Hydrobiological Aspects of Saturated, Methyl-Branched, and Cyclic Fatty Acids Derived from Aquatic Ecosystems: Origin, Distribution, and Biological Activity
Abstract
:1. Introduction
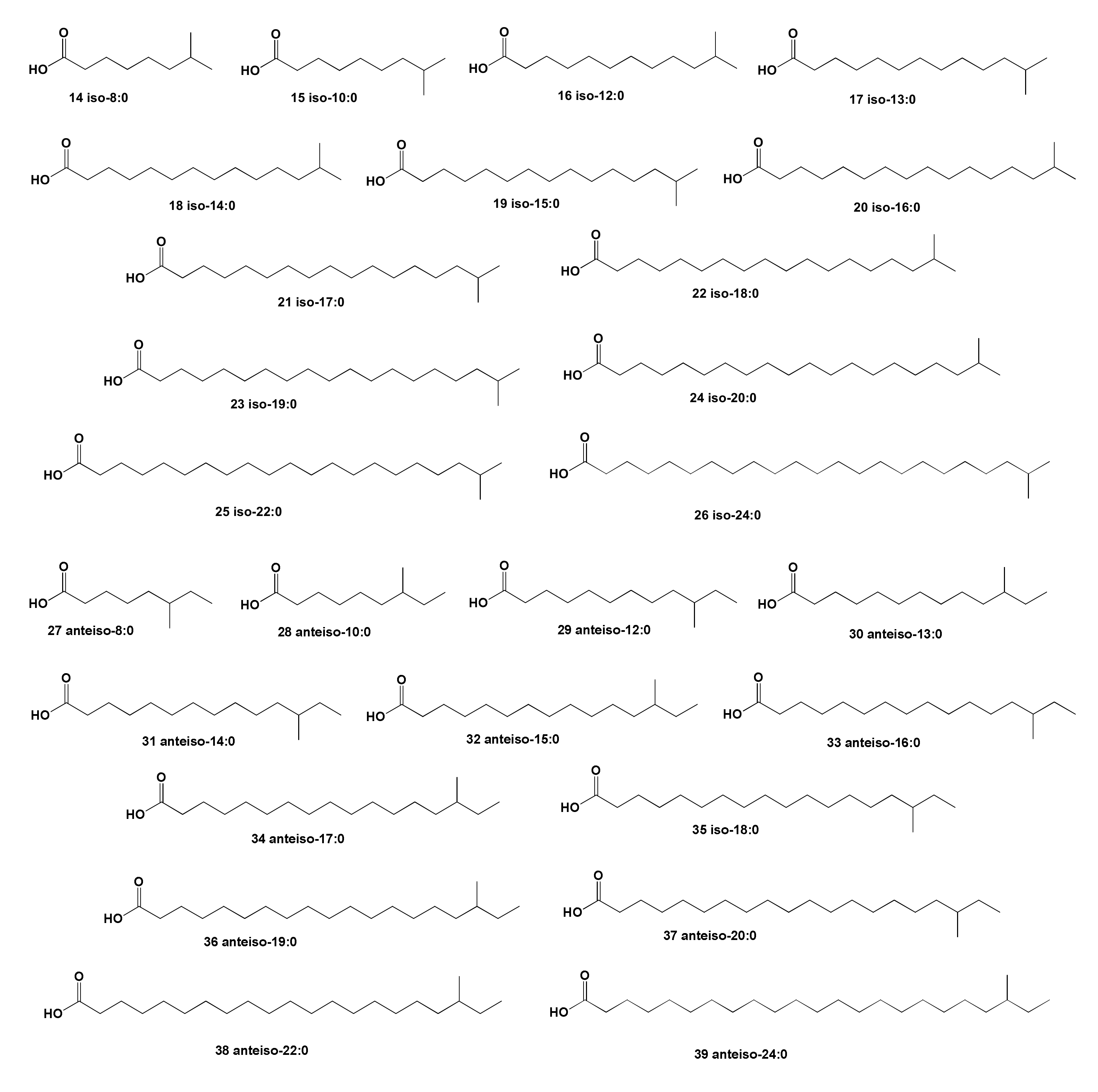
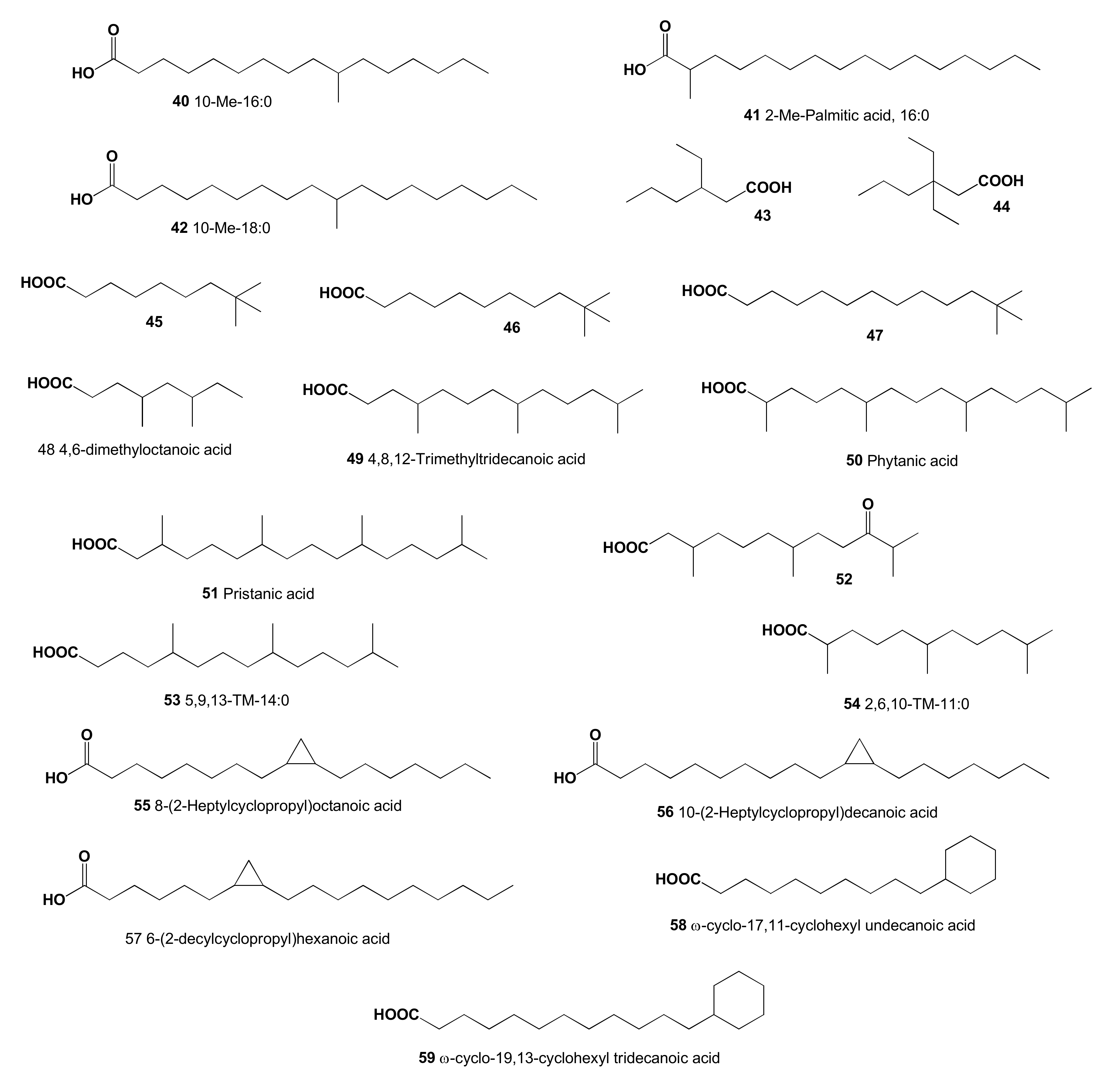
2. Acyclic Aliphatic Fatty (Carboxylic) Acids



3. Comparison of Biological Activities of Natural Saturated Fatty Acids
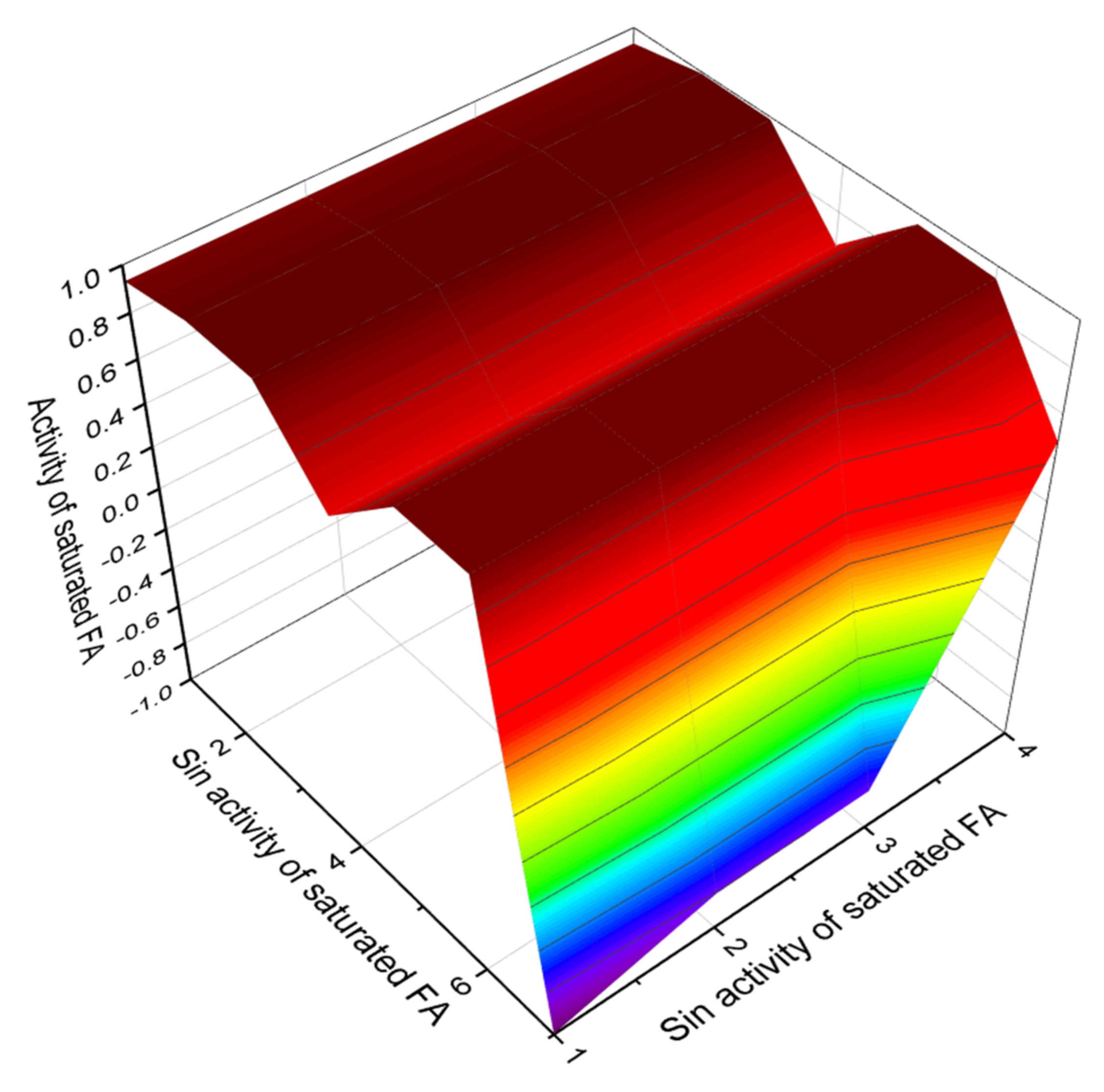
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackman, R.A. Marine Biogenic Lipids, Fats & Oils; CRC Press: Boca Raton, FL, USA, 1989; Volume 1. [Google Scholar]
- Eoin, F.; Shankar, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar]
- Cassim, A.M.; Gouguet, P.; Gronnier, J.; Laurent, N. Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res. 2019, 73, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.L.; Cummings, B.S. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomed. Chromatogr. 2006, 20, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, M. Phospholipids in marine environments: A review. Talanta 2005, 66, 422–434. [Google Scholar] [CrossRef]
- Motolese, P. Phospholipids do not have lipolytic activity. A critical review. J. Cosmet. Laser Ther. 2008, 10, 114–118. [Google Scholar] [CrossRef]
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R.A. Clinical review on triglycerides. Eur. Heart J. 2020, 41, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids: Their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef]
- Brockerhoff, H. Stereospecific analysis of triglycerides. Lipids 1971, 6, 942–956. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Ball, W.B.; Neff, J.K.; Gohil, V.M. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018, 592, 1273–1290. [Google Scholar] [CrossRef] [Green Version]
- Dyas, L.; Goad, L.J. Steryl fatty acyl esters in plants. Phytochemistry 1993, 34, 17–29. [Google Scholar] [CrossRef]
- Taylor, L.A.; Pletschen, L.; Arends, J. Marine phospholipids—A promising new dietary approach to tumor-associated weight loss. Support Care Cancer 2010, 18, 159–167. [Google Scholar] [CrossRef]
- Colin, L.A.; Jaillais, Y. Phospholipids across scales: Lipid patterns and plant development. Curr. Opin. Plant Biol. 2020, 53, 1–9. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V. Biosensing methods for determination of triglycerides: A review. Biosens. Bioelectron. 2018, 100, 214–227. [Google Scholar] [CrossRef]
- Rahim, N.; Ahmat, N.; Norrizah, J.S.; Aripin, N.F.K. A review on phase behaviour of glycolipids derived from plants. Materials Today Proceed. 2018, 5, S180–S185. [Google Scholar] [CrossRef]
- Vial, H.J.; Eldin, P.; Tielens, A.G.M.; van Hellemond, J.J. Phospholipids in parasitic protozoa. Mol. Biochem. Parasitol. 2003, 126, 143–154. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef]
- Simon, E.W. Phospholipids and plant membrane permeability. New Phytol. 1974, 73, 377–420. [Google Scholar] [CrossRef]
- Thompson, G.A., Jr.; Nozawa, Y. Lipids of protozoa: Phospholipids and neutral lipids. Ann. Rev. Microbiol. 1972, 26, 249–278. [Google Scholar] [CrossRef]
- Working, E.B.; Andrews, A.C. The structure of the phospholipids. Chem. Rev. 1941, 29, 245–256. [Google Scholar] [CrossRef]
- Nakamura, Y. Plant phospholipid diversity: Emerging functions in metabolism and protein–lipid interactions. Trends Plant Sci. 2017, 22, 1027–1040. [Google Scholar] [CrossRef]
