar to what one would expect eggs to experience as they are slowly exposed subaerially while the wetted perimeter of the spring retreats back into the spring openings during an extended drought.
We pooled eggs from the shredded swimbladders of several freshly wild-caught centrarchids from the SMS, stirred them into a container of artesian water, and pipetted a 100 µL aliquot of the egg suspension into each of two 100 mL beakers filled with the same artesian water. One beaker was randomly assigned to the desiccation treatment and the other established as a continuously hydrated control. Both beakers were maintained uncovered in the same conditioned room at about 22 °C and exposed to evaporation, differing only in that the control beaker was periodically topped off with deionized water to maintain original volume. At the end of the first week, the treatment beaker had become completely desiccated. We continued to incubate both beakers at 22 °C until the end of the second week (the treatment beaker remained dry while water lost from the control beaker was replenished daily). We then rehydrated the treatment beaker with aquifer water and incubated both beakers until the end of the third week, providing deionized water to both as needed.
Twelve adult lab-reared amphipods (Hyalella cf. azteca) of about the same size were randomly divided into two groups of six. One group was added to the beaker of control eggs and the other six to the treatment beaker and incubated for 5 d at 22 °C (amphipods exposed to eggs become infective to fish 5 d after exposure to eggs [45]). Each experimental amphipod was then carefully examined for Huffmanela larvae under a dissecting scope. All amphipods exposed to non-desiccated control eggs were infected with multiple active Huffmanela larvae (100% prevalence), while none (0% prevalence) of the amphipods exposed to desiccated eggs were observed to contain any Huffmanela larvae either dead or alive (Table 2).

Table 2.
Effects of desiccation on the ability of Huffmanela huffmani eggs to infect amphipods (Hyalella cf. azteca).
This finding is consistent with our expectation that the Huffmanela population that was present in CS prior to 1952 had been extirpated from CS when it stopped flowing for several months during the drought of record in 1956, and that the CS and SMS had otherwise never ceased to maintain groundwater at the surface since before the Last Glacial Maximum, even during the much more severe droughts of the Holocene.
The continued discharge from CS during the subsequent drought of 2008–2014, despite a near doubling of regional population between 1960 and 2000, means that the cessation of flow in the CS in 1956 was caused by over pumpage, rather than by the drought itself. The difference was the effectiveness of the staged pumpage restrictions mandated by Texas Senate Bill 1477 to control withdrawals from the aquifer during droughts [78] as they become more severe. Had this legislation been implemented early in the 1950′s, it would probably have also protected the Comal population of fountain darters that were extirpated, the now extinct Huffmanela sp., a hypothesized crenobiontic Hyalella clade similar to the SMS Hyalella, and probably other unknown crenobiontic endemics unique to the Comal River system.
3.1.2. More Unexpected Contributions from Texas Wild Rice (Zizania texana)
Another corroborating observation in support of the permanency of flow in the SMR spring run through the Holocene (relative to other springs of the Edwards Plateau) can be inferred from Texas wild rice (Zizania texana Hitchcock [79]), an endangered plant currently restricted to the upper 4 km of the SMR and never reported from the larger CS less than 30 km away.
Z. texana is thought to have been derived from a widespread clade of Zizania that had flourished during the Wisconsin glaciation when the southwestern USA was much wetter, but then retreated eastward during mid-Holocene aridity, with the isolated SMR population containing the only extant representatives [80].
We conjecture that another remnant population of Z. texana might have survived in the CR into perhaps the mid Holocene but was then extirpated from there by a drought that had stopped the flow of crenal water through the consequently stagnant shallows and marshes surrounding the spring openings where the plant had previously flourished when flowing. However, the fountain darter and the Huffmanela/Hyalella/centrarchid system had both survived these same conditions in crenal pools over the openings of CS. This conjecture is consistent with information obtained from two scientists having research experience with the plant. Hardy [81] (pers. comm.) said that, in experiments pertaining to the growth of potted Z. texana in varying velocities of spring water, the plants flourished in flows at characteristic spring-run velocities, but when cohorts were transferred to flow-through ponds fed with the same water but with no measurable velocity at the pot, the rice began yellowing within 1 week and then died. Heard [82] (pers. com.) also gave an account of a healthy stand of Z. texana observed in shallow flowing water in the SMR (at 29.888626, −97.934122). The root bed of the stand was later exposed to air during a low-flow event in 2018 which killed all the exposed plants within about 2 weeks. Additionally, Horne et al. [80] reported that both the roots and seeds of Z. texana are very sensitive to desiccation. Thus, it is possible that Holocene droughts had extirpated the remaining stands of Zizania texana from all springs lacking shallows permanently flooded with flowing crenal water for as little as 2 weeks, but had still allowed H. huffmani and the fountain darter to survive in groundwater-sourced pools in both CS and SMS, with the latter being the only spring maintaining a flowing springbrook continuously through the Holocene with enough velocity to support Z. texana.
3.2. Q2: Can the Occurrence of Endemic Hyalellids Similar to the SMS Hyalella in Other Springs Reveal Other CIdER Refugia Harboring Huffmanela?
A literature search for other large, mucronate, narrowly endemic Hyalella similar to the SMS Hyalella revealed one other species in Texas (H. texana [50]) at Clear Creek Springs (CCS; also known as Wilkinson Springs; Table 1, Figure 1). Subsequently, Clear Creek Springs (CCS), the type locality for the crenobiontic Hyalella texana, was found to be positive for Huffmanela, with species-specific infection patterns similar to the San Marcos River. This finding is consistent with our predictions that crenobiontic Hyalella in the Edwards Plateau are associated with CIdER refugia and that the endemic Hyalella coevolved in isolation with a local population of Huffmanela parasites, which is also endemic to that spring.
The CCS spring run (Clear Creek) is a short tributary (<5 km) of the San Saba River (SSR) and the headsprings of the SSR (30.825991, −100.119046) are only 23 km west and upstream of the confluence of Clear Creek with the SSR (Figure 1). Thus, centrarchids from the SSR headsprings were also checked for the presence of Huffmanela and found to be positive. Consistent with the hypothesis that springs bearing Huffmanela are former CIdER refugia, a crenobiontic population of mucronate Hyalella was found there as well. However, these crenobiontic amphipods are morphologically similar to, and were found to be reproductively compatible with, H. texana from CCS, while also being reproductively incompatible with the SMS Hyalella sp. from the San Marcos Springs [44].
Interestingly, the water level at Clear Creek Springs is about 25 m lower in elevation than any of the spring openings we have seen in the SSR headsprings, and so the mucronate crenobiontic Hyalella in the SSR headsprings is probably derived directly from H. texana, and the CCS probably becomes the permanent headsprings of the SSR during extended severe droughts such as those of the Holocene. Meanwhile, there are numerous submerged springs in the SSR between to two sites and plenty of opportunities for the Clear Creek Hyalella to exchange alleles with the headsprings clade. Thus, we consider the crenobiontic Hyalella from the SSR headsprings to simply represent an extension of the known range of Hyalella texana, and henceforth we will use that taxon to refer to the crenobiontic Hyalella from the SSR headsprings.
The South Concho River (SCR) headsprings (31.135647, −100.493471; also known as Anson Springs; Figure 1) just 50 air km NW of the CCS was also sampled and found to be positive for Huffmanela in the swimbladders of local centrarchids. However, after several thorough searches, all amphipods collected in the SCR appeared to be morphologically consistent with the widespread Hyalella cf. azteca, and we were unable to find a mucronate Hyalella similar to Hyalella texana or the SMS Hyalella. This observation makes the SCR and Comal Springs the only known sites for Huffmanela in the freshwaters of Texas where we have not also found a morphologically distinct crenobiontic Hyalella amphipod restricted to the same biotope.
However, since Huffmanela huffmani is obligately dependent on Hyalella for its intermediate host, it is almost certain that there was a hyalellid amphipod in the SCR that carried Huffmanela through the Holocene period, but that this species had not developed reproductive incompatibility with the Hyalella cf. azteca before the latter was repatriated back into the spring. In this case, the crenobiontic form would have been introgressively swamped into extinction by the returning H. cf. azteca, which now serves as intermediate host for the Huffmanela population in the SCR.
3.3. Q3: Are the Downstream Distributional Limits of Crenobionts in the SMR Determined by Change in Some Aspect of Spring Physicochemistry?
3.3.1. Overview of Contextual Data
Studies of spring endemics from all over the world have reported gradual declines in abundance of the endemics as distance downstream in the spring run from the springhead and the crenobiontic Hyalella/Huffmanela host–parasite system in the SMR is no exception.
While Cox et al. [77] had previously shown that Huffmanela prevalence drops off precipitously at around 3 km downstream, we sought to verify that this downstream depression was still operating at about the same distance downstream, to quantify the decline in abundance, and to assess changes in various physicochemical parameters as possible causes of the decline.
We collected, by angling with artificial lures, a total of 59 fish belonging to five centrarchid species from five sampling sites along the upper 5 km of the SMR spring run. Captured fish were maintained in buckets with at least 10 L of aerated artesian water at 22 °C until euthanized according to IACUC-approved protocols the same day.
Retia mirabilia were excised from the swimbladder of euthanized fish and examined with a dissecting microscope for evidence of Huffmanela eggs. Since Cox et al. [77] had determined that an infected fish might contain upwards of millions of the tiny eggs of H. huffmani, we used an egg-density rating protocol adapted from Worsham et al. [45] to serve as a proxy for the traditional parasite intensity as a measure of parasite load. The procedure per fish was to examine five non-overlapping fields of the rete that contained some of the densest deposits of eggs in the rete and rate each field according to the subjective ordinal scale in Table 3. The arithmetic mean of the five field ratings was then used to represent the load of that fish as its mean egg-density rating. We then averaged the egg-density ratings for all infected fish from a collection site to represent the mean egg-density rating (MnEggDensRat) for that site. Note that Worsham et al. [45] had used the term “intensity” rating, but we later determined that “egg-density” rating is probably more appropriate [49,83] since we were not actually counting eggs. Additionally, while egg number would accurately represent the history of Huffmanela infection of a fish, it would not necessarily represent the number of worms present at necropsy (intensity), which is usually zero.

Table 3.
Protocol and criteria used to determine egg-density ratings for Huffmanela infection levels in centrarchid swimbladders.
Prevalence at a site was determined by dividing the number of fish whose swim bladder contained at least one egg by the total number of fish examined for infection at that site.
There was a decline in both prevalence and mean egg-density ratings when values were plotted on a map showing collection stations (Figure 3).
