Abstract
The complex life cycle of the malaria parasite Plasmodium requires the parasite to adequately adapt to different conditions. For this reason, Plasmodium strictly controls its gene expression, and given its evolutionary distance from the human host, the involved factors may figure as attractive potential drug targets. In recent years, several unique transcription factors and chromatin modifiers have been identified and partially characterized in Plasmodium falciparum and in the murine species P. yoelii and P. berghei. This review unites data from studies focusing on drug development against enigmatic plant-like AP2-transcription factors and chromatin modifiers, such as histone acetyl transferases and deacetylases and histone methyltransferases and demethylases. Considering the reported success of inhibition of both factors, these may be included as targets to effectively combat the parasite by perturbing its control of gene expression.
1. Introduction
The intricate life cycle of malaria parasites, exemplified by Plasmodium falciparum, directly links to the ongoing challenge of malaria control. This mosquito-borne disease, primarily transmitted by infected female Anopheles mosquitoes during blood-feeding, poses a significant public health threat, with P. falciparum being the main species that causes deaths. Despite significant efforts, progress in reducing the burden of malaria has plateaued since 2018. In 2021, the World Health Organization reported a staggering 241 million clinical malaria cases worldwide, with disruptions caused by the COVID-19 pandemic contributing to approximately 68% of the 627,000 deaths in 2020 [1].
To truly develop effective interventions, it is crucial to delve into the intricacies of the malaria parasite’s life cycle. This journey begins when infected female Anopheles mosquitoes inject sporozoites into human skin during blood-feeding. These sporozoites swiftly travel to the liver, where they infect hepatocytes and multiply, generating numerous merozoites [2,3,4]. Subsequently, these merozoites are released into the bloodstream, where they invade red blood cells, undergoing a series of transformations as they progress through the stages of rings and trophozoites before culminating in fragmented schizonts, producing 16–32 merozoites upon completion. This cycle perpetuates as these merozoites initiate a new round of the intraerythrocytic life cycle [5], significantly contributing to the morbidity and mortality associated with malaria. Additionally, some parasites take an alternative route, developing into gametocytes, a crucial stage for mosquito transmission. Understanding the molecular details of this process is fundamental to devising strategies for malaria control and elimination [6].
Notably, Plasmodium stands out among eukaryotes due to several distinctive mechanisms of gene regulation. These include the absence of linker histone H1 [7], the lack of RNA interference machinery [8], and the existence of unusual histone variants with a unique array of modifications [9]. Unlike the majority of higher eukaryotes, the best studied Plasmodium species, P. falciparum chromatin, primarily adopts a euchromatic state, punctuated by only a few heterochromatic regions distinguished by the trimethylation of lysine 9 on histone 3 (H3K9me3).
In eukaryotes, the accessibility of specific DNA regions to the transcription machinery is influenced by the arrangement of nucleosomes along the double helix. This arrangement can be precisely adjusted through post-translational modifications (PTMs) occurring on either the exposed N- or C-terminal tails of histone proteins [10]. These PTMs, including acetylation, phosphorylation, methylation, SUMOylation, and ubiquitination, bring about changes in the electronic charge and structural characteristics of these histone tails. Consequently, they interact with DNA, reshaping the chromatin structure and ultimately governing the accessibility of the transcriptional machinery, thereby determining whether particular genes are transcribed or not [11]. Indeed, a remarkable total of 44 distinct post-translational covalent modifications in P. falciparum have been identified [9]. Of these, the repressive methyl mark on histone H3 lysine 9 contributes to gene silencing, while active genes display an enrichment of H3K9ac, H3K4me3 marks, and the H2A.Z histone variant. Nevertheless, the mechanisms governing these epigenetic marks and the transitions between them during subsequent reinvasion cycles of the parasite remain incompletely understood [12]. The significance of epigenetics in malaria parasite development underscores the potential of targeting the pathways involved in parasite chromatin modifications for drug development.
Another essential facet of gene expression regulation in eukaryotes is the role played by transcription factors. Initially, it was believed that the parasite’s genome contained few of these transcription factors [13,14]. However, in 2005, Balaji and colleagues made a groundbreaking discovery by identifying the presence of the ApiAP2 family of putative transcription factors across the apicomplexan phylum [15]. These ApiAP2 factors exhibit a structural similarity with the AP2/ERF domains present in the transcription factors of plants [16].
In the case of P. falciparum, researchers identified 27 members of the ApiAP2 family. Subsequent studies confirmed the regulated and cascading expression of these ApiAP2 factors throughout the parasite’s developmental stages. Moreover, the presence of their DNA-binding motifs upstream of various genes, including their own ApiAP2 genes, which pointed to their involvement in controlling the parasite’s life cycle. It is believed that the coordinated action of multiple ApiAP2 factors plays a pivotal role in transcriptional control [17,18,19]. In Figure 1, a simplified model of the interaction of nucleosomes, chromatin modifiers/associating proteins, and transcription factors is depicted.
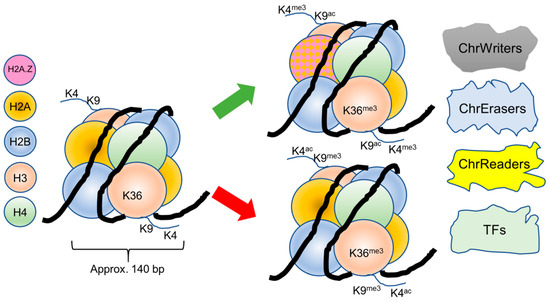
Figure 1.
A simplified scheme shows the basic nucleosome unit and 3 pivotal histone 3 modifications. A red arrow shows modifications occurring in silenced chromatin, while the green arrow shows the modifications leading to activated chromatin. Upon activation, H2A.Z is inserted into the nucleosomes which cover promoter regions upon activation [20], indicated as hatched colors. Importantly, there are more histone variants in Plasmodium with yet to be defined exact functions. Chromatin writers/erasers/readers and TFs comprise the factors described in the main text and may be responsible for both activating or inactivating genomic loci.
To a significant extent, the slowdown in global malaria control can be attributed to resistance against antimalarial drugs. The first cases of P. falciparum resistance to chloroquine were reported at the beginning of the 1960s [21]. The idea of a new compound emerged in 1981 during the Fourth Meeting of the Scientific Working Group on the Chemotherapy of Malaria, suggesting artemisinin as a potential new antimalarial treatment, which is now figuring as first line treatment in combination with other drugs [22]. Since then, artemisinin and its derivatives, including dihydroartemisinin, artemether, and artesunate, have become the preferred antimalarial drugs recommended by healthcare professionals worldwide. This shift led to a substantial reduction in malaria cases in the ensuing years. Nonetheless, artemisinin resistance initially surfaced in 2008 and has since expanded across Southeast Asia [23]. More seriously, a recent study reported that ART-resistant mutant strains of malaria parasites have also appeared in Africa [24]. This emergence of resistant malaria strains, along with the disease’s spreading prevalence, underscores the urgent need for novel antimalarial agents with improved safety and efficacy profiles, ideally featuring a different mechanism of action.
In this context, the present study aims to showcase the literature focusing on the pivotal research that has contributed to understanding the function of these epigenetic regulatory mechanisms and the key transcription factors present in P. falciparum. This review critically evaluates the potential to target these factors with antimalarial drugs, perhaps offering a promising new avenue for malaria treatment.