- Liu, B.; Benning, C. Lipid metabolism in microalgae distinguishes itself. Curr. Opin. Biotechnol. 2013, 24, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Natural neo acids and neo alkanes: Their analogues and derivatives. Lipids 2006, 41, 309–340. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.R.; Bordin, K.; Fernandes, A.M.; Rodrigues, C.E.C.; Corassin, C.H.; Cruz, A.G.; Oliveira, C.A.F. Storage of refrigerated raw goat milk affecting the quality of whole milk powder. J. Dairy Sci. 2013, 96, 4716–4724. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Ribeiro, S.D.A. Specialty products made from goat milk. Small Ruminant Res. 2010, 89, 225–233. [Google Scholar] [CrossRef]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 41, 288–302. [Google Scholar] [CrossRef]
- Kaneda, T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J. Bacteriol. 1967, 93, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Pedneault, K.; Angers, P.; Gosselin, A.; Tweddell, R.J. Fatty acid composition of lipids from mushrooms belonging to the family Boletaceae. Mycol. Res. 2006, 110, 1179–1183. [Google Scholar] [CrossRef]
- Moore, B.S.; Floss, H.G. Biosynthesis of cyclic fatty acid containing cyclopropyl, cyclopentyl, cyclohexyl and cycloheptyl rings. Reference Module in Chemistry, Molecular Sciences, and Chemical Engineering. Comprehen. Nat. Prod. Chem. 1999, 1, 61–82. [Google Scholar]
- Bao, X.; Katz, S.; Pollard, M.; John, O. Carbocyclic fatty acids in plants: Biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculia foetida. Plant Biol. 2002, 99, 7172–7177. [Google Scholar] [CrossRef] [Green Version]
- Grogan, D.W.; Cronan, J.E., Jr. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 1997, 14, 429–441. [Google Scholar]
- Ortiz, A.; Sansinenea, E. Chemical compounds produced by Bacillus sp. factories and their role in nature. Mini Rev. Med. Chem. 2019, 19, 373–380. [Google Scholar] [CrossRef]
- James, G.; Das, B.C.; Jose, S. Bacillus as an aquaculture friendly microbe. Aquacult. Int. 2021, 29, 323–353. [Google Scholar] [CrossRef]
- Cooper, W.J.; Blumer, M. Linear, iso and anteiso fatty acids in recent sediments of the North Atlantic. Deep Sea Res. Oceanogr. Abst. 1968, 15, 535–540. [Google Scholar] [CrossRef]
- Perry, G.J.; Volkman, J.K.; Johns, R.B.; Bavor, H.J., Jr. Fatty acids of bacterial origin in contemporary marine sediments. Geochim. Cosmochim. Acta 1979, 43, 1715–1725. [Google Scholar] [CrossRef]
- Opris, S.; Sicora, C.; Rusu, T.; Miclean, M. Identification and quantification of fatty acids in cyanobacteria cells. ProEnvironment 2013, 6, 402–406. [Google Scholar]
- Ötleş, S.; Pire, R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int. 2001, 84, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Řezanka, T.; Dor, I.; Prell, A.; Dembitsky, V.M. Fatty acid composition of six freshwater wild cyanobacterial species. Folia Microbiol. 2003, 48, 71–75. [Google Scholar] [CrossRef]
- Rezanka, T.; Víden, I.; Go, J.V.; Dor, I.; Dembitsky, V.M. Polar lipids and fatty acids of three wild cyanobacterial strains of the genus Chroococcidiopsis. Folia Microbiol. 2003, 48, 781–786. [Google Scholar] [CrossRef]
- Temina, M.; Rezankova, H.; Rezanka, T.; Dembitsky, V.M. Diversity of the fatty acids of the Nostoc species and their statistical analysis. Microbiol. Res. 2007, 162, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.C.; Sinha, R.P.; Hader, D.P. Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool. 2002, 41, 297–308. [Google Scholar]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Ananya, A.K.; Ahmad, I.Z. Cyanobacteria “the blue green algae” and its novel applications: A brief review. Int. J. Innov. Appl. Stud. 2014, 7, 251–261. [Google Scholar]