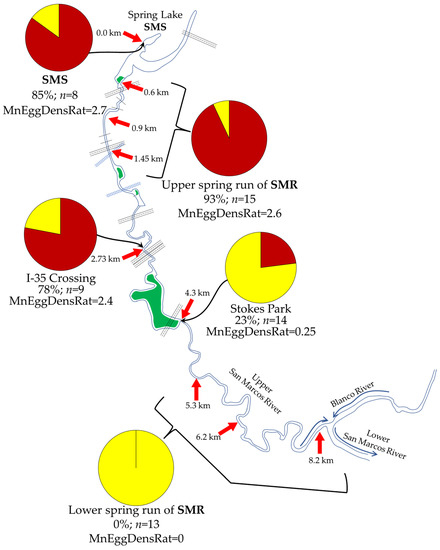
Figure 3.
Map of San Marcos River, Hays County, TX, showing decline in Huffmanela abundance at progressively more downstream stations as measured by prevalence and mean egg-density ratings in wild-caught fish (red pie slices = %fish positive; yellow slices = %fish negative for H. huffmani). Distances are approximate thalweg kilometers down-stream from headsprings (29.894038, −97.930154) based on a path traced in Google Earth Pro (map data ©12/21/2018 Google). n refers to number of fish sampled from each location.
The prevalence of Huffmanela huffmani infection in centrarchids wild-caught from various stations at or near the headsprings of the San Marcos River was consistently near 100% (Figure 3), but declined at sampled stations increasingly farther downstream from the headsprings and diminished to zero just beyond the 4.3 km station. The mean egg-density ratings for Huffmanela-infected fish at those stations tracked almost exactly in the same trend as the prevalence, with both suddenly dropping precipitously at about 3 km (Figure 4A). Indeed, over the distance downstream where Huffmanela declined to absent, the reach between 2.7 and 4.3 km revealed the sharpest declines relative to maximum values, with an 81% decrease in MnEggDensRat and a 59% decrease in prevalence (Figure 4a).
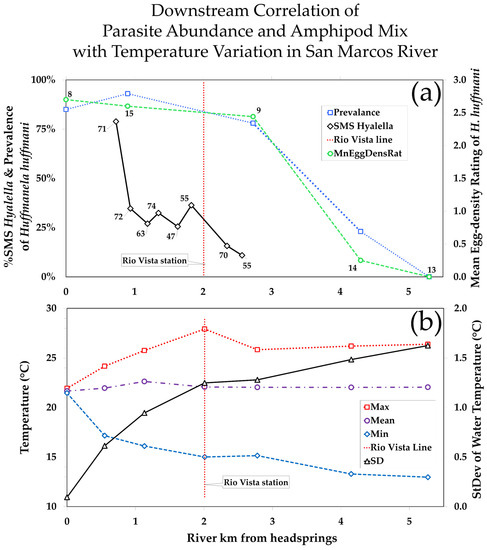
Figure 4.
(a) Variation in prevalence and mean egg-density ratings for Huffmanela huffmani in wild-caught centrarchids, and %relative abundance of amphipods sampled that were SMS Hyalella—all plotted against river kilometers down-stream from the headsprings of the San Marcos River; data labels are sample sizes. (b) Variation in mean temperature and standard deviation of temperature at various stations downstream from headsprings along with 14 y minima and maxima. All temperature data were recorded at 4 h intervals during years 2000–2014, which included years of severe drought followed by recovery.
Since we suspect the SMS Hyalella, also crenobiontic, of being the ancestral intermediate host that carried Huffmanela huffmani through the Holocene droughts, we also expected the SMS Hyalella to show a decline in abundance relative to the widespread H. cf. azteca downstream. We sampled habitats in eight near-shore low-flow stations varying from about 0.5–2.8 km downstream from the headsprings. Consistency among habitats sampled was maintained by finding beds of Ceratophyllum sp. and gently lifting single sprigs from the river at each station and swirling them around in a small bucket of locally obtained amphipod-free river water. This procedure was repeated at each station until about 50–75 amphipods had been captured. We temporally stored the amphipods from each station in separate labelled jars for transportation back to the lab. We estimated relative abundance by sorting the amphipods from each station into the two pans representing the species of Hyalella that occur in the river (SMS Hyalella and H. cf. azteca), calculated the percent of total hyalellids that we had identified as SMS Hyalella, and then plotted the percentages against river kilometer at each collection site.
Note in Figure 4a that the proportion of the amphipods that were identified as the SMS Hyalella at various stations downstream showed an even more pronounced decline downstream than did the prevalence of H. huffmani. This does not necessarily mean that the SMS Hyalella is more sensitive to physicochemical instability than H. huffmani, because the farther downstream distribution of Huffmanela-infected fish could also be explained by the relatively greater mobility and a much longer longitudinal home range than individual SMS Hyalella. A freshly infected fish host will be prepatent for nearly a year after its initial infection [45], and during that prepatent period, its infection cannot be detected by the gross inspection method we used. During the prepatent period, an infected fish would probably wander up and down the stream some distance from the site of initial infection before the fish (if caught) would be classified as infected. Nonetheless, the downstream decline in H. huffmani parasitism combined with the downstream decline in the relative abundance of the SMS Hyalella, the intermediate host thought to have carried the parasite’s life cycle through the Holocene droughts, adds circumstantial corroboration to our hypothesis that Huffmanela huffmani and the SMS Hyalella are coevolved endemics of the same CIdER refugium.
A collateral conclusion that can be drawn from these findings is that the centrarchid fishes in the study must have a surprisingly small home range over a period of several years. Indeed, given that (1) there were several flood events before and during the sampling period, (2) any prior Huffmanela infection remains grossly obvious in a fish for the rest of its life, and (3) the minimum time between a fish becoming infected and developing obvious signs of infection is 1 y, it then follows that any individual infected fish in the study had not moved up or down the stream more than a hundred meters or so in their entire life. Thus, Huffmanela could be used as permanent tags to indicate that a fish had at least once in its life been exposed to at least one Huffmanela-infected amphipod. Furthermore, since the eggs of many species of Huffmanela in a fish host have been shown by many authors to go through a predictable series of easily categorized changes as they age year after year, one could also determine approximately how long the fish had spent in a reach where it was exposed to infected amphipods.
One other noteworthy finding relative to the CIdER hypothesis is the uptick in the otherwise downstream decline in relative abundance of the SMS Hyalella at about river kilometer 1.8 (Figure 4a). At first we thought it was just sampling variability, but we noted that the same site where the uptick occurred was the only site sampled where we also collected any amphipods in the genus Crangonyx, which is hyporheic, but does not survive for long in surface water. Crangonyx amphipods are often collected near spring openings in Spring Lake, the impoundment over the SMS, but note that none were collected among the 452 amphipods collected at the other seven stations along the spring run, and yet 4 Crangonyx were collected among the 59 amphipods collected at this one station. Thus, the site at 1.8 km is probably receiving a local influx of cool hyporheic water from a seep from the rarely flowing Purgatory Creek that occasionally ejects Crangonyx amphipods, and that is corroborated by a leveling of the otherwise downstream-increasing slope in the standard deviation of temperature for a short reach starting at about km 2.0 (where the thalweg of Purgatory Creek joins the SMR). Indeed, the temperature spread over 14 y (Max-Min) upstream of the site is 12.9, 10.7 at the first station downstream from the site, and back up to 12.9 at the second site farther downstream. It is also very unlikely that Crangonyx would escape predation for more than a day after emerging into surface water, so there must be substantial hyporheic discharge from the Purgatory Creek channel to allow four to have been collected there in one grab sample. The uptick in SMS Hyalella there adds additional credibility to our conjecture that the general downstream decline in relative abundance of SMS Hyalella (Figure 4a) is due to some declining physicochemical attribute of spring water; but the question remains, “which attribute(s)?”
3.3.2. Downstream Increase in Temperature Variance Is Inversely (But Tightly) Correlated with Huffmanela huffmani Abundance Measures in Wild-Caught SMR Fish
We reviewed previous studies of patterns of downstream change in various physicochemical parameters in the SMR and found almost all of them were stable at the springhead but showed progressively increased diel, seasonal and precipitation-related variability with distance downstream [84,85,86]. However, water temperature, among all these parameters, seemed to exhibit the highest degree of contrast between low variability at the springhead relative to variability downstream, and therefore seems the most likely candidate to be the limiting factor preventing the crenobionts from surviving more than a few kilometers downstream. Changes in magnitudes of seasonal and diel variations in water temperature at various stations in the SMR over 14 y are demonstrated in Figure 5.
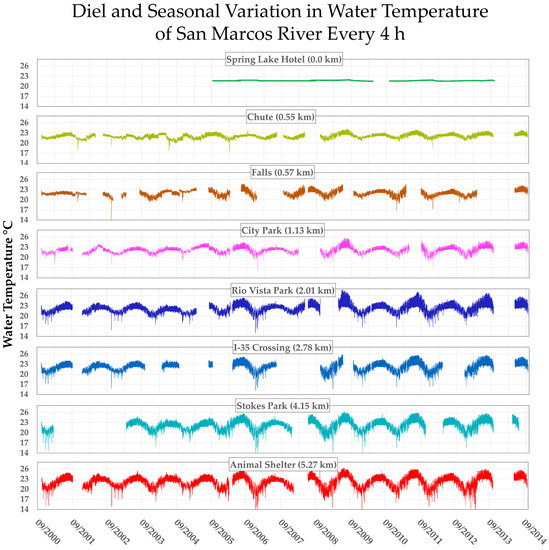
Figure 5.
Seasonal and diel variability of water temperature over 14 years recorded at various stations downstream from headspring (graciously shared by BIO-WEST, Inc.; gaps are memory-full errors, losses due to spates, or vandalism; transients are due to cold fronts and winter spates from flashy tributaries.
The discharge of the eucrenal zone of San Marcos Springs is currently almost entirely from groundwater; therefore, the resulting diel and seasonal variability of water temperature near the headsprings is biologically trivial (Figure 5); indeed, out of all the 15,252 temperature records at the spring head between 2006 and 2013, the difference between very highest temperature and very lowest temperature was 21.94 − 21.46 = 0.48 °C. It seems reasonable to assume that variation over a typical year during the geologic time frame under consideration would be similarly trivial, and that the invertebrate clades evolving in this headspring over many thousands of generations would not have been made sensitive to such minute temperature swings by their loss of thermal-shock recovery systems through genetic drift. One complication to this portrayal of springhead tranquility is that the current locality of the main springs supplying the SMS discharge can only be traced back by 14 ky of sediments, and prior to that, the spring head may have been at a site about 1.2 km NE (29.902652, −97.922809) on the other side of Sink Creek. However, the entire Sink Creek valley across the springs from the escarpment to about 0.5 km southeast was scoured to bedrock until deposition was re-started about 13 kya [87], so the record is uncertain as to when the SMS discharge first started at the current location.