2. Malaria Parasite Chromatin Dynamics and Therapeutic Prospects
2.1. Histone Acetylases and Histone Deacetylases
In eukaryotes, the finely tuned balance of the acetylation and deacetylation processes plays a pivotal role in the dynamic regulation of gene expression by modulating chromatin structure, switching between “open” and “closed” states. The malaria parasite possesses four histone acetylases (HATs; “writers”), such as MYST and PfGNC5, which are instrumental in governing its epigenetic landscape. Among these HATs, one particular enzyme, PfMYST, emerges as a central figure. Found as an essential gene in the malaria parasite, PfMYST exhibits a pronounced preference for acetylating histone H4 at specific lysine residues (K5, K8, K12, and, to a lesser extent, K16). It is intricately involved in regulating the parasite cell cycle, and its overexpression results in a schizogony defect. Moreover, PfMYST is crucial for DNA damage repair, further underscoring its essentiality for the intraerythrocytic development cycle (IDC). These characteristics position PfMYST as a potential drug target for malaria treatment, given its vital role in the parasite’s biology and its potential as a point of intervention for therapeutic strategies [25,26]. In cancer, histone acetyltransferases of the MYST family were successfully explored as drug targets [27]. Accordingly in 2020, Sen and colleagues could show that the compound NU9056, which is active against the human TIP60 acetyl transferase, was also inhibitory for PfMYST [28]. The histone acetylase PfGCN5 was also addressed as a target. Kumar and colleagues were able to define that the compound C14 inhibited at three-digit nanomolar concentrations P. falciparum growth in vitro, while the toxicity of C14 in human fibroblasts was at least 40 times lower [29]. It appears that the natural compound curcumin also inhibits histone acetylases, as shown by Cui and colleagues [30], besides the induction of reactive oxygen species.
As epigenetic “erasers”, P. falciparum possesses five deacetylases (HDACs) in its genome. Among these, three show homology to mammalian class I/II HDACs and utilize Zn2+ as a cofactor. These are named Pf HDAC1, Pf HDAC2, and Pf HDAC3. Additionally, two deacetylases in the parasite exhibit a similarity to class III mammalian HDACs and function as NAD+-dependent enzymes [31,32]. It is worth noting that the disruption or knockout of Zn2+-containing Pf HDACs results in lethal phenotypes; therefore, these compounds have become the primary focus of extensive studies aimed at discovering inhibitors that target these essential enzymes [33]. Inhibiting these enzymes leads to genome-wide transcriptional alterations, ultimately resulting in the death of the parasite [33,34,35]. There are also two non-essential histone acetylases termed SIR2A and SIR2B, which are involved in variant (var, see below) gene regulation [36,37], but also somehow in sensing external stimuli and sexual differentiation [38,39].
While no antimalarial drug designed specifically to target HDAC has gained approval to date, research has focused on repurposing HDAC inhibitor drugs that have already been validated for other diseases, mainly cancer. These were tested to assess their antimalarial potential. Within this framework, two distinct chemotypes that have garnered significant attention are hydroxamic acids and cyclic tetrapeptides [40,41,42,43], of which apicidin, trichostatin A, SBHA, SB939, and four FDA-approved anticancer HDAC inhibitors (vorinostat, belinostat, panobinostat, and romidepsin) have been explored [44,45,46]. However, these compounds are plagued by either significant cytotoxicity or insufficient antimalarial efficacy. In response to these limitations, Jian Li’s research group has undertaken extensive efforts to develop improved derivatives of quisinostat (Qui) that exhibit enhanced efficacy and safety. Among the more than 60 derivatives assessed, two compounds, internally designated as compound 11 and JX35, have emerged as particularly promising. These compounds have demonstrated substantial in vitro potency against a range of multidrug-resistant malaria parasites, including two clinical isolates resistant to artemisinin. Notably, both compounds have showcased the ability to eradicate both liver and erythrocytic parasites in vivo, effectively eliminating all morphological erythrocytic parasites with a notable specificity for schizonts. Additionally, the compounds have displayed favorable attributes such as acceptable metabolic stability and pharmacokinetic properties. Furthermore, its inhibitory activity of compound JX35 during the gametocyte stage has also been established, proposing this prototype for innovation in malaria treatment [42,47].
In the quest for drugs effective not only against intraerythrocytic forms of the parasite but also the exo-erythrocytic stage of Plasmodium parasites [46], the potential development of HDAC inhibitors as causal prophylactic and/or transmission-blocking agents has emerged. This aligns with the overarching goal of malaria eradication. As part of this pursuit, the activity of an HDAC inhibitor containing an alkoxyamide connecting-unit linker region [48] was assessed across various stages of the parasite’s life cycle. This study led to the identification of the compound LMK235, which displayed IC50 values ranging from 0.09 to 1.12 mM [43].
In the quest to discover inhibitors for PfHDAC1, researchers have employed molecular docking techniques. In a groundbreaking 2008 study, Andrews and her colleagues identified a set of 26 compounds specifically designed to target PfHDAC1. Remarkably, 16 of these compounds exhibited robust in vitro antimalarial activity. These compounds inhibited P. falciparum at IC50 values up to tenfold lower compared with their inhibitory action against human cell lines [49]. In addition, the same group identified 14 compounds showcasing favorable molecular interactions with PfHDAC1’s catalytic zinc-bound active site, alluding to predicted affinities in the micromolar-to-nanomolar range for this enzyme. Among these, six compounds, characterized by a straightforward cysteine scaffold fused to benzylamine and 4-butanoyl hydroxamate, have demonstrated IC50 values spanning from 48 to 339 nM against multidrug-resistant P. falciparum strains. Simultaneously, the remaining seven inhibitors, based on 2-aminosuberic acid, have exhibited potent capabilities in inhibiting P. falciparum growth in vitro, targeting both multidrug-resistant and drug-sensitive strains, all while preserving a considerable selectivity for the parasite over mammalian cells [50].
In recent years, researchers have turned their attention to natural compounds as potential inhibitors, notably delving into extracts like cyclic tetrapeptide analogs sourced from Wardomyces dimerus. These compounds have exhibited potent antimalarial properties with remarkably low nanomolar potency and exceptional selectivity. Their mechanism of action involves inducing hyperacetylation of histone H4 and chromatin remodeling, demonstrating a preference for activity during the early life stages of the parasite. This distinctive profile contributes to their superior efficacy in eliminating malaria parasites [41].
The array of inhibitory compounds identified for this deacetylase, which has been established as vital for P. falciparum, and their success in the previously mentioned candidate molecules underscore the promise of this drug target in the quest for malaria control and elimination. However, it is crucial to acknowledge that despite the emergence of promising compounds, there is still a substantial journey ahead. Evidence of their efficacy in animal models remains lacking, representing an essential step for validating their effectiveness.
2.2. Histone Methyltransferases and Histone Demethylases
Histone methylation is also one of the major posttranslational modifications and is regulated by histone methyltransferases (HMTs, “writers”) and histone demethylases (HDMs, “erasers”) by adding or removing methyl groups from histone proteins [51,52,53]. Similar to acetylases deacetylases, these modifications can affect the overall chromatin structure and therefore the interaction of the nucleosomes with the DNA, which in turn modulates the transcriptional machinery’s accessibility of promoter regions in the DNA, thus promoting or inhibiting the transcriptional activity of specific genes [12,54]. Therefore, HMTs and HDMs as drivers of specific chromatin modifications have emerged as a promising target candidate in antimalarial drug development [52,55].
HMTs are writer enzymes and responsible for the addition of methyl groups to specific lysine or arginine residues on histone proteins, while HDMs as eraser enzymes catalyze the removal of methyl groups from histone proteins. Bearing a similarity to SET and HDAC proteins, plasmodial HMTs and HDMs function as important regulators of gene expression. The methylation patterns are of importance when it comes to maintaining the transcriptional state, and their modification can lead to a change in the steady state [54,56]. The coordination of gene expression has particularly been studied in the erythrocytic stages of the Plasmodium life cycle in the human host during the expression of virulence-associated var genes, and hereby turned out to be of particular importance in terms of controlling key developmental processes of the parasite [12,57,58]. In this context, the precise control of expression patterns affects various aspects of the parasite life cycle, including host invasion, differentiation, and antigenic variation [55,56,59].