- Kong, F.; Romero, I.T.; Warakanont, J. Lipid catabolism in microalgae. New Phytol. 2018, 218, 1340–1348. [Google Scholar] [CrossRef]
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Srebnik, M. Variability of hydrocarbon and fatty acid components in cultures of the filamentous cyanobacterium Scytonema sp. isolated from microbial community ‘Black Cover’ of limestone walls in Jerusalem. Biochemistry 2002, 67, 1276–1282. [Google Scholar]
- Dembitsky, V.M.; Dor, I.; Shkrob, I. Variability of lipid constituents of the soil cyanobacterium Microcoleus vaginatus from the Dead Sea basin and Negev Desert. Biochemistry 2000, 65, 1403–1408. [Google Scholar]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X. The diversity of cyanobacterial toxins on structural characterization, distribution, and identification: A Systematic Review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef] [Green Version]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Mnif, I.; Ghribi, D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Peptide Sci. 2015, 104, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. Anti-Cancer Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rozentsvet, O.A.; Pechenkina, E.E. Glycolipids, phospholipids, and fatty acids of some brown algae species from the Black Sea. Phytochemistry 1990, 29, 3417–3421. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rozentsvet, O.A. Phospholipid composition of some marine red algae. Phytochemistry 1990, 29, 3149–3152. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Pechenkina-Shubina, E.E.; Rozentsvet, O.A. Glycolipids and fatty acids of some seaweeds and marine grasses from Black Sea. Phytochemistry 1991, 30, 2279–2283. [Google Scholar] [CrossRef]
- Harwood, J.L. Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Chen, Y.; Wu, Y.; Hao, H.; Liang, W.; Liu, J.; Huang, R. Marine unsaturated fatty acids: Structures, bioactivities, biosynthesis, and benefits. RSC Adv. 2019, 9, 35312–35327. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Pham, H.D.; Seon, J.; Woo, H.C. Marine brown algae: A conundrum answer for sustainable biofuels production. Renew. Sustain. Energy Rev. 2015, 50, 782–792. [Google Scholar] [CrossRef]
- Santos, S.; Oliveira, A.; Lopes, C. Systematic review of saturated fatty acids on inflammation and circulating levels of adipokines. Nutr. Res. 2013, 33, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezankova, H.; Rezanka, T.; Hanus, L.O. Variability of the fatty acids of the marine green algae belonging to the genus Codium. Biochem. System. Ecol. 2003, 31, 1125–1145. [Google Scholar] [CrossRef]
- Khotimchenko, S.V. Fatty acids of species in the genus Codium. Bot. Marine 2003, 46, 456–460. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Šarkanj, B. Chemical diversity of Codium bursa (Olivi) C. Agardh headspace compounds, volatiles, fatty acids, and insight into its antifungal activity. Molecules 2019, 24, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Shoubaky, G.A.; El Rahman Salem, A. Active ingredients fatty acids as antibacterial agent from the brown algae Padina pavonica and Hormophysa triquetra. J. Coast. Life Med. 2014, 2, 431–438. [Google Scholar]