While the water at the headsprings of SMS was found to be physicochemically stable year-round, the diel and seasonal variability increased, as expected, downstream. Interestingly, despite the local weather inducing major temperature swings and transients in downstream water, the annual means of water temperature at these stations varied only slightly from 22 °C at progressively more downstream stations, with the maximum difference between any two 14 y station means being 1 °C. However, the variance of the temperatures recorded at the station 2 km downstream during the same time interval (1.5595 C2) was 175 times larger than the variance at the headsprings (0.0089 C2) even though the annual means at the two stations differed by only 0.4 °C.
Downstream trends in prevalence and the mean egg-density ratings of H. huffmani at (Figure 4a) are almost exact inverses of the trend in temperature variation (as standard deviation) at nearby stations (Figure 4b). Note in Figure 5 that the diel variation in temperature (thickness of the trace) increases consistently with downstream distance until at about 2 km where it more-or-less stops increasing. The amplitude of seasonal variation also more or less stabilizes at near 2 km. Then, notice in Figure 4a that both prevalence and egg density also drop precipitously just downstream from that location.
This observation suggests that increasing amplitude of variations in water temperature (or something else covarying closely with it) at progressively more downstream stations is reducing the likelihood that centrarchids captured at those downstream stations will be infected.
We statistically compared the mean egg-density ratings and prevalences from five sampling sites at varying distances downstream (from Figure 3) to the combined seasonal and diel variation in temperature at seven nearby monitoring stations. Variation in temperature was expressed as the standard deviation of temperatures in degrees Celsius. Spearman’s Rho correlation procedure [88] was used to determine the likelihood that the apparent relationship between the prevalence of Huffmanela and the standard deviation of temperature could be explained by random variation, and the correlation was very highly significant with p(rs = |−1|)→0.
3.3.3. Downstream Depression of Huffmanela Abundance Probably Caused by Progressively Increasing Thermal Instability: Experimental Corroboration
Even though we detected significant correlations between declining downstream H. huffmani abundance measures and increasing downstream variation in temperature, such a correlation would not provide direct evidence of a cause–effect temperature-sensitivity mechanism, since there are many other water-quality factors in the SMR that also change predictably within the first few kilometers of a spring run [84,86]. Variation in water temperature can only be concluded to be a factor contributing to the endemism of H. huffmani if we can experimentally demonstrate some sort of sensitivity to naturally occurring off-mean water temperatures similar to those that occur near where the abundance of Huffmanela infections begins to decline (3–4 km; Figure 4a). Thus, we designed an experiment that might demonstrate temperature sensitivity in a parasitologically meaningful way.
We examined temperature data at a station just upstream from where the abundance of the parasite declined sharply and determined that the minimum and maximum water temperatures there that were sustained for at least 4 h at any time in the 14 y period of monitoring were approximately 15 °C and 28 °C. The monitoring period included the second most severe drought of record followed by return average temperature.
We designed an elaborate experiment to evaluate our hypothesis that the distribution of H. huffmani is primarily limited by temperature extremes. The experiment was designed to assess varying subsets of the temperature transients observed at the chosen site for varying durations to determine, by titration, the temperatures at which the eggs (1) began to be affected and (2) at which they were all killed. We predicted that temperature swings to one extreme or another for several hours will depress the ability of H. huffmani eggs to infect the intermediate host, Hyalella cf. azteca.
When we were in the final stages of implementing the experiment, our university announced an impending lockout of all labs due to COVID-19, which severely compromised how much of the original design we could complete in the two weeks remaining before the lockout. However, we decided to go forth with a simplified experiment, so that there might be some level of experimental findings pertaining to our hypothesized temperature sensitivity, albeit with threats to validity brought on by the compromises. The design of the simplified experiment is diagrammed in Figure 6.
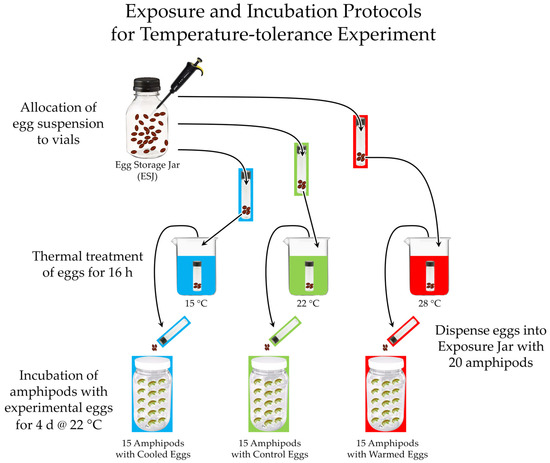
Figure 6.
Design of experiment to determine if temperature extremes recorded at the site where Huffmanela huffmani prevalence declined at the greatest rate can help explain the limited downstream distribution of the parasite in the San Marcos River.
The source of Huffmanela eggs for the experiment was a batch that had been pooled from several freshly caught SMR centrarchids and that had been validated for infectivity to amphipods. We gently stirred the eggs to suspend them and pipetted about equal aliquots (based on titrations by [45]) into each of three vials. We randomly assigned each vial into one of three experimental baths (15, 22, and 28 °C).
Then, we added to each bath a HOBO U-2 v2 thermal data logger programmed to record bath temperature every 60 s. At about 14:15 on 18 March 2020, we began ramping down the temperature of the 15-C bath over a period of 3.7 h to a mean treatment temperature of 14.6 (14.5–15.4) °C and held for 16 h before ramping the temperature back up to 22 °C. During the same period, we ramped up the temperature of the 18-C bath over a period of 2.5 h to a mean treatment temperature of 27.6 (27.4–28.0) °C, which was held for 16 h before ramping back down to 22 °C. Meanwhile, the 22 °C bath was held at a mean of 21.8 (21.7–21.9) °C (close to mean annual river temperature) for the same length of time. The temperature ramps were not smooth (because of the impending lockout, we resorted to controlling temperature in the cold-treatment bath by adding ice chips to the outer bath and tracking cooling progress real-time with a mercury thermometer) and the overall rates of change in temperature ramps between start and end of a ramp was between 1.8 and 2.4 °C/h (Figure 7).
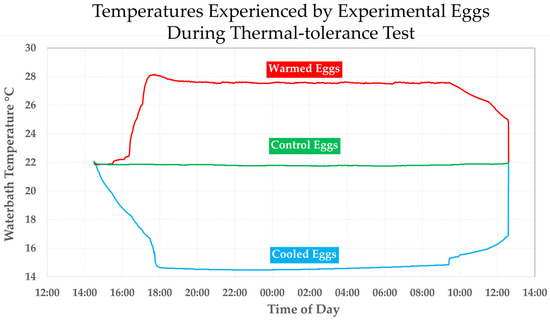
Figure 7.
Record of the water temperatures experienced by the three experimental groups of Huffmanela eggs prior to feeding eggs to experimental amphipods.
The ramp rate we employed is 10 to 20 times faster than the fastest rate we observed in naturally occurring thermal transients at the site and in experiments involving animals adapted to environments within ambient temperature swings; such ramp rates would be a severe threat to validity because they would swamp the heat-shock response system. However, many stenothermal animals have lost an inducible heat-shock response [89], and extended ramp times (more gradual slope) would probably not have resulted in reduced effects; indeed, other investigators have reported that extending the ramp time to targeted hold temperature (slowing the rate of change) while maintaining the same exposure duration at the targeted temperature often increases the number of stenothermal subjects responding negatively to the targeted temperature because the extended ramps also extend the exposure of the subjects to off-mean temperature (see [90,91]). In other words, if we had ramped temperatures more slowly, we would likely have seen stronger effects of reduced infectivity, even at less extreme targeted temperatures.
Prior to the end of the thermal treatment of the egg vials, we placed 15 lab-reared amphipods (Hyalella cf. azteca) into each of four 1-L jars, three of which we had labelled to match the three experimental groups, and the fourth was to serve as a Death-rate Control (in case exposed amphipods started dying before they were ready for dissection, we could determine if the deaths were caused by over-dosing the treated amphipods). Immediately after the thermal treatment of experimental eggs concluded, we transferred the eggs from the vials in each experimental group to their corresponding amphipod exposure jars. After the amphipods had been exposed to the eggs for 4 d, we rinsed the amphipods and incubated them for an additional 24 h to allow any eggs consumed on the fourth day of exposure to migrate to the hemocoel prior to dissection. We then coded the amphipod source containers, and had previously trained workers dissect all the amphipods live over a period of 6 h, with workers dissecting an amphipod from each of the four containers before repeating. Immediately after counting worm larvae in the experimental amphipods, we were locked out of the lab for several months.
The number of larval worms recovered from each amphipod was then used as a data point for that group and the three groups of data were analyzed with a one-way ANOVA with Tukey’s HSD test for multiple-comparisons. Our expectation was that the amphipods in either (or both) of the off-mean-temperature groups would show significantly diminished larval counts relative to those in the control group (22 °C).
Both treatment groups (15 °C and 28 °C) showed a reduced mean infectivity of eggs relative to that of the 22-C control group (Figure 8). A one-way ANOVA of the three groups was highly significant [p(F0.05(1),2,39 ≥ 9.50) < 0.001]. A Tukey’s HSD test for multiple comparisons was then applied, and infectivity of eggs for both the 15-C and 28-C treatment groups were significantly lower than that of the 22-C control group [p(q0.05,3,39 = 3.44) < 0.01] for both groups. However, the difference in infectivity effects of the 15 °C and 28 °C groups did not differ significantly from each other, indicating that both summer and winter temperature extremes may be operating together to restrict downstream colonization of the spring run. None of the amphipods in the Death-rate Control jar died before the experimental amphipods were dissected, suggesting that all amphipods that died in the exposed groups died of infection-related causes.
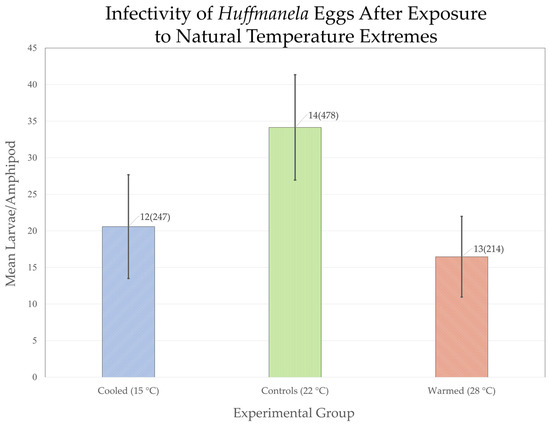
Figure 8.