While the acetylation H3K9ac is found to be associated with transcriptionally active genes throughout the entire erythrocytic life cycle, the methylation H3K4me3 is a typical posttranslational modification for active and poised genes in the blood stage of Plasmodium. Furthermore, H3K9me3 as a generally repressive histone modification is only detected in association with clonally variant gene families and telomeric regions [60]. In contrast to other organisms, this methylation does therefore not appear to be involved in the transcriptional repression of housekeeping genes in P. falciparum [58]. More precisely, the PTMs of H3K9me3 and H3K4me2/3 are especially involved in the monoallelic expression of the var gene family [59,60,61]. This gene family contains approximately 60 members encoding the erythrocyte membrane protein P. falciparum erythrocyte membrane protein 1 (PfEMP1) in the plasmodial blood stage. The expression of only one version of PfEMP1 at a time is associated with the pathogenesis and immune evasion by the parasite. Thus, this mutually exclusive expression requires a tight regulation to ensure that the majority of var genes are repressed, while only one var gene is actively transcribed [62,63]]. The expression of var genes depends on the epigenetic mechanisms described above, with the active var gene assuming an euchromatic and the silenced var genes being in a heterochromatic state. This also involves changes in the nuclear distribution of var gene loci [64,65]. Several experimental approaches revealed that the tri-methylation of lysine 4 on histone 3 (H3K4me3) is present in the active var gene, while var gene silencing is associated with H3K9 and H3K36 tri-methylation (H3K9me3, H3K36me3) [59,66,67]]. Furthermore, these methylation patterns especially differ between different stages of plasmodial development. The unique biology of each stage is underpinned by the expression of stage-specific gene sets, regulated at the epigenetic level [55]. Different machine learning models suggested, for example, that high levels of H4K20me3 marks correlate strongly with high expression in schizonts, while H3K4me3 marks are more present in active genes in the ring and trophozoite stages of Plasmodium [54,68].
Several histone modifiers have been described to be directly involved in the modifications of these histone marks localized on the var gene loci. Today, ten SET (Su(var)3–9-′Enhancer of zeste-Trithorax)-domain-containing lysine-specific HMTs (SET1 to SET10) and three arginine-specific HMTs (PRMT1, PRMT5 and CARM1) are known in P. falciparum [52,69]. In addition, four HDMs (LSD1, JmjC1, JmjC2, and Jmj3) are encoded in the genome of P. falciparum [67,70], and a second LSD was postulated [71]. Targeting these plasmodial enzymes with small molecule inhibitors offers a promising strategy to perhaps disrupt var gene expression control and potentially inhibits the parasite survival [12,54,72]. The selective inhibition of HMTs and HDMs activity can hereby either lead to an increase or decrease in transcription, depending on the position and degree of methylation, and ultimately contributes to transcriptional de-regulation and cell death. Since aberrant histone modifications are also involved in many forms of human cancers, HDMs have already been investigated as a promising class of targets for the development of new anti-cancer chemotherapies [73]. The development of selective inhibitors against the histone-modifying enzymes has already shown a promising reduction of the parasite’s viability and virulence in an intraerythrocytic stage-independent manner [60]. These inhibitors are proposed to perturb the balance of activating and repressing histone methylation marks, leading to altered gene expression profiles that hinder the proper development of the parasite [74]. Regarding HMTs, a recent report examined the effect of the G9a HMT inhibitor BIX-01294 on gene expression in gametocytic phases and its influence on transmission, plus the effect on asexual replication. A strong inhibition in two-digit nanomolar concentration was found for asexual parasites, and equally important, gametocyte stages were also impaired in their progress [75]. Recently, a study tested inhibitors against the Jumonji-class of demethylases in P. falciparum, and one inhibitor (JIB-04 E) successfully interfered in higher nanomolar concentrations of asexual and lower nanomolar concentrations of sexual parasite forms [70], probably by inhibiting PfJmj3 activity. The same study also found a strong effect on histone methylation, which explained the huge changes in the resulting transcriptome of JIB04-E-treated parasites. Differentially expressed genes also included transcriptional regulators such as PfAP-I (see below) and bromodomain protein 1, but also variant genes of the rifin family.
2.3. ApiAP2 Transcription Factors as Drug Targets: Implications for Malaria Control
The Apetala 2 factors, originally identified in plants, have expanded into the Apicomplexa phylum [15]. The ApiAP2 family has been the subject of ongoing studies and has been identified as an important class of transcriptional regulators that act as transcriptional activators or silencers in Plasmodium [19,76,77,78,79,80,81]. A total of 27 factors with AP2 domains were identified, and these factors do not have homologues in the human genome or the transmitting Anopheles vectors. In-depth studies that individually examine the function of these transcription factors, such as the recent paper by Russell and colleagues [82], are of great importance for strengthening and developing anti-malarial strategies. P. falciparum appears to utilize transcription factors from the ApiAP2 family to regulate gene expression, and at least 80% of protein-coding transcripts are regulated during the intraerythrocytic cycle [19]. Consequently, proteins belonging to the AP2 family play central roles in the genetic regulation of these parasites and assist in maintaining the complex life cycle of this apicomplexan. The transcription factors ApiAP2 that were individually approached in their function in the parasite’s life cycle within the vertebrate host organism are AP2-L [76], SIP2 [83], AP2-I [84], AP2SP3-TEL [85], AP2-O [86], AP2-EXP [81], AP2-G [87,88], AP2-G2 [89], AP2-G3 [78,90], AP2-G4 [71], and AP2-G5 [91]. The AP2-L factor is known to be essential for the development of the parasite within hepatocytes, and as of now, there are no records of another transcription factor (TF) acting during the hepatic phase of Plasmodium [76]. Described as recognizing motifs AATTTCC, the ortholog of PF3D7_0730300 (gene ID code in www.PlasmoDB.org) encodes a 1272-amino acid protein that possesses two AP2 domains. Studies on transcriptional and histone modifications have reported that AP2-L is overexpressed in sporozoite stages in P. falciparum [92], although its expression also occurs in the trophozoite and schizont stages [76,92]. The knockout of AP2-L in the P. berghei model of infection does not affect the invasion of sporozoites into hepatocytes; however, it causes disruptions in growth and cell division after 24 and 36 h, respectively [78]. Despite the hepatic phase of the parasite being a potent target for malaria infection drugs and vaccines, genetic regulation during this stage is not yet well elucidated.
During the phase of merozoite invasion into erythrocytes within the vertebrate host, the parasite expresses numerous surface proteins to facilitate this process. The transcription factor AP2-I, along with bromo domain protein 1 (PfBDP1) [93], is closely associated with the control of the expression of these genes [84]. This AP2-I protein is detected in the nuclei of trophozoites and schizonts, but is absent in newly invaded forms (ring stage), and its coding gene seems to possess the capability for autoregulation, a feature attributed to the inclusion of its own DNA-binding motif among its regulatory targets, as elucidated by Santos et al. in 2017 [84].
Due to its importance for the intraerythrocytic developmental stage, Oladejo et al. (2023) conducted in silico predictions of various molecules with potential inhibitors of the binding of the AP2-I domain to its DNA-binding motif. Five compounds were considered suitable for post-docking studies, with the molecule MCULE-7146940834 being indicated as the main candidate. Experimental preclinical validations are required to assess its effectiveness as an AP2-I inhibitor [94].