- Fleurence, J.; Gutbier, G.; Mabeaul, S.; Leray, C. Fatty acids from 11 marine macroalgae of the French Brittany coast. J. Appl. Phycol. 1994, 6, 527–532. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Bychek, I.A.; Afonina, O.M. Acetylenic acids and lipids of some mosses from Russia. Phytochemistry 1993, 33, 1021–1027. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Bychek, I.A.; Afonina, O.M. Chemical constituents of some moss species. J. Hattori Bot. Lab. 1994, 75, 161–172. [Google Scholar]
- Dembitsky, V.M.; Rezanka, T. Distribution of diacylglycerylhomoserines, phospholipids and fatty acids in thirteen moss species from South-Western Siberia. Biochem. Syst. Ecol. 1995, 23, 71–78. [Google Scholar] [CrossRef]
- Kornprobst, J.M.; Barnathan, G. Demospongic acids revisited. Mar. Drugs 2010, 8, 2569–2577. [Google Scholar] [CrossRef] [Green Version]
- Litchfield, C.; Morales, R.W. Are demospongiae membranes unique among living organisms? In Aspects of Sponge Biology; Harrison, F.W., Cowden, R.R., Eds.; Academic Press: New York, NY, USA, 1976; pp. 183–200. [Google Scholar]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Rezanka, T.; Sigler, K. Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. Prog. Lipid Res. 2009, 48, 206–238. [Google Scholar] [CrossRef]
- Bergé, J.P.; Barnathan, G. Fatty acids from lipids of marine organisms: Molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biotechnol. 2005, 96, 49–125. [Google Scholar]
- Dembitsky, V.M.; Rezanka, T.; Srebnik, M. Lipid compounds of freshwater sponges: Family Spongillidae, class Demospongiae. Chem. Physics Lipids 2003, 123, 117–155. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Comparative study of the endemic freshwater fauna of Lake Baikal. 2. Unusual lipid composition of two sponges Baicalospongia bacilifera and B. intermedia (Family Lubomirskiidae, Class Demospongiae). Comp. Biochem. Physiol. 1993, 106B, 825–831. [Google Scholar]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Comparative study of the endemic freshwater fauna of Lake Baikal. 6. Unusual fatty acid and lipid composition of the endemic sponge Lubomirskia baicalensis and sponge’s amphipod crustacean parasite Brandtia (Spinacantus) parasitica. Comp. Biochem. Physiol. 1994, 109B, 415–426. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T. Unusually high levels of eicosatetraenoic, eicosapentaenoic and docosahexaenoic fatty acids in Palestinian freshwater sponges. Lipids 1996, 31, 647–650. [Google Scholar] [CrossRef]
- Rezanka, T.; Dembitsky, V.M. Isoprenoid polyunsaturated fatty acids from freshwater sponges. J. Nat. Prod. 1993, 56, 1898–1904. [Google Scholar] [CrossRef]
- Rezanka, T.; Dembitsky, V.M. Multibranched polyunsaturated and very-long-chain fatty acids of freshwater Israeli sponges. J. Nat. Prod. 2002, 65, 709–713. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Maldonado, L.; Porras, B. Isoprenoid fatty acids from marine sponges. Are sponges selective? Lipids 1987, 22, 767–769. [Google Scholar] [CrossRef]
- Rod’kina, S.A. Fatty acids and other lipids of marine sponges. Russ. J. Mar. Biol. 2005, 31, S49–S60. [Google Scholar] [CrossRef]
- Carballeira, N.; Thompson, J.E.; Ayanoglu, E.; Djerassi, C. Biosynthetic studies of marine lipids. 5. The biosynthesis of long-chain branched fatty acids in marine sponges. J. Org. Chem. 1986, 51, 2751–2756. [Google Scholar] [CrossRef]
- Gillan, F.T.; Stoilov, I.L.; Thompson, J.E.; Hogg, R.W.; Wilkinson, C.R.; Djerassi, C. Fatty acids as biological markers for bacterial symbionts in sponges. Lipids 1988, 23, 1139–1145. [Google Scholar] [CrossRef]