Effects of natural temperature extremes (15 °C and 28 °C) for 16 h on the ability of thermally treated Huffmanela eggs to establish infections in exposed amphipods relative to controls (22 °C); value labels are: # surviving amphipods (recovered larvae); error bars are 95% confidence intervals.
3.4. Q4: Are There Corroborating Data Consistent with the Two Cenobiontic Host/Parasite Systems Having Been Separated for Thousands of Amphipod Generations?
3.4.1. River Flow Patterns
The South Concho and the San Saba Rivers both flow east for about 150–250 river kilometers where they are confluent with the much larger Colorado River, which drains the eastern and northern margins of the Edwards Plateau (Figure 1). In contrast, the Comal and San Marcos rivers are confluent with the Guadalupe River, which drains much of the southern edge of the Plateau. Therefore, the two Huffmanela populations in CCS and the SCR are not nearly as isolated from each other as they both are from the populations on the south side of the Edwards Plateau (SMS H. huffmani and the now extinct CS Huffmanela). Likewise for Hyalella texana of the CCS vs. the SMS Hyalella. Indeed, ever since probably well before the Pleistocene, the San Saba and South Concho river populations have been separated from the two southern populations (San Marcos and Comal rivers) by what currently amounts to over 1000 river kilometers and 100 km of saltwater bays, and by 230 air km with no known intervening Huffmanela or crenobiontic Hyalella populations (Figure 1).
3.4.2. COI Genetics of Crenobiontic Hyalellids
It is also worth noting that the CCS Hyalella texana is an ancient clade according to COI phylogeny, and much older than the more recent SMS Hyalella [44]. The Hyalella cf. azteca of the SMR is phylogenetically intermediate between the two crenobiontic forms.
Regarding the absence of a crenobiontic Hyalella in Comal Springs, it would be interesting to return to the museum collection of [5] and check out those centrarchids in which the now extinct Comal Springs clade of Huffmanela was discovered, and look to see if their stomachs contain evidence of a mucronate, crenobiontic Hyalella that went extinct along with its Huffmanela parasite during the drought of 1956.
3.4.3. Reproductive Isolation of the Crenobiontic Hyalellids
Assuming that the crenobiontic Hyalella population in the Clear Creek Springs and its Huffmanela parasite have been genetically isolated for thousands of amphipod generations from their respective counterparts in the San Marcos Springs, one would also assume that some prezygotic or postzygotic reproductive isolation to have occurred. It turns out that [44] had already demonstrated that size-matched adults of these two crenobiontic amphipod species (Hyalella texana from CCS and the SMS Hyalella) occasionally entered amplexus in symmetrical cross experiments, but never produced first generation offspring (F1) after 8 weeks together (neither ♂SMS × ♁CCS nor ♂CCS × ♁ SMS). Likewise with symmetrical crosses of either crenobiont with the Hyalella cf. azteca from the San Marcos River. However, conspecifics of all three clades readily mated and produced F1 in as little as 2 weeks under identical conditions.
3.5. Q5: Are There Corroborating Data Consistent with the Two Crenobiontic Hyalellids Having Coevolved with Their Local Huffmanela Parasites Prior to the Repatriation of Hyalella cf. azteca to Both Springs?
There are two aspects of this question that we were able to test: (i) an experimental comparison of the two crenobiontic Hyalella/Huffmanela host/parasite systems with each other, and (ii) a retrospective comparison of one crenobiontic Hyalella/Huffmanela system with its sympatric repatriated Hyalella/Huffmanela system in the same spring.
3.5.1. The Two Crenobiontic Host/Parasite Systems: Exposing Both Crenobiontic Hyalellids to Heavy Doses of Local vs. Exotic Huffmanela Eggs
While it would be useful to determine if adult Huffmanela worms of the SMS and CCS clades could cross successfully, such an experiment would require either isolating rare adult worms of separate sexes of both species in rete tissue culture, or learning how to reliably sex viable eggs without harming them, and would take years to complete, if even feasible. However, if the two Huffmanela clades in CCS and SMS have diverged genetically (as have their amphipod hosts; Section 3.4.3), then it would seem that an attempt to cross-infect the CCS hyalellid with Huffmanela eggs harvested from the SMS centrarchids (and the reciprocal cross infection), the cross-infections should exhibit detectable differences in infectivity or some other manifestation of host reaction compared to infections of each crenobiontic hyalellid with eggs from its home spring. Worsham et al. [45] had already demonstrated that eggs from the CCS and SMS Huffmanela clades would infect the crenobiontic amphipod from either spring, but what we needed to show was some kind of differences in the host/parasite dynamics that would be consistent with the two host/parasite systems having evolved separately over thousands of generations in separate CIdER refugia.
We hypothesized that in order for such host parasites systems to survive through many boom-and-bust cycles in such a tiny, isolated biotope, the host amphipod clade in each system must have evolved some way of managing unusually high challenges with Huffmanela eggs. For instance, if a disturbance kills a substantial number of infected fish hosts in a short period, the amphipods would soon be challenged with ingestion of potentially orders of magnitude more Huffmanela eggs than they normally had to deal with, unless they had developed an immunological defense against such occasional excesses of eggs. We anticipated that the amphipod clade that had coevolved in a spring with the local Huffmanela clade would be more resistant to being swamped by eggs of that Huffmanela clade than from an equal dose of eggs from an exotic Huffmanela clade.
With these factors in mind, we designed an experiment to determine if the crenobiontic hyalellids (CCS & SMS) would show detectable resistance to a near lethal challenge with eggs of the local Huffmanela with which they presumably coevolved, but would be relatively immunologically naïve to an approximately equal challenge with eggs from the exotic Huffmanela clade from the other spring. Fortunately, in the life-cycle work of [45], an approximate lethal level of Huffmanela huffmani eggs in the SMS Hyalella had already been titrated, and we decided to use that approximate abundance of eggs in the experiment.
A 2 × 2 experiment was set up consisting of four identical containers of amphipods: two containers with 20 each lab-reared crenobiontic CCS Hyalella, and the other two containers with 20 each lab-reared crenobiontic SMS Hyalella. Two containers of Huffmanela eggs were prepared: one harvested from fish naturally infected with the CCS Huffmanela clade and one harvested from fish naturally infected with the SMS Huffmanela clade. The CCS eggs were suspended by stirring and an aliquot of the suspension then split about equally, with half delivered to a container with 20 CCS amphipods and the other half to a container with 20 SMS amphipods. The procedure was then repeated with the other two containers of amphipods but with the SMS eggs (Table 4). It was not critical that the dose of CCS Huffmanela eggs be equal to the dose of SMS Huffmanela eggs, as long as both doses killed some amphipods and allowed others to survive. However, the dose of one egg source must be very close to the other dose from the same source, and great care was taken to split each source equally between the two recipient amphipod containers.

Table 4.
Setup of differential mortality experiment to test for evidence of long-term genetic isolation and coevolution of the SMS and CCS Hyalella/Huffmanela host/parasite systems. Values represent number of Hyalella amphipods from the side-indicated spring being challenged with a heavy dose of Huffmanela eggs from the top-indicated spring.
The four beakers of exposed amphipods were monitored over a 2-week exposure period to determine the mortality rates of amphipods in the four cells of the experiment. Significantly higher mortality rates in the two cross infections (CCS/SMS and SMS/CCS) would be interpreted as consistent with our assertion that the two host/parasite systems had diverged genetically over many generations of isolation in CIdER refugia.
The mortality rates of the two crenobiontic amphipods, when over-exposed to Huffmanela eggs from both springs, were almost exactly opposite (Figure 9). Significantly more native Hyalella from each spring survived exposure to eggs of local Huffmanela than to about equal doses of eggs of exotic Huffmanela. A chi-square contingency test with Yates correction for continuity (Table 5) returned a very highly significant result. These results show that both crenobiontic Hyalella clades are capable of withstanding massive exposure to eggs of the Huffmanela species with which they presumably coevolved, but not as well with eggs of the Huffmanela species to which they are naïve.
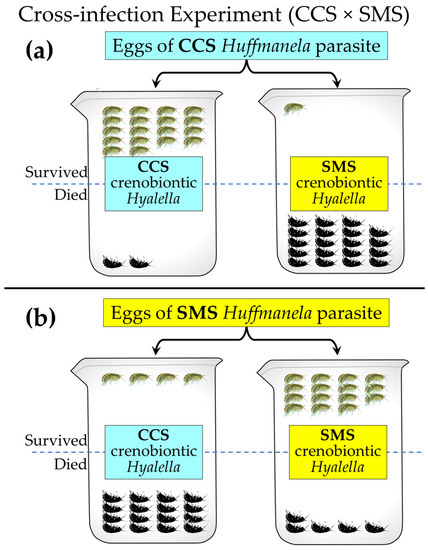
Figure 9.
Results of a 2 × 2 cross-infection experiment to determine differential mortality rates of the two crenobiontic Hyalella clades when over exposed to eggs of Huffmanela clades from the local vs. the exotic springs. Panel (a) is exposure of Hyalellla to CCS Huffmanela while panel (b) is exposure to SMS Huffmanela.

Table 5.
Chi-square contingency analysis of the data from the differential survivability experiment depicted in Figure 9.
3.5.2. Comparison of One Crenobiontic Amphipod/Huffmanela System with the Local Repatriated Amphipod/Huffmanela System: Differences in Chronic Reactions to the Parasite
This test was not a dedicated experiment but consisted of reviewing the records from many unpublished exposures of amphipods to Huffmanela eggs during the [45] life cycle experiments. Most of that data were derived from exposures of either of the two San Marcos Springs amphipods (SMS Hyalella or Hyalella cf. azteca) to eggs of H. huffmani from the SMS, and we focus our analysis thereon.