The transcription factor PfSIP2 (PF3D7_0604100) is associated with heterochromatin formation and genome integrity rather than transcriptional regulation. During schizogony, the 60 kDa PfSIP2 protein binds to SPE2 motifs in the N-terminal portion using only one of its two DNA-binding domains, the D1 domain, and its deletion is refractory [19,83]. The recognition sequence of PfSIP2 is located upstream of subtelomeric var genes in UpsB regions associated with telomeres [19]. In an in-silico study, approximately 700 sites were targeted by PfSIP2, of which 94% correspond to two distinct regions of subtelomeric heterochromatin, one to tandem sequences located upstream of this region in var genes, and the other associated with 2/3 of repetitive elements within telomeric regions (TAREs). These results suggest a multifunctional role for this protein and its potential role in heterochromatin formation [83]. It is important to highlight that in the case of both P. falciparum and the murine species P. berghei, the SIP2 transcription factor cannot be deleted, indicating its essential role in the intraerythrocytic cycle of the parasite [83,95]. This warrants further studies for finding inhibitors of PfSIP2.
PfAP2-Tel, encoded by PF3D7_0622900, has a size of 237 kDa and contains a single 46-amino acid AP2 domain. Despite this AP2 domain showing a smaller size than the average of other AP2 domains in P. falciparum, this domain enables PfAP2-Tel to directly bind to telomeric repeats [85]. Its expression occurs during the blood stage with peaks of enrichment at all 28 telomeres of P. falciparum, and also in important gene families such as var, rifin, stevor, and Pfmc-2TM [85]. This indicates that PfAP2-Tel may have a function related to the maintenance of chromosomal ends. However, this protein is deemed non-essential, given that no change in parasite fitness was observed [96]. Along with PfSIP2 and PfAP2-Tel, PfAP2-HC also has a primary function related to heterochromatin and the end biology of the chromosome [97]. The results regarding the expression pattern of PfAP2-HC, for a better understanding of its function in the parasite’s genome, showed that the protein was detectable in trophozoites and in immature schizonts, but was not enriched in the predicted target motifs CACACA [19]. It was also found that its AP2 domain is dispensable for DNA binding. In order to associate with heterochromatin, PfAP2-HC is dependent on PfHP1 which, like the trimethylation of histone 3 lysine 9 (H3K9me3), is closely associated with condensed chromatin regions [98]. Interestingly, the knockout of PfAP2-HC leads to the almost complete silencing of var genes, suggesting an interaction with chromatin modifiers [99], such as the non-recruiting of SET/HMT proteins to var loci. The efficient blockage of PfAP2-HC may make IRBC less adhesive, given that var genes are suppressed and the corresponding PfEMP1, which mediates cytoadherence to endothelial cells [100], may be not properly expressed. Theoretically, this inhibition would render IRBC vulnerable to splenic clearance.
Gametocytes/gamonts are the forms ingested by the mosquito vector which ensure the transmission of the parasite to this host. These originate from the differentiation of asexual cells and undergo a series of developmental stages, encompassing commitment, conversion, and sexual maturation [101]. ApiAP2-G is the master regulator of sexual commitment [87,88,102], a stage that may occur before schizogony, referred to as the conversion route in the next cycle of the expression of AP2-G. In P. falciparum, it can also occur at the beginning of the ring stage, resulting in the same cycle conversion [88,103]. In parasites that do not express AP2-G, the gene encoding this protein, PF3D7_1222600 (ortholog PBANKA_143750/ PY17X_1440000), is found in a heterochromatic state, regulated by the epigenetic mark H3K9me3, histone deacetylase 2 (PfHda2), and associations with heterochromatin protein 1 (PfHP1) [37,104]. The stabilization of AP2-G expression is achieved by the removal of these bindings through mechanisms involving the protein GDV1 (gametocyte development 1), which acts as a positive regulator of sexual commitment.
Predicted to bind to DNA through the recognition of the motif (Gx)GTACNC [19], it was identified that these binding regions were also present upstream of the AP2-G-encoding gene itself, indicating the existence of the control of its expression through positive feedback [87]. Furthermore, AP2-G appears to be located at the top of a specific transcriptional cascade of gametocytogenesis, in which AP2-G2, AP2-FG, and AP2-O3 are directly influenced by this master regulator [105].
Many genes regulated by PfAP2-G are also targets of the transcription factor PfAP2-I, and studies indicate their possible direct and joint action on these promoters, an important mechanism that increases gene regulation specificity [106]. It is important to note that while AP2-G is continuously observed in P. falciparum erythrocytic stages at low levels, in P. berghei and P. yoelii, its expression occurs only for a short period, suggesting divergence in the sexual development between these species [90,105]. Although the genomic loci where PfAP2-G associates were discovered [106], it is currently not known which factors physically interact with the factor and if PfAP2-G binds as a multimer or heteromer. This lack of information is also true for all other plasmodial AP2 factors.
Another transcription factor that was identified in Plasmodium spp. with important functions during gametogenesis is AP2-G2 (PF3D7_1408200/PBANKA_1034300), which is expressed in sexually committed ring forms and demonstrates protein expression during the trophozoite and schizont stages [107]. Knockout assays revealed that even in the absence of its gene disruption, there is no discernible impact on the sexual commitment phase of the parasites. However, AP2-G2 knockout parasites were unable to develop beyond the stage III of gametocytes, leading to a blockage in transmission to the vector [78,89,107]. Consequently, AP2-G2 appears to assume a critical role after the activation of AP2-G within the cascade of ApiAP2 proteins associated with gametogenesis [87]. ChIP-seq analyses revealed that the binding motif of AP2-G2 was identified in regulatory regions of 1500 genes, and although many of these targets were related to proliferation in the blood stage [89], no negative effects were observed in these genes during the knockout of AP2-G2. Another transcription factor, ApiAP2-L, which is critical for liver stage development, positively regulated AP2-G2 knocked-out gametocytes in P. falciparum and P. berghei, resulting in the inability of these parasites to trigger liver infection [95,107]. Together, these data suggest that AP2-G2 acts as a repressor during the sexual and asexual stages. In contrast, Xu and colleagues (2021) [108] identified that the deletion of PfAP2-G2 in P. falciparum had a repressive effect on the expression of PfMDV-1 (male development gene) in asexual stages, suggesting the possibility of a dual role for this protein as a repressor and activator of specific genes [101]. Furthermore, PfAP2-G2 shares 80% of its binding sites with epigenetic silencing marks H3K36me3, indicating the potential for collaborative interactions between them [107]. A third AP2 factor related to the gametogenesis phase, AP2-G3, appears to act upstream of AP2-G, translating cytosolic signals into the nucleus and influencing AP2-G transcription [90,109]. In P. berghei, this transcription factor is essential for the regulation of genes specific to the formation of female gametocytes, and is named AP2-FG for this species [110]. PfAP2-G5, identified by Shang et al. [91], was considered indispensable for the gametocyte development phase as it binds to the regulatory region of PfAP2-G, inhibiting its activation and that of its target genes, preventing the initiation of sexual commitment. According to the authors, the role of AP2-G5 may be involved in various physiological processes beyond gametogenesis, such as parasite–host interaction remodeling, pathogenesis, and others [91]. The blockage or inhibition of the function of PfAP2-G5 may therefore be useful to render parasites into sexual stage forms which can no longer replicate, nor be transmitted, due to its dual role in controlling AP2-G production and gametocyte maturation. This issue has not yet been approached.
Studies conducted with AP2-exp (PF3D7_1466400) suggest that this protein is involved in the asexual cycle of P. falciparum, while its ortholog is a strong regulator of sporogony in a murine model for P. berghei (PbAP2-Sp). PfAP2-exp appears to be also involved in the regulation of multigene families, including Rif, Stevor, and Pfmc-2tm [81]. This important transcription factor may be related to the control of parasite virulence, as binding sites in the promoters of var genes have been predicted [19]. The association of PfAP2-exp with accessible chromatin regions [99] and its participation in the PfGCN5 acetyltransferase complex [111] are responsible for preferentially acetylating histone 3 at lysine 9 and lysine 14 (K9, K14), which is considered an important mark related to active genes. The presence of the protein is detectable in the nucleus of schizonts in P. falciparum. Apparently, this transcription factor is also involved in the asexual growth of the parasite, as the complete deletion of PfAP2-exp failed [81,99]. This reinforces the hypothesis that PfAP2-exp may also be considered as a useful drug target, as recently shown by Russell and colleagues [82].