- Mishra, P.M.; Sree, A.; Panda, P.K. Fatty acids of marine sponges. Fatty Acids of Marine Sponges. In Springer Handbook of Marine Biotechnology; Springer Handbooks; Kim, S.K., Ed.; Springer: Berlin, Heidelberg, 2015. [Google Scholar]
- Pereira, M.; Vinholes, J.D.; Correia-da-Silva, G.; Valentao, P.; Teixeira, N.; Andrade, P.B. Fatty acids in marine organisms: In the Pursuit of bioactive agents. Curr. Pharm. Anal. 2011, 7, 108–119. [Google Scholar] [CrossRef]
- Aguiar, A.C.C.; Parisi, J.R.; Granito, R.N.; de Sousa, L.R.F. Metabolites from marine sponges and their potential to treat malarial protozoan parasites infection: A Systematic Review. Mar. Drugs 2021, 19, 134. [Google Scholar] [CrossRef]
- Mioso, R.; Marante, F.J.T.; Bezerra, R.S.; Borges, F.V.P. Cytotoxic compounds derived from marine sponges. A review (2010–2012). Molecules 2017, 22, 208. [Google Scholar] [CrossRef] [Green Version]
- Kiem, P.V.; Minh, C.V.; Nhiem, N.X.; Tai, B.H. The chemical constituents and biological activity of some sponges in Northern Vietnam: A review. Vietnam J. Chem. 2019, 57, 261–271. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Riccardi, N.; Urbanska, M. Major shortfalls impairing knowledge and conservation of freshwater molluscs. Hydrobiologia 2021, 848, 2831–2867. [Google Scholar] [CrossRef]
- Kappes, H.; Haase, P. Slow, but steady: Dispersal of freshwater molluscs. Aquat. Sci. 2012, 74, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.W. Aspects of freshwater mollusc ecological biogeography. Palaeogeograph. Palaeoclim. Palaeoecol. 1988, 62, 511–576. [Google Scholar] [CrossRef]
- Balian, E.V.; Segers, H.; Martens, K.; Lévéque, C. The Freshwater Animal Diversity Assessment: An Overview of the Results. In Freshwater Animal Diversity Assessment. Developments in Hydrobiology; Balian, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 198. [Google Scholar]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. Camb. Philos. Soc. 2010, 85, 757–775. [Google Scholar] [CrossRef]
- Smith, A.B. Marine diversity through the Phanerozoic: Problems and prospects. J. Geol. Soc. 2007, 164, 731–745. [Google Scholar] [CrossRef]
- Zhukova, N.V. Fatty acids of marine mollusks: Impact of diet, bacterial symbiosis, and biosynthetic potential. Biomolecules 2019, 9, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.R.; Scheibling, R.E. Fatty acids as dietary tracers in benthic food webs. Mar. Ecol. Prog. Ser. 2012, 446, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Parrish, C.C. Determination of Total Lipid, Lipid Classes, and Fatty Acids in Aquatic Samples. In Lipids in Freshwater Ecosystems; Arts, M.T., Wainman, B.C., Eds.; Springer: New York, NY, USA, 1999; pp. 4–20. [Google Scholar]
- Parrish, C.C. Lipids in Marine Ecosystems. ISRN Oceanogr. 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.D. Distribution, and composition of lipids in marine invertebrates. Mar. Biog. Lipids Fats Oils 1989, 2, 49–144. [Google Scholar]