The review revealed that anytime individual SMS Hyalella had been exposed to eggs from local SMS Huffmanela, the amphipods rather quickly, starting at about 2 w post-exposure, had immobilized, or killed many of the Huffmanela larvae from the experimental exposure to local eggs. In contrast, when individuals from the other local amphipod clade (Hyalella cf. azteca) had been exposed to the same local eggs of SMS Huffmanela, the larvae resulting from the exposures had survived for over 6 w in the Hyalella cf. azteca. A related finding from the review was that when either of the mucronate crenobiontic hyalellids from either the CCS or SMS had survived experimental exposure to Huffmanela eggs from their local springs, subsequent observations of the surviving amphipods had revealed that the larval Huffmanela from local eggs had become calcified and yellowed after 2–3 w. In contrast, not a single Hyalella cf. azteca that had been exposed to Huffmanela eggs was observed with yellowed larvae, even >6 w post-exposure. These finding from the review (summarized in Table 6) are consistent with our hypothesis that the SMS Hyalella and its Huffmanela parasite had coevolved in isolation for probably thousands of amphipod generations in the absence of Hyalella cf. azteca, the latter having recolonized the San Marcos Springs since the Holocene droughts.

Table 6.
Differential reaction of the two SMS amphipod species to infection with eggs of local Huffmanela.
Another observation related to this test is the change in relative proportions of the two sympatric Hyalella clades at progressively more downstream stations from the SMS springhead (Figure 4a). This progressive downstream replacement of the crenobiontic amphipod, which had presumably survived the Holocene droughts in the spring, by the widespread amphipod, which had presumably reinvaded the springs after the droughts, is exactly what one would expect, and such patterns have been observed with other clades that were presumably brought back into contact after the Holocene droughts [8]. In such cases, the crenobionts would be so perfectly specialized for competition in the crenobiontic biocenosis of the springhead that the repatriated congener could not eliminate them there, but the crenobiont would lose this competitive advantage as it encountered more and more species downstream that have evolved to be competitive under more widely varying conditions and also cyclical variations in community structure on a seasonal basis.
4. Conclusions
4.1. Paleoecological Inference
How aquatic taxa evolve to become crenobiontic has yet to be directly tested but we hypothesize that, because Edwards Aquifer-fed springs are well known to experience little to no daily and seasonal variation in physicochemical conditions, the evolution of crenobionts is the result of extended periods of evolution in extremely stable spring refugia. Paleoclimatological evidence suggests that two Holocene thermal maxima occurred prior to the present warming trend and these thermal maxima coincided with severe droughts. The finding that the drought of the present warming trend extirpated spring endemic fountain darters and Huffmanela from Comal Springs in the 1950s [45,72] clearly demonstrates that drought conditions have the capacity to sculpt the distribution of crenobiontic taxa. It should be noted that during the drought of the 1950s Comal Springs ceased flowing in fall of 1955 and early winter of 1956 but still had small patches of standing surface water that probably tracked ambient temperatures. Thermal tolerance studies suggest that ambient temperature swings are not survivable by Huffmanela eggs (Figure 8) which reinforces the notion that drought and thermal variability have sculpted the distribution of crenobionts; this finding is reinforced by the correlation between the contemporary biogeography of Huffmanela and temperature in the San Marcos River. The finding of a second endemic Huffmanela population in centrarchids from a spring it shares with the only other known crenobiontic Hyalella in Texas is consistent with our conjecture that the CIdER refugium hypothesis is a reasonable explanation for the present distribution of many endemic crenobiont clades in Central Texas; this finding is reinforced by evidence of coevolution between cooccurring Huffmanela and Hyalella. With this in mind, this study suggests that parasites with at least two distinct obligate hosts are far more useful for palaeoecological inference than most free-living taxa because the contemporary distribution of such a parasite indicates that the environmental and ecological conditions necessary to support all host species, as well as meeting the environmental and ecological requirements of the parasite itself, were met without interruption since the arrival of the parasite to that geographic region. The coupled coevolution of parasites and their obligate hosts provides rich insight into palaeoecological conditions; especially aquatic parasites with multiple hosts required to complete their life cycle. To our knowledge, this is only the third study to use biogeographic data on parasites with multiple hosts to inform palaeoecological reconstructions [67,68] and we suggest applying this strategy can prove insightful in other model systems.
4.2. A Proposed New Subcategory for Evolutionary Refugia
The term “Crenal Interglacial-drought Evolutionary Refugia” (CIdER) is proposed as a label under which research data pertinent to this special category of refugia can be kept separate from data pertaining to terrestrial glacial and interglacial evolutionary refugia, the latter two being driven by completely different forces, even while developing in the same region at the same time. Extant CIdER are characterized by the following generalized historical narrative:
- The starting point is a diverse community of obligately aquatic plants and mostly invertebrate animals that are widely distributed among interconnected streams during a period of high regional precipitation associated with extensive glaciation just poleward of the region.
- The community becomes challenged by region-wide gradually reduced precipitation as the glacial margins retreat poleward.
- Evapotranspiration begins to exceed precipitation and many streams and springs that were formerly perennial become progressively more intermittent, and the distribution of the obligate aquatic community becomes progressively more fragmented.
- Many smaller springs stop flowing and lotic habitats become restricted to isolated flows fed by a few perennially rheocrenic springs, and perennial high-order rivers draining remote regions.
- The largest rheocrenic springs have spring runs extending from the springhead but disappear into ground and air, never connecting to other surface waters; others shrink back to limnocrenes in which the water remains thermally constant a meter or so deep where there is movement of groundwater.
- This condition is maintained for thousands of generations of the obligate aquatic invertebrates, which are now members of an isolated biocenosis continually bathed in a thermally constant environment with a stable bottom undisturbed by spates.
- The surviving clades eventually lose (to drift or reassignment) genetic loci for heat shock proteins that once allowed the organisms to adapt to varying temperatures, but also become extremely efficient competitors under these rigidly constrained physicochemical conditions.
- The drought breaks, and dependable precipitation in excess of evapotranspiration returns to the region.
- Spring-streams lengthen, eventually forming permanent connections with surface-fed streams, but crenobionts that survived through the drought in the springs can no longer survive through the temperature swings of progressively more ambient waters downstream.
- Sister clades that are relatives of the crenobionts, but which had retreated to other regions with dependable precipitation during the drought, now return and intermingle with the crenobionts.
- If the crenobionts are now reproductively isolated from the repatriated sister clades, a longitudinal density gradient is established, with the repatriated sister clades diminishing in density toward the spring head, and the crenobiontic clades diminishing downstream with physicochemical instability.
- The obligate aquatic community at the springhead now consists of a highly diverse mixture of surviving, but still endemic crenobionts and repatriated sister clades.
Author Contributions
Conceptualization: A.B., D.G.H., J.R.G. and M.L.D.W.; methodology: A.B., D.G.H. and M.L.D.W.; software: A.B., D.G.H. and M.L.D.W.; validation, D.G.H. and M.L.D.W.; formal analysis, A.B., D.G.H. and M.L.D.W.; investigation A.B. and M.L.D.W.; resources: D.G.H.; data curation, A.B., D.G.H. and M.L.D.W.; writing—original draft preparation: A.B., D.G.H. and M.L.D.W.; writing—review and editing: A.B., D.G.H., J.R.G. and M.L.D.W.; visualization, D.G.H. and M.L.D.W.; supervision, D.G.H. and J.R.G.; project administration: D.G.H. and J.R.G.; funding acquisition, n/a. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This research was conducted using procedures approved by the Texas State University Institutional Animal Care and Use Committee (IACUC) under permits #73 (Bond) and #0519_0620_11 (Worsham). Fish were collected under Texas Parks and Wildlife Department Diversity Permit #SPR-0913-124 (Huffman).
Acknowledgments
We would like to acknowledge Harlan Nicols, Stephen Harding, Helen McKennon, Alex Zalmat, and Sungyoung Kim for invaluable help in the field and/or the lab, and Randy Gibson for his invaluable help with the taxonomy and ecology of aquatic invertebrates. We would also like to thank Mackenzie Barnett for providing access to the sampling location at Clear Creek Springs; Bio-West Inc. for providing us with their water temperature databases; the USFWS for support in conducting this research and The Meadows Center for Water and the Environment for providing access to Spring Lake and boat/diving support. Generous research-oriented access to Anson Spring, a private property on Head of the River Ranch near Christoval, TX, USA was graciously provided by Ryland Howard. The views expressed herein are those of the authors and do not necessarily represent those of the United States Fish and Wildlife Service. This study is dedicated to the late Eric Julius and Emmett Worsham who both devoted their young lives to the study, conservation, and husbandry of rare and imperiled aquatic and marine organisms.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Review of Terminology Related to Refugia, Relicts, and Endemics
This section addresses a confusing array of ambiguous terms that we had no intention of dealing with, but which will continue to unnecessarily encumber the communication and integration of related findings unless some attempt is made to clear the terminological fog.
Appendix A.1. Terms Related to Endemics
Much of the recent attention to the concept of endemism has been from the perspective of how best to focus limited mitigation resources for maximum conservation impact. Conservationists have developed algorithms that can purportedly identify sensitive geographic hotspots of endemism that are contributing substantially to global diversity and then triage them for prioritization in preparation for anticipated rapid climate change. The CANAPE method for the Categorical Analysis of Neo- and Paleo-endemism is claimed by Mishler et al. [92] to allow, “… for the first time, a clear, quantitative distinction between centres of neo- and paleo-endemism…” Given the urgency of biodiversity conservation concerns and the need to prioritize regions of endemism for the establishment of reserves, this appears to be a reasonable way to go about parsing the growing body of ecological and taxonomic data available, and then prioritizing resource allocation. However, some authorities have concluded that the protocol for classifying regions by these terms (neo-vs. paleo-endemism) introduces investigator bias into the decision-making process [56] because the investigator must impose artificial boundaries on the limits of time and space under consideration. Another complication is that the clades we are studying would transition from one category to the other over the time interval we are considering for our narrative. Fattorini [93] provides a detailed history of the endemism concept and some of the problems associated with the varied uses of paleo- and neoendemism while Myers et al. [56] proposed a detailed classification of different applications of endemism. However, we finally chose to avoid the use of these modifiers and will simply refer to our clades as endemics sensu Darwin’s glossary entry [94], “peculiar to a given locality,” with the qualifier “peculiar” distinguishing our use of the term from the way it is sometimes used to imply “indigenous to” or “native to.” Thus, we will avoid the prefixes “Neo” and “Paleo-.” Furthermore, we will only characterize an ecosystem as being the habitat of an endemic if it is the smallest geographic area to which at least two endemic clades are restricted, following criteria established by Platnick et al. [95] and Harold et al. [57].