Another member of the ApiAP2 family, AP2-O (PF3D7_1143100), was the first member of the AP2 family with a function specifically related to the morphology or formation of ookinetes and oocysts, and these functions were characterized in P. berghei (PBANKA_0905900) [76,79]. To date, only few studies describe the function or active participation of this transcription factor in the asexual phase of the parasite, specifically during the intraerythrocytic stage. Blocking the AP2-O protein through knockout in P. berghei was demonstrated in Anopheles mosquitoes, where the ookinete phenotype was aberrant, showing an inability to invade the mosquito’s midgut [79]. Additional data demonstrated that AP2-O binds to the promoters of most genes dysregulated in the parasite [77,79], and it may have a role in the asexual cycle, influencing the parasite’s growth within erythrocytes [78]. In P. falciparum, AP2-O is involved in the transmission stages and regulation of virulence genes, such as var genes. However, the knockout of this transcription factor is refractory, and the knockdown via a destabilizing domain resulted in no visible phenotype in the erythrocytic stages of the parasite [86], probably due to the leakiness of the type of knockdown used. Recently, another AP2 factor – PfAP2-P - was found to be essential during the blood stage. The factor apparently controlled a vast number of transcripts important not only for egress/invasion, but also transcripts from variant family genes and gametocyte developmental markers [112], indicating a very important general role for this factor, at least in P. falciparum. Surely, inhibitors of PfAP2-P would probably have an immediate and huge impact on parasite proliferation, warranting further research on this factor. The inferred function and tested druggability of all AP2 factors are resumed in Table 1.

Table 1.
Regulation of Plasmodium Parasites by the ApiAP2 factors.
Taken together, despite the significant importance of the transcription factors described here for the parasite’s development cycle in humans, few studies have been conducted so far using these proteins as targets for new drugs [82,94]. The absence of AP2 domains in humans makes them an attractive target to block the binding of these proteins to DNA or to their mostly unknown cofactors, which may result in profound transcriptional changes and ultimately parasite death.
3. Conclusions
The accurate regulation of transcription in the haploid protozoan Plasmodium is of pivotal importance for its survival in different, mostly hostile environments. A major handicap for drug development against the factors discussed herein is probably the current lack of detailed structural information, such as crystal structures—this makes structure-guided drug development a difficult task. The development of more accurate computational structure predictions may help in this issue. Also, the current gap in information concerning how writing/reading/erasing complexes form at the molecular level hinders the rational development of molecules which may crucially interfere with the formation of these complexes. In this regard, the elucidation of temporarily interacting components may be approached by pulldown experiments or by proximity-based biotinylation, as shown by Birnbaum and colleagues [115]. Unfortunately, both techniques are time-consuming and rely on the systematic creation of transfectant lines, which is faster in murine Plasmodium species, but have the disadvantage that the results for P. berghei possibly may not translate directly to the human Plasmodium species.
Author Contributions
L.F.O.S., G.M.d.O., E.H.N.C., N.W. and F.S.d.P. wrote the draft, G.W. conceptualized, reviewed and edited the manuscript, and acquired funding, C.W. acquired funding and reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP (Fundação de Apoio a Pesquisa do Estado de São Paulo), grant numbers 2021/13727-2 (G.W.), 2015/26722-8 (C.W.), and master or doctoral fellowships provided by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) within the the post-graduation programme “Biology of Host-Pathogen Interactions”.G.W. and C.W. are CNPq Research fellows.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used were from public databases, see references.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- WHO. Word Malaria Report 2021; WHO: Genva, Switzerland, 2021. [Google Scholar]
- Burda, P.C.; Roelli, M.A.; Schaffner, M.; Khan, S.M.; Janse, C.J.; Heussler, V.T. A Plasmodium Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane. PLoS Pathog. 2015, 11, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Formaglio, P.; Thiberge, S.; Mordelet, E.; Van Rooijen, N.; Medvinsky, A.; Ménard, R.; Amino, R. Role of Host Cell Traversal by the Malaria Sporozoite during Liver Infection. J. Exp. Med. 2013, 210, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Amino, R.; van de Sand, C.; Regen, T.; Retzlaff, S.; Rennenberg, A.; Krueger, A.; Pollok, J.-M.; Menard, R.; Heussler, V.T. Manipulation of Host Hepatocytes by the Malaria Parasite for Delivery into Liver Sinusoids. Science 2006, 313, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.Y.; Blackman, M.J. Malaria Parasite Egress at a Glance. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef] [PubMed]
- Sinden, R.E. The Biology of Malaria Transmission. In Recent Advances in Malaria Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 87–124. ISBN 9781118493816. [Google Scholar]
- Miao, J.; Fan, Q.; Cui, L.; Li, J.; Li, J.; Cui, L. The Malaria Parasite Plasmodium Falciparum Histones: Organization, Expression, and Acetylation. Gene 2006, 369, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Papenfuss, A.T.; Mair, G.R.; Janse, C.J.; Vlachou, D.; Waters, A.P.; Cowman, A.F.; Crabb, B.S.; de Koning-Ward, T.F. Molecular Genetics and Comparative Genomics Reveal RNAi Is Not Functional in Malaria Parasites. Nucleic Acids Res. 2009, 37, 3788–3798. [Google Scholar] [CrossRef]
- Trelle, M.B.; Salcedo-Amaya, A.M.; Cohen, A.M.; Stunnenberg, H.G.; Jensen, O.N. Global Histone Analysis by Mass Spectrometry Reveals a High Content of Acetylated Lysine Residues in the Malaria Parasite Plasmodium Falciparum. J. Proteome Res. 2009, 8, 3439–3450. [Google Scholar] [CrossRef]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Jabeena, C.A.; Rajavelu, A. Epigenetic Players of Chromatin Structure Regulation in Plasmodium Falciparum. Chembiochem 2019, 20, 1225–1230. [Google Scholar] [CrossRef]
- Callebaut, I.; Prat, K.; Meurice, E.; Mornon, J.-P.; Tomavo, S. Prediction of the General Transcription Factors Associated with RNA Polymerase II in Plasmodium Falciparum: Conserved Features and Differences Relative to Other Eukaryotes. BMC Genom. 2005, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Coulson, R.M.R.; Hall, N.; Ouzounis, C.A. Comparative Genomics of Transcriptional Control in the Human Malaria Parasite Plasmodium Falciparum. Genome Res. 2004, 14, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Babu, M.M.; Iyer, L.M.; Aravind, L. Discovery of the Principal Specific Transcription Factors of Apicomplexa and Their Implication for the Evolution of the AP2-Integrase DNA Binding Domains. Nucleic Acids Res. 2005, 33, 3994–4006. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Meyerowitz, E.M. The AP2/EREBP Family of Plant Transcription Factors. Biol. Chem. 1998, 379, 633–654. [Google Scholar] [CrossRef]
- Jeninga, M.D.; Quinn, J.E.; Petter, M. ApiAP2 Transcription Factors in Apicomplexan Parasites. Pathogens 2019, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- De Silva, E.K.; Gehrke, A.R.; Olszewski, K.; León, I.; Chahal, J.S.; Bulyk, M.L.; Llinás, M. Specific DNA-Binding by Apicomplexan AP2 Transcription Factors. Proc. Natl. Acad. Sci. USA 2008, 105, 8393–8398. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.L.; De Silva, E.K.; Olszewski, K.L.; Elemento, O.; Llinás, M. Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite. PLoS Pathog. 2010, 6, e1001165. [Google Scholar] [CrossRef] [PubMed]
- Bartfai, R.; Hoeijmakers, W.A.; Salcedo-Amaya, A.M.; Smits, A.H.; Janssen-Megens, E.; Kaan, A.; Treeck, M.; Gilberger, T.W.; Francoijs, K.J.; Stunnenberg, H.G. H2A.Z Demarcates Intergenic Regions of the Plasmodium Falciparum Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3. PLoS Pathog. 2010, 6, e1001223. [Google Scholar] [CrossRef]
- Foley, M.; Tilley, L. Quinoline Antimalarials: Mechanisms of Action and Resistance and Prospects for New Agents. Pharmacol. Ther. 1998, 79, 55–87. [Google Scholar] [CrossRef]
- Malaria Policy Advisory Committee Meeting. Available online: https://www.who.int/groups/malaria-policy-advisory-group (accessed on 11 January 2023).