- Dembitsky, V.M.; Kashin, A.G.; Stefanov, K. Comparative investigation of phospholipids and fatty acids of freshwater molluscs from the Volga River basin. Comp. Biochem. Physiol. 1992, 102, 193–198. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Comparative examination phospholipids and fatty acids from some Caspian invertebrates. Comp. Biochem. Physiol. 1993, 104, 617–622. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Fatty acid and phospholipid compo¬sitions of freshwater molluscs Anadonta piscinalis and Limnaea fragilis from the river Volga. Comp. Biochem. Physiol. 1993, 105, 597–601. [Google Scholar]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Comparative study of the endemic freshwater fauna of Lake Baikal. 1. Phospholipid and fatty acid composition of two molluscs species Baicalia oviformis and Benedictia baicalensis. Comp. Biochem. Physiol. 1993, 106, 819–823. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Comparative study of the endemic freshwater fauna of Lake Baikal. 4. Phospholipid and fatty acid composition two gastropod molluscs of genus Valvata. Comp. Biochem. Physiol. 1994, 107, 325–330. [Google Scholar] [CrossRef]
- Brown, A.C.; Fraser, T.R. The connection of chemical constitution and physiological action. Trans. Roy. Soc. Edinburg 1868, 25, 224–242. [Google Scholar]
- Cros, A.F.A. Action de l’Alcohol Amylique Sur l’Organisme. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 1863. [Google Scholar]
- Richet, M.C. Note sur le rapport entre la toxicité et les propriétes physiques des corps. Compt. Rend. Soc. Biol. 1893, 45, 775–776. [Google Scholar]
- Meyer, H. Zur Theorie der AIkoholnarkose. Arch. Exp. Path. Pharm. 1899, 42, 109–118. [Google Scholar] [CrossRef]
- Overton, C.E. Studien über die Narkose; Fischer: Jena, Germany, 1901. [Google Scholar]
- Hammett, L.P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 1935, 17, 125–136. [Google Scholar] [CrossRef]
- Hammett, L.P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Taft, R.W. Separation of Polar, Steric and Resonance Effects in Reactivity. In Steric Effects in Organic Chemistry; Newman, M.S., Ed.; Wiley: Hoboken, NJ, USA, 1956; pp. 556–675. [Google Scholar]
- Hansch, C.; Fujita, T. p-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Exploring QSAR; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W., Jr. Computational methods in drug discovery. Pharm. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef] [Green Version]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef] [Green Version]
- Kokh, D.B.; Amaral, M.; Bomke, J.; Grädler, U.; Musil, D. Estimation of drug-target residence times by τ-random acceleration molecular dynamics simulations. J. Chem. Theor. Comput. 2018, 14, 3859–3869. [Google Scholar] [CrossRef]
- Cherkasov, A.M.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef] [Green Version]
- Burov, Y.V.; Poroikov, V.V.; Korolchenko, L.V. National system for registration and biological testing of chemical compounds: Facilities for new drugs search. Bull. Natl. Center Biol. Act. Comp. 1990, 1, 4–25. [Google Scholar]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.; Filimonov, D.; Poroikov, V.; Oprea, T. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Druzhilovskiy, D.S.; Rudik, A.V. Computer-aided prediction of biological activity spectra for organic compounds: The possibilities and limitations. Russ. Chem. Bull. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V.; Gloriozova, T.A.; Imbs, A.B. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 4663–4674. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Savidov, N. Steroid phosphate esters and phosphonosteroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 7679–7692. [Google Scholar] [CrossRef]
- PASS. Available online: http://www.way2drug.com/passonline/ (accessed on 30 November 2021).