Appendix A.2. Terms Related to Relicts
Some of the endemic clades in our study have been loosely referred to as relicts; but, unfortunately, the jaded application history, and the almost sensational intrigue sometimes associated with the term “relict,” have reduced the utility of the term to that of a colloquialism. Grandcolas et al. [96] attempted to break out the functionally different historical applications of the term and apply unique names to them, but did not provide for a term that would apply to a geographically restricted population of “left-behind” survivors of a formerly widespread but climatically displaced and still extant sister group which is later repatriated; a situation we appear to be dealing with in our study (along with many other researchers dealing with glacial “relicts”). Since almost any definition of any kind of relict will technically require that the relict population represents the sole survivors of a formerly widespread (but now extinct) source population, we have avoided using the term “relict” for the endemic clades we are studying.
Appendix A.3. Terms Related to “Refugia”
Appendix A.3.1. Misapplications of “Refug-”
Frequencies of words derived from ‘refuge’ and ‘refugium’ have seen dramatic increases in life-science literature in recent years. Keppel et al. [97] searched for literature containing references to keywords ‘refugia,’ ‘refugium,’ and ‘refuge’ in the title, abstract, or keywords and published between 1991 and 2010. They noted that the number of papers using the terms derived from “refuge” increased by about 5-fold in that time interval, but papers using latinized terms derived from “refugium” increased by about 13-fold. Unfortunately, these terms have been increasingly applied indiscriminately to a variety of scenarios having presumptive origins that are functionally unrelated [53,54,98,99]. Indeed, we know of cases where the term “refugia” has been conflated with many other terms, including refuges, remnants, and reserves [97]; captive or artificial cultures maintained by humans [100]; an anthelmintic treatment strategy wherein a portion of stock in a pasture had been treated with anthelmintics and then all stock rotated out of that pasture (which becomes “the refugia” pasture) [101]; diurnal retreats for nocturnal species (and v.v.); and is sometimes even used as the plural form of refuge, i.e., using “refugia” where one would expect to see “refuges” [102]. Clearly, a literature search for findings pertaining to a specific functional type of refugium will inevitably return hits bloated with ambiguity.
Appendix A.3.2. Problems with “Evolutionary Refugia”
The modifier “evolutionary” has been applied to refugia since 1970 and perhaps before. Davis et al. [60] attempted to refine the application of the term in arid regions as, “permanent, groundwater-dependent habitats (subterranean aquifers and springs) supporting vicariant relicts and short-range endemics.” However, they unnecessarily (in our opinion) included the term “vicariant” in the definition, thus technically excluding its application to climate-change endemics, and ignored the applications of the same term to terrestrial habitats. Davis et al. [60] also defined the minimum tenure of the refugia in chronological units (millennia) rather than potential number of life-cycles completed during the tenure, despite the fact that a given chronological time span will impose profoundly different effects on clades that typically produce four generations per year vs. those that require a minimum of 4 years per generation. Despite these reservations, we consider the modifier “evolutionary” to be useful (less the constraints referenced above) in that it implies an isolation experience sufficient to have an evolutionarily persistent effect on the clade(s) of interest.
Appendix A.3.3. “Refugia” with “Glacial” Modifiers
The term refugia has often been used with Pleistocene modifiers in the past several decades to refer specifically to “glacial refugia,” in which the distribution of a formerly widespread temperate clade is subsequently compressed, by the colder temperatures of an approaching glaciation, into geographically much more limited patches or margins where conditions are milder, and from which the species ultimately disperses to widespread occurrence again following glacial retreat [54]. Later, the inverse term “interglacial refugia” was used to refer to limited areas into which the distribution of a once widespread boreal species that thrives in low temperature conditions (e.g., Dryas integrifolia) is subsequently compressed into north-facing alpine patches or retreating glacial margins by increasingly warmer interglacial conditions, only to return to abundance during the next glacial advance [103]. However, both refugial types are driven by changing thermal patterns, and the terms, as they have been defined, should not be used to refer to glacial or interglacial refugia driven by regional-scale variation in other factors such as meteoric moisture, even though the factors usually covary with temperature. Indeed, conservation plans designed for a refugium formed by changing thermal regimes require dramatically different approaches compared to management plans for refugia formed by changing precipitation patterns. Thus, the term “interglacial refugium” alone does not provide sufficient specificity to distinguish between scenarios representing dramatically different functional models of refugia.
Appendix A.3.4. Recent Attempts to Rein in Refugial Misuses
Recent papers have attempted, with marginal success, to rein in how these terms are used by defining boundaries for them (e.g., “What do we mean by ‘refugia’?” [54]. Ashcroft [53], in an extensive review of the application and misapplication of terms derived from refugium, indicated that they are “used without distinguishing between macrorefugia and microrefugia, ex situ refugia and in situ refugia, glacial and interglacial refugia or refugia based on habitat stability and refugia based on climatic stability.” This is a sad state of affairs for a discipline determined to sort out, clarify, and prioritize the issues related to diversity conservation. Ashcroft’s [53] recommendation to rectify the situation was, “More care needs to be taken to properly define the context when referring to refugia … so that the validity of methods and the conservation significance of refugia can be assessed.”
Appendix A.3.5. The Need New Refugial Categories
There are functionally different subcategories of refugia that have been identified by researchers but which are not adequately distinguished by any of the frequently used terms. For instance, glacial refugia and interglacial refugia are both cyclical in nature, with a few major and many minor cycles involving the mass displacement of entire communities, often leaving behind remnants in small climatological alcoves. This is followed by the return of the main sister population which then either swamps the left-behind endemics, or sympatrically contends with them, (these outcomes varying from one refugium or cycle to another). Yet, there is no specific term that we can find to represent the cyclically precipitation-restricted endemics in our study and their kilo-generational crenal (freshwater spring) refugia. Indeed, most related terms we can find refer to refuges that either originated from one-time dispersal driven processes or as one-time vicariance-driven processes. Furthermore, these terms have been hijacked by workers studying the cyclical glacial/interglacial processes in which cyclical trends in either precipitation or temperature (or both) are the primary displacing stimuli. Some investigators have attempted to mitigate this problem by declaring that climate change is a form of vicariance, but that just muddies the literary water, because landform vicariance by tectonic disjunction is usually on a very long time scale and cannot be merged with climate-induced cyclical displacement/return scenarios that involve very different implications for population dynamics, perturbation resilience, and habitat management plans.
Appendix A.4. Consequences of Terminological Ambiguity and Recommended Solutions
Continued ambiguity in the use of these short-term safe-haven and long-term refugial terminologies complicates literature searches and confounds attempts to advance understanding of the evolutionary pressures experienced by occupants of functionally distinct types of refugia. This, in turn, complicates ongoing attempts to prioritize application of limited resources set aside for conservation of biodiversity and can result in application of ineffective or even counter-productive management strategies.
We consider terminological ambiguity to be a substantial cause of the confusion that has historically confounded attempts to develop effective management strategies for protecting the biodiversity of widely varying types of refugia, especially those associated with delicate spring ecosystems. This is a functional, rather than merely a semantic ivory-tower problem, because properly established and well-thought-out categorical terms can serve as virtual repositories into which pertinent descriptive and cause–effect information can be accumulated and efficiently retrieved with less confusion and conflation.
Future work in the arena of evolutionary refugia should be required to either (1) use an established and unambiguous compound term with a representative abbreviation and use explicit language to describe the reasons why the term applies to the situation, or else (2) develop a new compound term with a recommended abbreviation and describe in explicit language how subsequent workers can know when they are dealing with a case that comes under the new refugial type, and also describe how the refugial type being named differs from established unambiguous refugial terms in use.
References
- Wallace, A.R. The Malay Archipelago; The Land of the Orang-Utan and the Bird of Paradise; A Narrative of Travel with Studies of Man and Nature; Macmillan & Co.: London, UK, 1869; Volumes 1 and 2. [Google Scholar]
- Huxley, T.H. On the classification and distribution of the Alectoromorphae and Heteromorphae. Proc. Zool. Soc. Lond. 1868, 1868, 294–319. [Google Scholar]
- Cracraft, J. Historical biogeography and earth history: Perspectives for a future synthesis. Ann. Mo. Bot. Gard. 1975, 62, 227–250. [Google Scholar] [CrossRef]
- Cantonati, M.; Füreder, L.; Gerecke, R.; Jüttner, I.; Eileen, J.C. Crenic habitats, hotspots for freshwater biodiversity conservation: Toward an understanding of their ecology. Freshw. Sci. 2012, 31, 463–480. [Google Scholar] [CrossRef]
- Hubbs, C.; Kuehne, R.A.; Ball, J.C. The fishes of the upper Guadalupe River, Texas. Tex. J. Sci. 1953, 5, 216–244. [Google Scholar]
- George, P.G.; Mace, R.E.; Petrossian, R. Aquifers of Texas; Report 380; Texas Water Development Board: Austin, TX, USA, 2011; p. viii+172.
- Jones, I.C. The northern segment of the Edwards (Balcones Fault Zone) Aquifer. In The Edwards Aquifer: The Past, Present, and Future of a Vital Water Resource; Sharp, J.M., Jr., Green, R.T., Schindel, G.M., Eds.; The Geological Society of America: Boulder, CO, USA, 2019; Volume mwr215, pp. 119–130. [Google Scholar]
- Craig, C.A.; Kollaus, K.A.; Behen, K.P.K.; Bonner, T.H. Relationships among spring flow, habitats, and fishes within evolutionary refugia of the Edwards Plateau. Ecosphere 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Gibson, J.R.; Harden, S.J.; Fries, J.N. Survey and distribution of invertebrates from selected springs of the Edwards Aquifer in Comal and Hays Counties, Texas. Southwest. Nat. 2008, 53, 74–84. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T.; Schneider, K. Vicariance, dispersal and scale in the aquatic subterranean fauna of karst regions. Freshw. Biol. 2009, 54, 918–929. [Google Scholar] [CrossRef]
- Kauffman, E.G. Paleobiogeography and evolutionary response dynamic in the Cretaceous Western Interior Seaway of North America. In Jurassic-Cretaceous Biochronology and Paleogeography of North America; Westermann, G.E., Ed.; Geological Association of Canada: St. John’s, NL, Canada, 1984; Special Paper 27; Volume 27, pp. 273–306. [Google Scholar]
- Schindel, G.M. Genesis of the Edwards (Balcones Fault Zone) Aquifer. In The Edwards Aquifer: The Past, Present, and Future of a Vital Water Resource; The Geological Society of America: Boulder, CO, USA, 2019; pp. 9–18. [Google Scholar]
- Laubach, S.E.; Jackson, M.L.W. Origin of arches in the northwestern Gulf of Mexico basin. Geology 1990, 18, 595–598. [Google Scholar] [CrossRef]
- Barker, R.A.; Bush, P.W.; Baker, E.T. Geologic History and Hydrogeologic Setting of the Edwards-Trinity Aquifer System, West-Central Texas; US Department of the Interior, US Geological Survey: Reston, VA, USA, 1994.