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The Birth of Artemisinin. Pharmacol. Ther. 2020, 216, 107658. [Google Scholar] [CrossRef] [PubMed]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.B.; Munguti, K.; et al. Emergence and Clonal Expansion of in Vitro Artemisinin-Resistant Plasmodium Falciparum Kelch13 R561H Mutant Parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J. Chromatin-Mediated Epigenetic Regulation in the Malaria Parasite Plasmodium Falciparum. Eukaryot. Cell 2010, 9, 1138–1149. [Google Scholar] [CrossRef]
- Joshi, M.B.; Lin, D.T.; Chiang, P.H.; Goldman, N.D.; Fujioka, H.; Aikawa, M.; Syin, C. Molecular Cloning and Nuclear Localization of a Histone Deacetylase Homologue in Plasmodium Falciparum. Mol. Biochem. Parasitol. 1999, 99, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Leaver, D.J.; Hermans, S.J.; Kelly, G.L.; Brennan, M.S.; Downer, N.L.; Nguyen, N.; Wichmann, J.; McRae, H.M.; Yang, Y.; et al. Inhibitors of Histone Acetyltransferases KAT6A/B Induce Senescence and Arrest Tumour Growth. Nature 2018, 560, 253–257. [Google Scholar] [CrossRef]
- Sen, U.; Nayak, A.; Khurana, J.; Sharma, D.; Gupta, A. Inhibition of PfMYST Histone Acetyltransferase Activity Blocks Plasmodium Falciparum Growth and Survival. Antimicrob. Agents Chemother. 2020, 65. [Google Scholar] [CrossRef]
- Kumar, A.; Bhowmick, K.; Vikramdeo, K.S.; Mondal, N.; Subbarao, N.; Dhar, S.K. Designing Novel Inhibitors against Histone Acetyltransferase (HAT: GCN5) of Plasmodium falciparum. Eur. J. Med. Chem. 2017, 138, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Miao, J.; Cui, L. Cytotoxic Effect of Curcumin on Malaria Parasite Plasmodium Falciparum: Inhibition of Histone Acetylation and Generation of Reactive Oxygen Species. Antimicrob. Agents Chemother. 2007, 51, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Kanyal, A.; Rawat, M.; Gurung, P.; Choubey, D.; Anamika, K.; Karmodiya, K. Genome-Wide Survey and Phylogenetic Analysis of Histone Acetyltransferases and Histone Deacetylases of Plasmodium falciparum. FEBS J. 2018, 285, 1767–1782. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Pradhan, A.; Shah, F.; Tekwani, B.L.; Avery, M.A. Structural Insights into the Plasmodium Falciparum Histone Deacetylase 1 (PfHDAC-1): A Novel Target for the Development of Antimalarial Therapy. Bioorg Med. Chem. 2008, 16, 5254–5265. [Google Scholar] [CrossRef] [PubMed]
- Chaal, B.K.; Gupta, A.P.; Wastuwidyaningtyas, B.D.; Luah, Y.H.; Bozdech, Z. Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Plasmodium falciparum Life Cycle. PLoS Pathog. 2010, 6, e1000737. [Google Scholar] [CrossRef]
- Hu, G.; Cabrera, A.; Kono, M.; Mok, S.; Chaal, B.K.; Haase, S.; Engelberg, K.; Cheemadan, S.; Spielmann, T.; Preiser, P.R.; et al. Transcriptional Profiling of Growth Perturbations of the Human Malaria Parasite Plasmodium falciparum. Nat. Biotechnol. 2010, 28, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Gupta, A.P.; Tran, T.N.; Fairlie, D.P.; Gobert, G.N.; Bozdech, Z. Comparative Gene Expression Profiling of P. falciparum Malaria Parasites Exposed to Three Different Histone Deacetylase Inhibitors. PLoS ONE 2012, 7, e31847. [Google Scholar] [CrossRef]
- Tonkin, C.J.; Carret, C.K.; Duraisingh, M.T.; Voss, T.S.; Ralph, S.A.; Hommel, M.; Duffy, M.F.; Silva, L.M.d.; Scherf, A.; Ivens, A.; et al. Sir2 Paralogues Cooperate to Regulate Virulence Genes and Antigenic Variation in Plasmodium falciparum. PLoS Biol. 2009, 7, e84. [Google Scholar] [CrossRef]
- Coleman, B.I.; Skillman, K.M.; Jiang, R.H.Y.; Childs, L.M.; Altenhofen, L.M.; Ganter, M.; Leung, Y.; Goldowitz, I.; Kafsack, B.F.C.; Marti, M.; et al. A Plasmodium falciparum Histone Deacetylase Regulates Antigenic Variation and Gametocyte Conversion. Cell Host Microbe 2014, 16, 177–186. [Google Scholar] [CrossRef]
- Mancio-Silva, L.; Slavic, K.; Grilo Ruivo, M.T.; Grosso, A.R.; Modrzynska, K.K.; Vera, I.M.; Sales-Dias, J.; Gomes, A.R.; MacPherson, C.R.; Crozet, P.; et al. Nutrient Sensing Modulates Malaria Parasite Virulence. Nature 2017, 547, 213–216. [Google Scholar] [CrossRef]
- Harris, C.T.; Tong, X.; Campelo, R.; Marreiros, I.M.; Vanheer, L.N.; Nahiyaan, N.; Zuzarte-Luís, V.A.; Deitsch, K.W.; Mota, M.M.; Rhee, K.Y.; et al. Sexual Differentiation in Human Malaria Parasites Is Regulated by Competition between Phospholipid Metabolism and Histone Methylation. Nat. Microbiol. 2023, 8, 1280–1292. [Google Scholar] [CrossRef]
- Huang, Z.; Li, R.; Tang, T.; Ling, D.; Wang, M.; Xu, D.; Sun, M.; Zheng, L.; Zhu, F.; Min, H.; et al. A Novel Multistage Antiplasmodial Inhibitor Targeting Plasmodium falciparum Histone Deacetylase 1. Cell Discov. 2020, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.E.; Lee, J.W.; Bohmer, M.J.; Welden, J.D.; Arshadi, A.K.; Du, L.; Cichewicz, R.H.; Chakrabarti, D. Cyclic Tetrapeptide HDAC Inhibitors with Improved Plasmodium falciparum Selectivity and Killing Profile. ACS Infect. Dis. 2021, 7, 2889–2903. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ling, D.; Tang, T.; Huang, Z.; Wang, M.; Ding, Y.; Liu, T.; Wei, H.; Xu, W.; Mao, F.; et al. Discovery of Novel Plasmodium falciparum HDAC1 Inhibitors with Dual-Stage Antimalarial Potency and Improved Safety Based on the Clinical Anticancer Drug Candidate Quisinostat. J. Med. Chem. 2021, 64, 2254–2271. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.; Sumanadasa, S.; Stenzel, K.; Duffy, S.; Meister, S.; Marek, L.; Schmetter, R.; Kuna, K.; Hamacher, A.; Mordmüller, B.; et al. Discovery of HDAC Inhibitors with Potent Activity against Multiple Malaria Parasite Life Cycle Stages. Eur. J. Med. Chem. 2014, 82, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Haque, A.; Jones, M.K. HDAC Inhibitors in Parasitic Diseases. Immunol. Cell Biol. 2012, 90, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.J.; Arnold, M.S.J.; Xu, W.; Lancelot, J.; Lamotte, S.; Späth, G.F.; Prina, E.; Pierce, R.J.; Fairlie, D.P.; Skinner-Adams, T.S.; et al. Effect of Clinically Approved HDAC Inhibitors on Plasmodium, Leishmania and Schistosoma Parasite Growth. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Sumanadasa, S.D.M.; Goodman, C.D.; Lucke, A.J.; Skinner-Adams, T.; Saham, I.; Haque, A.; Do, T.A.; McFadden, G.I.; Fairlie, D.P.; Andrews, K.T. Antimalarial Activity of the Anticancer Histone Deacetylase Inhibitor SB939. Antimicrob. Agents Chemother. 2012, 56, 3849–3856. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, T.; Li, R.; Huang, Z.; Ling, D.; Zheng, L.; Ding, Y.; Liu, T.; Xu, W.; Zhu, F.; et al. Drug Repurposing of Quisinostat to Discover Novel Plasmodium falciparum HDAC1 Inhibitors with Enhanced Triple-Stage Antimalarial Activity and Improved Safety. J. Med. Chem. 2022, 65, 4156–4181. [Google Scholar] [CrossRef] [PubMed]