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef]
- Vil, V.; Terent’ev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Oxetane-containing metabolites: Origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 2449–2467. [Google Scholar] [CrossRef]
- Vil, V.A.; Gloriozova, T.A.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Hydroperoxides derived from marine sources: Origin and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 1627–1642. [Google Scholar] [CrossRef]
- Vil, V.A.; Gloriozova, T.A.; Poroikov, V.V.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Naturally occurring of α,β-diepoxy-containing compounds: Origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 3249–3264. [Google Scholar] [CrossRef]
- Vil, V.A.; Terent’ev, A.O.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Pounina, T.A.; Dembitsky, V.M. Hydroperoxy steroids and triterpenoids derived from plant and fungi: Origin, structures and biological activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Harada, H.; Yamashita, U.; Kurihara, H. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004, 50, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-chain fatty acids—An underexplored class of dairy-derived fatty acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef]
- Ciuffreda, E.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Alicyclobacillus spp.: New insights on ecology and preserving food quality through new approaches. Microorganisms 2015, 3, 625–640. [Google Scholar] [CrossRef] [Green Version]
- Oshima, M.; Ariga, T. Omega-cyclohexyl fatty acids in acidophilic thermophilic bacteria. Studies on their presence, structure, and biosynthesis using precursors labeled with stable isotopes and radioisotopes. J. Biol. Chem. 1975, 250, 6963–6968. [Google Scholar] [CrossRef]
- De Rosa, M.; Gambacorta, A.; Minale, L.; Bu’lock, J.D. The formation of ω-cyclohexyl-fatty acids from shikimate in an acidophilic thermophilic bacillus. A new biosynthetic pathway. Biochem. J. 1972, 128, 751–754. [Google Scholar] [CrossRef] [Green Version]








| No. | Predicted Biological Activity, Pa * | Reported Activity | Ref. |
|---|---|---|---|
| 1 | Lipid metabolism regulator (0.860), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) Atherosclerosis treatment (0.634), Antiprotozoal (Coccidial) (0.514) | Antibacterial Anti-fungal Anti-protozoan Antiviral | [132] |
| 2 | Lipid metabolism regulator (0.860), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) Atherosclerosis treatment (0.634), Antiprotozoal (Coccidial) (0.514) | Antibacterial Anti-protozoan Anti-fungal Antiviral | [132] |
| 3 | Lipid metabolism regulator (0.860), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) | Antibacterial Anti-fungal Anti-protozoan | [132] |
| 4 | Lipid metabolism regulator (0.860), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) | Antibacterial Antiviral | [132] |
| 5 | Lipid metabolism regulator (0.860), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) | Antibacterial Anti-fungal | [132] |
| 6 | Lipid metabolism regulator (0.860), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) | Antibacterial | [132] |
| 7 | Preneoplastic conditions treatment (0.836), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) | Antibacterial Anticancer | [132,133] |
| 8 | Lipid metabolism regulator (0.860), Antiviral (Arbovirus) (0.833) Anti-inflammatory (0.709), Antiviral (Picornavirus) (0.706) Anti-hypercholesterolemic (0.646), Antibacterial (0.638) | Antibacterial Anti-breast cancer | [132,134] |
| 9 | Lipid metabolism regulator (0.913), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Antiviral (Arbovirus) (0.705), Antiviral (Picornavirus) (0.617) | Antibacterial Antiviral Hemolytic | [132] |
| 10 | Lipid metabolism regulator (0.913), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Antiviral (Arbovirus) (0.705), Antiviral (Picornavirus) (0.617) | No experimental data | |
| 11 | Lipid metabolism regulator (0.913), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Antiviral (Arbovirus) (0.705), Antiviral (Picornavirus) (0.617) | No experimental data | |
| 12 | Lipid metabolism regulator (0.913), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Antiviral (Arbovirus) (0.705), Antiviral (Picornavirus) (0.617) | No experimental data | |
| 13 | Lipid metabolism regulator (0.817), Hypolipemic (0.784) Antiviral (Arbovirus) (0.768), Antineurotic (0.752) Antiviral (Picornavirus) (0.683), Antifungal (0.567) | No experimental data |
| No. | Predicted Biological Activity, Pa * | Reported Activity | Ref. |
|---|---|---|---|
| 14 | Sclerosant (0.878), Anesthetic general (0.849), Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805) Acute neurologic disorders treatment (0.723), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | No experimental data | |
| 15 | Sclerosant (0.878), Anesthetic general (0.849), Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805) Acute neurologic disorders treatment (0.723), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | No experimental data | |
| 16 | Sclerosant (0.878), Anesthetic general (0.849), Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805) Acute neurologic disorders treatment (0.723), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134,135] |
| 17 | Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134,135] |
| 18 | Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134] |
| 19 | Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134,135] |
| 20 | Lipid metabolism regulator (0.810), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | ||
| 21 | Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134,135] |