- Woodruff, C.M., Jr.; Abbott, P.L. Drainage-basin evolution and aquifer development in a karstic limestone terrain South-Central Texas, USA. Earth Surf. Process. Landf. 1979, 4, 319–334. [Google Scholar] [CrossRef]
- White, K.; Davidson, G.R.; Paquin, P. Hydrologic evolution of the Edwards Aquifer recharge zone (Balcones fault zone) as recorded in the DNA of eyeless Cicurina cave spiders, south-central Texas. Geology 2009, 37, 339–342. [Google Scholar] [CrossRef]
- Collins, E.W. Geologic Map of the New Braunfels, Texas, 30 × 60 Minute Quadrangle, Texas: Geologic Framework of an Urban-Growth Corridor along the Edwards Aquifer, South-Central Texas; Miscellaneous Map #39; The University of Texas at Austin: Austin, TX, USA, 2000. [Google Scholar]
- Hart, M.B.; Yancey, T.E.; Leighton, A.D.; Miller, B.; Liu, C.; Smart, C.W.; Twitchett, R.J. The Cretaceous-Paleogene boundary on the Brazos River, Texas: New stratigraphic sections and revised interpretations. GCAGS J. 2012, 1, 69–80. [Google Scholar]
- Yancey, T.E. Depositional trends in siliciclastic deposits of the Stone City transgressive systems tract, middle Eocene, Texas. Trans.-Gulf Coast Assoc. Geol. Soc. 1995, 45, 581–586. [Google Scholar]
- Haq, B.U.; Hardenbol, J.; Vail, P.R. Chronology of fluctuating sea levels since the Triassic. Science 1987, 235, 1156–1167. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Miller, K.G.; Fairbanks, R.G.; Mountain, G.S. Tertiary oxygen isotope synthesis, sea level history, and continental margin erosion. Paleoceanography 1987, 2, 1–19. [Google Scholar] [CrossRef]
- Kominz, M.A.; Browning, J.V.; Miller, K.G.; Sugarman, P.J.; Mizintseva, S.; Scotese, C.R. Late Cretaceous to Miocene sea-level estimates from the New Jersey and Delaware coastal plain coreholes: An error analysis. Basin Res. 2008, 20, 211–226. [Google Scholar] [CrossRef]
- Kominz, M.A.; Miller, K.G.; Browning, J.V. Long-term and short-term global Cenozoic sea-level estimates. Geology 1998, 26, 311–314. [Google Scholar] [CrossRef]
- Cooke, M.J.; Stern, L.A.; Banner, J.L.; Mack, L.E.; Stafford Jr, T.W.; Toomey III, R.S. Precise timing and rate of massive late Quaternary soil denudation. Geology 2003, 31, 853–856. [Google Scholar] [CrossRef]
- Toomey III, R.S.; Blum, M.D.; Valastro Jr, S. Late Quaternary climates and environments of the Edwards Plateau, Texas. Glob. Planet. Change 1993, 7, 299–320. [Google Scholar] [CrossRef]
- Blum, M.D.; Valastro, S. Response of the Pedernales River of central Texas to late Holocene climatic change. Ann. Assoc. Am. Geogr. 1989, 79, 435–456. [Google Scholar] [CrossRef]
- Nordt, L.C. Archaeological Geology of the Fort Hood Military Reservation, Fort Hood, Texas; 25; Texas A&M Univ College Station Archeological Research Lab: College Station, TX, USA, 1992. [Google Scholar]
- Nickels, D.L.; Bousman, C.B. Texas River Center Archaeology, Test Excavations at 41HY160, Hays County, Texas; Texas State University: San Marcos, TX, USA, 2010. [Google Scholar]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.I.; Banner, J.L.; Musgrove, M. Holocene climate variability in Texas, USA: An integration of existing paleoclimate data and modeling with a new, high-resolution speleothem record. Quat. Sci. Rev. 2015, 127, 155–173. [Google Scholar] [CrossRef]
- Booth, R.K.; Jackson, S.T.; Forman, S.L.; Kutzbach, J.E.; Bettis, E.A.; Kreigs, J.; Wright, D.K. A severe centennial-scale drought in midcontinental North America 4200 years ago and apparent global linkages. Holocene 2005, 15, 321–328. [Google Scholar] [CrossRef]
- Asmerom, Y.; Polyak, V.; Burns, S.; Rassmussen, J. Solar forcing of Holocene climate: New insights from a speleothem record, southwestern United States. Geology 2007, 35, 1–4. [Google Scholar] [CrossRef]
- Anderson, R.Y.; Allen, B.D.; Menking, K.M. Geomorphic expression of abrupt climate change in southwestern North America at the glacial termination. Quat. Res. 2002, 57, 371–381. [Google Scholar] [CrossRef]
- Mensing, S.A.; Benson, L.V.; Kashgarian, M.; Lund, S. A Holocene pollen record of persistent droughts from Pyramid Lake, Nevada, USA. Quat. Res. 2004, 62, 29–38. [Google Scholar] [CrossRef]
- Russ, J.; Loyd, D.H.; Boutton, T.W. A paleoclimate reconstruction for southwestern Texas using oxalate residue from lichen as a paleoclimate proxy. Quat. Int. 2000, 67, 29–36. [Google Scholar] [CrossRef]
- Hall, S.A.; Penner, W.L. Stable carbon isotopes, C3–C4 vegetation, and 12,800 years of climate change in central New Mexico, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 369, 272–281. [Google Scholar] [CrossRef]
- Ellwood, B.B.; Gose, W.A. Heinrich H1 and 8200 yr B.P. climate events recorded in Hall’s Cave, Texas. Geology 2006, 34, 753–756. [Google Scholar] [CrossRef]
- Smith, G.I.; Street-Perrott, F.A. Pluvial lakes of the western United States. Late-Quat. Environ. U. S. 1983, 1, 190–212. [Google Scholar]
- Sylvia, D.A.; Galloway, W.E. Morphology and stratigraphy of the late Quaternary lower Brazos valley: Implications for paleo-climate, discharge and sediment delivery. Sediment. Geol. 2006, 190, 159–175. [Google Scholar] [CrossRef]
- Musgrove, M.; Banner, J.L.; Mack, L.E.; Combs, D.M.; James, E.W.; Cheng, H.; Edwards, R.L. Geochronology of late Pleistocene to Holocene speleothems from central Texas: Implications for regional paleoclimate. GSA Bull. 2001, 113, 1532–1543. [Google Scholar] [CrossRef]
- Holsinger, J.R.; Longley, G. The Subterranean Amphipod Crustacean Fauna of an Artesian Well in Texas; Smithsonian Institution Press: Washington, DC, USA, 1980; Volume 308, p. 62. [Google Scholar]
- Hutchins, B.T. The conservation status of Texas groundwater invertebrates. Biodivers. Conserv. 2018, 27, 475–501. [Google Scholar] [CrossRef]
- Worsham, M.L.D.; Julius, E.P.; Nice, C.C.; Diaz, P.H.; Huffman, D.G. Geographic isolation facilitates the evolution of reproductive isolation and morphological divergence. Ecol. Evol. 2017, 7, 10278–10288. [Google Scholar] [CrossRef] [PubMed]
- Worsham, M.L.D.; Huffman, D.G.; Moravec, F.; Gibson, J.R. The life cycle of Huffmanela huffmani Moravec, 1987 (Nematoda: Trichosomoididae), an endemic marine-relict parasite of Centrarchidae from a Central Texas spring. Folia Parasitol. 2016, 63, 1. [Google Scholar] [CrossRef] [PubMed]
- Moravec, F. Revision of Capillariid Nematodes (Subfamily Capillariinae) Parasitic in Fishes. Studie ČSAV No. 3; Academia: Praha, Czech Republic, 1987; p. 144. [Google Scholar]
- Cox, M.K. The Distribution and Life Cycle of Huffmanela huffmani (Nematoda: Trichosomoididae). Master’s Thesis, Southwest Texas State University, San Marcos, TX, USA, 1998. [Google Scholar]
- Worsham, M.L.D. Huffmanela huffmani: Life Cycle, Natural History, and Biogeography. Master’s Thesis, Texas State University-San Marcos, San Marcos, TX, USA, 2015. [Google Scholar]
- Bond, A.T. Investigations into Huffmanela (Nematoda: Trichosomoididae): New Populations, Life Cycles, and Eggshell Ultrastructure. Master’s Thesis, Texas State University, San Marcos, TX, USA, 2020. [Google Scholar]
- Stevenson, M.M.; Peden, A.E. Description and ecology of Hyalella texana n. sp.(Crustacea: Amphipoda) from the Edwards Plateau of Texas. Am. Midl. Nat. 1973, 89, 426–436. [Google Scholar] [CrossRef]
- Bullard, S.A.; Moravec, F.; Ksepka, S.P.; Warren, M.B.; Dutton, H.R.; Huffman, D.G.; Yanong, R.P.E. Huffmanela cf. huffmani (Nematoda: Trichosomoididae) infecting swim bladder, peritoneum, and gonad of variable platyfish, Xiphophorus variatus (Cyprinodontiformes: Poeciliidae) and eastern mosquitofish, Gambusia holbrooki (Poeciliidae) in Florida; taxonomy, phylogenetic analysis, and pathological changes. Parasitol. Res. 2022; in press. [Google Scholar]
- Longley, G. The subterranean aquatic ecosystem of the Balcones Fault Zone Edwards Aquifer in Texas-threats from overpumping. In Proceedings of the First International Conference on Ground Water Ecology, Tampa, FL, USA, 26–27 April 1992; pp. 26–29. [Google Scholar]
- Ashcroft, M.B. Identifying refugia from climate change. J. Biogeogr. 2010, 37, 1407–1413. [Google Scholar] [CrossRef]
- Bennett, K.; Provan, J. What do we mean by ‘refugia’? Quat. Sci. Rev. 2008, 27, 2449–2455. [Google Scholar] [CrossRef]
- Cantonati, M.; Fensham, R.J.; Stevens, L.E.; Gerecke, R.; Glazier, D.S.; Goldscheider, N.; Knight, R.L.; Richardson, J.S.; Springer, A.E.; Tockner, K. Urgent plea for global protection of springs. Conserv. Biol. 2020, 35, 378–382. [Google Scholar] [CrossRef]
- Myers, A.A.; De Grave, S. Endemism: Origins and implications. Vie Et Milieu 2000, 50, 195–204. [Google Scholar]
- Harold, A.S.; Mooi, R.D. Areas of endemism: Definition and recognition criteria. Syst. Biol. 1994, 43, 261–266. [Google Scholar] [CrossRef]