- Marek, L.; Hamacher, A.; Hansen, F.K.; Kuna, K.; Gohlke, H.; Kassack, M.U.; Kurz, T. Histone Deacetylase (HDAC) Inhibitors with a Novel Connecting Unit Linker Region Reveal a Selectivity Profile for HDAC4 and HDAC5 with Improved Activity against Chemoresistant Cancer Cells. J. Med. Chem. 2013, 56, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, N.C.; Andrews, K.T.; Tran, T.L.; Lucke, A.J.; Reid, R.C.; Fairlie, D.P. Antimalarial Histone Deacetylase Inhibitors Containing Cinnamate or NSAID Components. Bioorg Med. Chem. Lett. 2010, 20, 7080–7084. [Google Scholar] [CrossRef]
- Andrews, K.T.; Tran, T.N.; Lucke, A.J.; Kahnberg, P.; Le, G.T.; Boyle, G.M.; Gardiner, D.L.; Skinner-Adams, T.S.; Fairlie, D.P. Potent Antimalarial Activity of Histone Deacetylase Inhibitor Analogues. Antimicrob. Agents Chemother. 2008, 52, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef]
- Cui, L.; Fan, Q.; Miao, J. Histone Lysine Methyltransferases and Demethylases in Plasmodium falciparum. Int. J. Parasitol. 2008, 38, 1083–1097. [Google Scholar] [CrossRef]
- Martin, C.; Zhang, Y. The Diverse Functions of Histone Lysine Methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Hollin, T.; Le Roch, K.G. From Genes to Transcripts, a Tightly Regulated Journey in Plasmodium. Front. Cell Infect. Microbiol. 2020, 10, 618454. [Google Scholar] [CrossRef]
- Connacher, J.; von Grüning, H.; Birkholtz, L. Histone Modification Landscapes as a Roadmap for Malaria Parasite Development. Front. Cell Dev. Biol. 2022, 10, 848797. [Google Scholar] [CrossRef]
- Coetzee, N.; Sidoli, S.; Van Biljon, R.; Painter, H.; Llinás, M.; Garcia, B.A.; Birkholtz, L.M. Quantitative Chromatin Proteomics Reveals a Dynamic Histone Post-Translational Modification Landscape That Defines Asexual and Sexual Plasmodium falciparum Parasites. Sci. Rep. 2017, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.F.; Selvarajah, S.A.; Josling, G.A.; Petter, M. Epigenetic Regulation of the Plasmodium falciparum Genome. Brief. Funct. Genom. 2014, 13, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, J.J.; Mancio-Silva, L.; Scherf, A. Genome-Wide Analysis of Heterochromatin Associates Clonally Variant Gene Regulation with Perinuclear Repressive Centers in Malaria Parasites. Cell Host Microbe 2009, 5, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Comeaux, C.A.; Duraisingh, M.T. Unravelling a Histone Code for Malaria Virulence. Mol. Microbiol. 2007, 66, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Malmquist, N.A.; Moss, T.A.; Mecheri, S.; Scherf, A.; Fuchter, M.J. Small-Molecule Histone Methyltransferase Inhibitors Display Rapid Antimalarial Activity against All Blood Stage Forms in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2012, 109, 16708–16713. [Google Scholar] [CrossRef]
- Volz, J.C.; Bártfai, R.; Petter, M.; Langer, C.; Josling, G.A.; Tsuboi, T.; Schwach, F.; Baum, J.; Rayner, J.C.; Stunnenberg, H.G.; et al. PfSET10, a Plasmodium falciparum Methyltransferase, Maintains the Active Var Gene in a Poised State during Parasite Division. Cell Host Microbe 2012, 11, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Scherf, A.; Hernandez-Rivas, R.; Buffet, P.; Bottius, E.; Benatar, C.; Pouvelle, B.; Gysin, J.; Lanzer, M. Antigenic Variation in Malaria: In Situ Switching, Relaxed and Mutually Exclusive Transcription of Var Genes during Intra-Erythrocytic Development in Plasmodium falciparum. EMBO J. 1998, 17, 5418–5426. [Google Scholar] [CrossRef]
- Chen, Q.; Fernandez, V.; Sundstrom, A.; Schlichtherle, M.; Datta, S.; Hagblom, P.; Wahlgren, M. Developmental Selection of Var Gene Expression in Plasmodium falciparum. Nature 1998, 394, 392–395. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Zhang, Y.; Fang, X.; Claes, A.; Duchateau, M.; Namane, A.; Lopez-Rubio, J.J.; Pan, W.; Scherf, A. A Critical Role of Perinuclear Filamentous Actin in Spatial Repositioning and Mutually Exclusive Expression of Virulence Genes in Malaria Parasites. Cell Host Microbe 2011, 10, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.A.; Scheidig-Benatar, C.; Scherf, A. Antigenic Variation in Plasmodium falciparum Is Associated with Movement of Var Loci between Subnuclear Locations. Proc. Natl. Acad. Sci. USA 2005, 102, 5414–5419, ISBN 0027-8424 (Print) 0027-8424 (Linking). [Google Scholar] [CrossRef]
- Duraisingh, M.T.; Voss, T.S.; Marty, A.J.; Duffy, M.F.; Good, R.T.; Thompson, J.K.; Freitas-Junior, L.H.; Scherf, A.; Crabb, B.S.; Cowman, A.F. Heterochromatin Silencing and Locus Repositioning Linked to Regulation of Virulence Genes in Plasmodium falciparum. Cell 2005, 121, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Mu, J.; Zhang, Q.; Ni, T.; Srinivasan, P.; Rayavara, K.; Yang, W.; Turner, L.; Lavstsen, T.; Theander, T.G.; et al. PfSETvs Methylation of Histone H3K36 Represses Virulence Genes in Plasmodium falciparum. Nature 2013, 499, 223–227. [Google Scholar] [CrossRef]
- Read, D.F.; Cook, K.; Lu, Y.Y.; Le Roch, K.G.; Noble, W.S. Predicting Gene Expression in the Human Malaria Parasite Plasmodium falciparum Using Histone Modification, Nucleosome Positioning, and 3D Localization Features. PLoS Comput. Biol. 2019, 15, e1007329. [Google Scholar] [CrossRef]