| 22 | Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134] |
| 23 | Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134] |
| 24 | Lipid metabolism regulator (0.810), Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649) | Anti-breast cancer | [134] |
| 25 | Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649), Antimutagenic (0.532) | No experimental data | |
| 26 | Preneoplastic conditions treatment (0.805), Antiviral (Arbovirus) (0.716), Antiviral (Picornavirus) (0.649), Antimutagenic (0.532) | No experimental data |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 27 | Anti-hypercholesterolemic (0.801), Preneoplastic conditions treatment (0.793) Hypolipemic (0.768), Acute neurologic disorders treatment (0.718) Atherosclerosis treatment (0.679), Antineoplastic (0.566), Antiparasitic (0.526) |
| 28 | Anti-hypercholesterolemic (0.801), Preneoplastic conditions treatment (0.793) Hypolipemic (0.768), Acute neurologic disorders treatment (0.718) Atherosclerosis treatment (0.679), Antineoplastic (0.566), Antiparasitic (0.526) |
| 29 | Anti-hypercholesterolemic (0.801), Preneoplastic conditions treatment (0.793) Hypolipemic (0.768), Acute neurologic disorders treatment (0.718) Atherosclerosis treatment (0.679), Antineoplastic (0.566), Antiparasitic (0.526) |
| 30 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801); Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 31 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 32 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 33 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 34 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718); Anticonvulsant (0.717) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 35 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 36 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 37 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 38 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| 39 | Lipid metabolism regulator (0.913), Anti-hypercholesterolemic (0.801), Hypolipemic (0.768) Acute neurologic disorders treatment (0.718), Anticonvulsant (0.717) Atherosclerosis treatment (0.679), Antifungal (0.592), Antiparasitic (0.526) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 40 | Lipid metabolism regulator (0.905), Anti-hypercholesterolemic (0.789), Hypolipemic (0.757) |
| 41 | Lipid metabolism regulator (0.817), Anti-hypercholesterolemic (0.674) Atherosclerosis treatment (0.643) |
| 42 | Lipid metabolism regulator (0.905), Anti-hypercholesterolemic (0.789) Atherosclerosis treatment (0.682) |
| 43 | Lipid metabolism regulator (0.853), Hypolipemic (0.767) Anti-hypercholesterolemic (0.699), Atherosclerosis treatment (0.694) |
| 44 | Hypolipemic (0.863), Lipid metabolism regulator (0.803) Atherosclerosis treatment (0.655), Anti-hypercholesterolemic (0.610) |
| 45 | Lipid metabolism regulator (0.877), Hypolipemic (0.809) Atherosclerosis treatment (0.701), Anti-hypercholesterolemic (0.656) |
| 46 | Lipid metabolism regulator (0.877), Hypolipemic (0.809) Atherosclerosis treatment (0.701), Anti-hypercholesterolemic (0.656) |
| 47 | Lipid metabolism regulator (0.877); Hypolipemic (0.809) Atherosclerosis treatment (0.701), Anti-hypercholesterolemic (0.656) |
| 48 | Lipid metabolism regulator (0.865), Hypolipemic (0.781) Anti-hypercholesterolemic (0.721), Atherosclerosis treatment (0.659) |
| 49 | Lipid metabolism regulator (0.854), Anti-hypercholesterolemic (0.766) Hypolipemic (0.740), Atherosclerosis treatment (0.685) |
| 50 | Lipid metabolism regulator (0.860), Anti-hypercholesterolemic (0.790) Hypolipemic (0.769), Atherosclerosis treatment (0.692) |
| 51 | Hypolipemic (0.833), Acute neurologic disorders treatment (0.830) Lipid metabolism regulator (0.771), Anti-hypercholesterolemic (0.701) Atherosclerosis treatment (0.698), Anti-inflammatory (0.662) |
| 52 | Lipid metabolism regulator (0.790) Anti-hypercholesterolemic (0.757), Hypolipemic (0.677) |
| 53 | Lipid metabolism regulator (0.854), Anesthetic general (0.793) Anti-hypercholesterolemic (0.766), Hypolipemic (0.740) |
| 54 | Hypolipemic (0.833), Acute neurologic disorders treatment (0.830) Lipid metabolism regulator (0.771), Neuroprotector (0.676) |
| 55 | Mucositis treatment (0.842), Fibrinolytic (0.821); Antithrombotic (0.635), Antimutagenic (0.566) |
| 56 | Anti-eczematic (0.893), Mucositis treatment (0.842); Fibrinolytic (0.821), Anti-inflammatory (0.704) |
| 57 | Anti-eczematic (0.893), Mucositis treatment (0.842); Fibrinolytic (0.821), Anti-inflammatory (0.704) |
| 58 | Fibrinolytic (0.915), Cardiovascular analeptic (0.715), Antithrombotic (0.688) Anti-ischemic, cerebral (0.655), Platelet antagonist (0.581), Anticoagulant (0.515) |
| 59 | Fibrinolytic (0.915), Cardiovascular analeptic (0.715), Antithrombotic (0.688) Anti-ischemic, cerebral (0.655), Platelet antagonist (0.581), Anticoagulant (0.515) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembitsky, V.M. Hydrobiological Aspects of Saturated, Methyl-Branched, and Cyclic Fatty Acids Derived from Aquatic Ecosystems: Origin, Distribution, and Biological Activity. Hydrobiology 2022, 1, 89-110. https://doi.org/10.3390/hydrobiology1010007
Dembitsky VM. Hydrobiological Aspects of Saturated, Methyl-Branched, and Cyclic Fatty Acids Derived from Aquatic Ecosystems: Origin, Distribution, and Biological Activity. Hydrobiology. 2022; 1(1):89-110. https://doi.org/10.3390/hydrobiology1010007
Chicago/Turabian StyleDembitsky, Valery M. 2022. "Hydrobiological Aspects of Saturated, Methyl-Branched, and Cyclic Fatty Acids Derived from Aquatic Ecosystems: Origin, Distribution, and Biological Activity" Hydrobiology 1, no. 1: 89-110. https://doi.org/10.3390/hydrobiology1010007