- Heenan, P.B.; Millar, T.R.; Smissen, R.D.; McGlone, M.S.; Wilton, A.D. Phylogenetic measures of neo-and palaeo-endemism in the indigenous vascular flora of the New Zealand archipelago. Aust. Syst. Bot. 2017, 30, 124–133. [Google Scholar] [CrossRef]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Davis, J.; Pavlova, A.; Thompson, R.; Sunnucks, P. Evolutionary refugia and ecological refuges: Key concepts for conserving Australian arid zone freshwater biodiversity under climate change. Glob. Change Biol. 2013, 19, 1970–1984. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.; Hochachka, P.W. Functional significance of isoenzymes in thermal acclimatization. Acetylcholinesterase from trout brain . Biochem. J. 1970, 116, 883–887. [Google Scholar] [PubMed]
- Somero, G.N. Thermal physiology and vertical zonation of intertidal animals: Optima, limits, and costs of living. Integr. Comp. Biol. 2002, 42, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Tellier, F.; Tapia, J.; Faugeron, S.; Destombe, C.; Valero, M. The Lessonia nigrescens species complex (Laminariales, Phaeophyceae) shows strict parapatry and complete reproductive isolation in a secondary contact zone. J. Phycol. 2011, 47, 894–903. [Google Scholar] [CrossRef]
- Adams, N.E.; Inoue, K.; Seidel, R.A.; Lang, B.K.; Berg, D.J. Isolation drives increased diversification rates in freshwater amphipods. Mol. Phylogenetics Evol. 2018, 127, 746–757. [Google Scholar] [CrossRef]
- Krejca, J.K. Stygobite Phylogenetics as a tool for Determining Aquifer Evolution. Doctoral Dissertation, University of Texas, Austin, TX, USA, 2005. [Google Scholar]
- Abbott, P.L. On the hydrology of the Edwards Limestone, south-central Texas. J. Hydrol. 1975, 24, 251–269. [Google Scholar] [CrossRef]
- Mejía-Madrid, H.H.; Vázquez-Domínguez, E.; Pérez-Ponce de León, G. Phylogeography and freshwater basins in central Mexico: Recent history as revealed by the fish parasite Rhabdochona lichtenfelsi (Nematoda). J. Biogeogr. 2007, 34, 787–801. [Google Scholar] [CrossRef]
- Martínez-Aquino, A.; Ceccarelli, F.S.; Eguiarte, L.E.; Vázquez-Domínguez, E.; de León, G.P.-P. Do the historical biogeography and evolutionary history of the digenean Margotrema spp. across central Mexico mirror those of their freshwater fish hosts (Goodeinae)? PLoS ONE 2014, 9, e101700. [Google Scholar]
- Lumme, J.; Mäkinen, H.; Ermolenko, A.V.; Gregg, J.L.; Ziętara, M.S. Displaced phylogeographic signals from Gyrodactylus arcuatus, a parasite of the three-spined stickleback Gasterosteus aculeatus, suggest freshwater glacial refugia in Europe. Int. J. Parasitol. 2016, 46, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Brune, G.M. Springs of Texas, Volume 1; Branch-Smith: Fort Worth, TX, USA, 1981. [Google Scholar]
- Musgrove, M.; Crow, C.L. Origin and Characteristics of Discharge at San Marcos Springs Based on Hydrologic and Geochemical Data (2008-10), Bexar, Comal, and Hays Counties, Texas; 141133454X; US Department of the Interior, US Geological Survey: Reston, VA, USA, 2012; p. viii+94.
- Schenck, J.R.; Whiteside, B.G. Distribution, habitat preference and population size estimate of Etheostoma fonticola. Copeia 1976, 1976, 697–703. [Google Scholar] [CrossRef]
- Jordan, D.S.; Gilbert, C.H. List of fishes collected in Arkansas, Indian Territory, and Texas, in September, 1884, with notes and descriptions. Proc. U. S. Natl. Mus. 1886, 1886, 1–25. [Google Scholar] [CrossRef]
- USFWS. National Wild Fish Health Survey Results for the Comal River, TX (CHN 15-16); FWS/R2/FR-SFHU/780; San Marcos Aquatic Resources Center (USFWS): San Marcos, TX, USA, 2015; p. 1.
- Michel, G.D. The Biology of Capillaria sp. (Nematoda: Capillariidae) from Swim Bladders of Sunfishes of the Upper San Marcos River. Master’s Thesis, Southwest Texas State University, San Marcos, TX, USA, 1984. [Google Scholar]
- O’Docharty, E.M. Studies on the life cycle of Huffmanela huffmani (Nematoda: Trichosomoididae). Master’s Thesis, Texas State University-San Marcos, San Marcos, TX, USA, 2007. [Google Scholar]
- Cox, M.K.; Huffman, D.G.; Moravec, F. Observations on the distribution and biology of Huffmanela huffmani (Nematoda: Trichosomoididae). Folia Parasitol. 2004, 51, 50–54. [Google Scholar] [CrossRef]
- EAA. The EAA Act: A Success Story. Available online: https://www.edwardsaquifer.org/business-center/legislation-rules/the-eaa-act-a-success-story/ (accessed on 25 February 2020).
- Hitchcock, A.S. New species and new names of grasses from Texas. J. Wash. Acad. Sci. 1933, 23, 449–456. [Google Scholar]
- Horne, F.; Kahn, A. Phylogeny of North American wild rice, a theory. Southwest. Nat. 1997, 42, 423–434. [Google Scholar]
- Hardy, T. (Chief Science Officer, Meadows Center for Water & the Environment, San Marcos, TX, USA) (Meadows Professor of Environmental Flows, Texas State University, San Marcos, TX, USA). Personal communication regarding ecological requirements of Texas wild rice, 2021.
- Heard, T.C. (Aquatic Biologist, Meadows Center for Water & the Environment, San Marcos, TX, USA). Personal communication regarding ecological requirements of Texas wild rice, 2021.
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Groeger, A.W.; Brown, P.F.; Tietjen, T.E.; Kelsey, T.C. Water quality of the San Marcos River. Tex. J. Sci. 1997, 49, 16. [Google Scholar]
- Saunders, K.S.; Mayes, K.B.; Jurgensen, T.A.; Trungale, J.T.; Kleinsasser, L.J.; Aziz, K.; Fields, J.R.; Moss, R.E. An Evaluation of Spring Flows to Support the Upper San Marcos River Spring Ecosystem, Hays County, Texas; Resource Protection Division, Texas Parks and Wildlife Department: Austin, TX, USA, 2001.
- Hannan, H.H.; Dorris, T.C. Succession of a macrophyte community in a constant temperature river. Limnol. Oceanogr. 1970, 15, 442–453. [Google Scholar] [CrossRef]
- Nickels, D.L.; Bousman, C.B. Archaeological testing at San Marcos Springs (41HY160) for the Texas Rivers Center, Hays County, Texas. Index Tex. Archaeol. Open Access Gray Lit. Lone Star State 2010, 2010, 8. [Google Scholar] [CrossRef]
- Social_Science_Statistics. Spearman’s Rho Correlation Coefficient Calculator. Available online: https://www.socscistatistics.com/tests/pearson/default2.aspx (accessed on 4 March 2020).
- Logan, C.A.; Buckley, B.A. Transcriptomic responses to environmental temperature in eurythermal and stenothermal fishes. J. Exp. Biol. 2015, 218, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.B. Climatic change and the evolution of cave invertebrates in the Grand Canyon, Arizona; J. Caves Karst Stud. 1980, 42, 53–60. [Google Scholar]
- Chown, S.L.; Jumbam, K.R.; Sørensen, J.G.; Terblanche, J.S. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 2009, 23, 133–140. [Google Scholar] [CrossRef]
- Mishler, B.D.; Knerr, N.; González-Orozco, C.E.; Thornhill, A.H.; Laffan, S.W.; Miller, J.T. Phylogenetic measures of biodiversity and neo-and paleo-endemism in Australian Acacia. Nat. Commun. 2014, 5, 4473. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Endemism in historical biogeography and conservation biology: Concepts and implications. Biogeogr. J. Integr. Biogeogr. 2017, 32, 47–75. [Google Scholar] [CrossRef]
- Darwin, C.R. The Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray. 6th Edition, with Additions and Corrections, 6th ed.; William Clowes and Sons: London, UK, 1872; p. xxii+460. [Google Scholar]
- Platnick, N.I.; Nelson, G. Composite areas in vicariance biogeography. Syst. Zool. 1984, 33, 328–335. [Google Scholar] [CrossRef]
- Grandcolas, P.; Nattier, R.; Trewick, S. Relict species: A relict concept? Trends Ecol. Evol. 2014, 29, 655–663. [Google Scholar] [CrossRef]
- Keppel, G.; Wardell-Johnson, G.W. Refugia: Keys to climate change management. Glob. Change Biol. 2012, 18, 2389–2391. [Google Scholar] [CrossRef]
- Robson, B.J.; Chester, E.T.; Mitchell, B.D.; Matthews, T.G. Disturbance and the role of refuges in mediterranean climate streams. Hydrobiologia 2013, 719, 77–91. [Google Scholar] [CrossRef]
- Burk, R.A.; Kennedy, J.H. Invertebrate communities of groundwater-dependent refugia with varying hydrology and riparian cover during a supraseasonal drought. J. Freshw. Ecol. 2013, 28, 251–270. [Google Scholar] [CrossRef]
- EAHCP. Habitat Conservation Plan 2018—Annual Report; Edwards Aquifer Authority: San Antonio, TX, USA, 2019. [Google Scholar]
- Van Wyk, J.A. Refugia-overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. Onderstepoort J. Vet. Res. 2001, 68, 55–67. [Google Scholar] [PubMed]
- Deuel, N.R.; Conner, L.M.; Miller, K.V.; Chamberlain, M.J.; Cherry, M.J.; Tannenbaum, L.V. Habitat selection and diurnal refugia of gray foxes in southwestern Georgia, USA. PLoS ONE 2017, 12, e0186402. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalén, L. Refugia revisited: Individualistic responses of species in space and time. Proc. R. Soc. B Biol. Sci. 2010, 277, 661–671. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).