- Volz, J.; Carvalho, T.G.; Ralph, S.A.; Gilson, P.; Thompson, J.; Tonkin, C.J.; Langer, C.; Crabb, B.S.; Cowman, A.F. Potential Epigenetic Regulatory Proteins Localise to Distinct Nuclear Sub-Compartments in Plasmodium falciparum. Int. J. Parasitol. 2010, 40, 109–121. [Google Scholar] [CrossRef]
- Matthews, K.A.; Senagbe, K.M.; Nötzel, C.; Gonzales, C.A.; Tong, X.; Rijo-Ferreira, F.; Bhanu, N.V.; Miguel-Blanco, C.; Lafuente-Monasterio, M.J.; Garcia, B.A.; et al. Disruption of the Plasmodium falciparum Life Cycle through Transcriptional Reprogramming by Inhibitors of Jumonji Demethylases. ACS Infect. Dis. 2021, 6, 1058–1075. [Google Scholar] [CrossRef] [PubMed]
- Poran, A.; Nötzel, C.; Aly, O.; Mencia-Trinchant, N.; Harris, C.T.; Guzman, M.L.; Hassane, D.C.; Elemento, O.; Kafsack, B.F.C. Single-Cell RNA Sequencing Reveals a Signature of Sexual Commitment in Malaria Parasites. Nature 2017, 551, 95. [Google Scholar] [CrossRef]
- Salcedo-Amaya, A.M.; van Driel, M.A.; Alako, B.T.; Trelle, M.B.; van den Elzen, A.M.; Cohen, A.M.; Janssen-Megens, E.M.; van de Vegte-Bolmer, M.; Selzer, R.R.; Iniguez, A.L.; et al. Dynamic Histone H3 Epigenome Marking during the Intraerythrocytic Cycle of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2009, 106, 9655–9660. [Google Scholar] [CrossRef]
- Copeland, R.A.; Solomon, M.E.; Richon, V.M. Protein Methyltransferases as a Target Class for Drug Discovery. Nat. Rev. Drug Discov. 2009, 8, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, N.; von Grüning, H.; Opperman, D.; van der Watt, M.; Reader, J.; Birkholtz, L.M. Epigenetic Inhibitors Target Multiple Stages of Plasmodium falciparum Parasites. Sci. Rep. 2020, 10, 2355. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, C.J.; Kiesow, M.J.; Orchard, L.M.; Farrukh, A.; Llinás, M.; Pradel, G. The G9a Histone Methyltransferase Inhibitor BIX-01294 Modulates Gene Expression during Plasmodium falciparum Gametocyte Development and Transmission. Int. J. Mol. Sci. 2019, 20, 5087. [Google Scholar] [CrossRef]
- Iwanaga, S.; Kaneko, I.; Kato, T.; Yuda, M. Identification of an AP2-Family Protein That Is Critical for Malaria Liver Stage Development. PLoS ONE 2012, 7, e47557. [Google Scholar] [CrossRef]
- Kaneko, I.; Iwanaga, S.; Kato, T.; Kobayashi, I.; Yuda, M. Genome-Wide Identification of the Target Genes of AP2-O, a Plasmodium AP2-Family Transcription Factor. PLoS Pathog. 2015, 11, e1004905. [Google Scholar] [CrossRef]
- Modrzynska, K.; Pfander, C.; Chappell, L.; Yu, L.; Suarez, C.; Dundas, K.; Gomes, A.R.; Goulding, D.; Rayner, J.C.; Choudhary, J.; et al. A Knockout Screen of ApiAP2 Genes Reveals Networks of Interacting Transcriptional Regulators Controlling the Plasmodium Life Cycle. Cell Host Microbe 2017, 21, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yuda, M.; Iwanaga, S.; Shigenobu, S.; Mair, G.R.; Janse, C.J.; Waters, A.P.; Kato, T.; Kaneko, I. Identification of a Transcription Factor in the Mosquito-Invasive Stage of Malaria Parasites. Mol. Microbiol. 2009, 71, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Yuda, M.; Iwanaga, S.; Shigenobu, S.; Kato, T.; Kaneko, I. Transcription Factor AP2-Sp and Its Target Genes in Malarial Sporozoites. Mol. Microbiol. 2010, 75, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Macpherson, C.R.; Claes, A.; Scheidig-Benatar, C.; Sakamoto, H.; Yam, X.Y.; Preiser, P.; Goel, S.; Wahlgren, M.; Sismeiro, O.; et al. An ApiAP2 Member Regulates Expression of Clonally Variant Genes of the Human Malaria Parasite Plasmodium falciparum. Sci. Rep. 2017, 7, 14042. [Google Scholar] [CrossRef]
- Russell, T.J.; De Silva, E.K.; Crowley, V.M.; Shaw-Saliba, K.; Dube, N.; Josling, G.; Pasaje, C.F.A.; Kouskoumvekaki, I.; Panagiotou, G.; Niles, J.C.; et al. Inhibitors of ApiAP2 Protein DNA Binding Exhibit Multistage Activity against Plasmodium Parasites. PLoS Pathog. 2022, 18, e1010887. [Google Scholar] [CrossRef] [PubMed]
- Flueck, C.; Bartfai, R.; Niederwieser, I.; Witmer, K.; Alako, B.T.F.; Moes, S.; Bozdech, Z.; Jenoe, P.; Stunnenberg, H.G.; Voss, T.S. A Major Role for the Plasmodium falciparum ApiAP2 Protein PfSIP2 in Chromosome End Biology. PLoS Pathog. 2010, 6, e1000784. [Google Scholar] [CrossRef]
- Santos, J.M.; Josling, G.; Ross, P.; Joshi, P.; Orchard, L.; Campbell, T.; Schieler, A.; Cristea, I.M.; Llinás, M. Red Blood Cell Invasion by the Malaria Parasite Is Coordinated by the PfAP2-I Transcription Factor. Cell Host Microbe 2017, 21, 731–741.e10. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Miranda, M.; Vembar, S.S.; Delgadillo, D.M.; Ávila-López, P.A.; Herrera-Solorio, A.M.; Lozano Amado, D.; Vargas, M.; Hernandez-Rivas, R. PfAP2Tel, Harbouring a Non-Canonical DNA-Binding AP2 Domain, Binds to Plasmodium falciparum Telomeres. Cell Microbiol. 2017, 19, e12742. [Google Scholar] [CrossRef]
- Cubillos, E.F.G.; Prata, I.O.; Fotoran, W.L.; Ranford-Cartwright, L.; Wunderlich, G. The Transcription Factor PfAP2-O Influences Virulence Gene Transcription and Sexual Development in Plasmodium falciparum. Front. Cell Infect. Microbiol. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Sinha, A.; Hughes, K.R.; Modrzynska, K.K.; Otto, T.D.; Pfander, C.; Dickens, N.J.; Religa, A.A.; Bushell, E.; Graham, A.L.; Cameron, R.; et al. A Cascade of DNA-Binding Proteins for Sexual Commitment and Development in Plasmodium. Nature 2014, 507, 253–257. [Google Scholar] [CrossRef]
- Kafsack, B.F.C.; Rovira-Graells, N.; Clark, T.G.; Bancells, C.; Crowley, V.M.; Campino, S.G.; Williams, A.E.; Drought, L.G.; Kwiatkowski, D.P.; Baker, D.A.; et al. A Transcriptional Switch Underlies Commitment to Sexual Development in Malaria Parasites. Nature 2014, 507, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Yuda, M.; Iwanaga, S.; Kaneko, I.; Kato, T. Global Transcriptional Repression: An Initial and Essential Step for Plasmodium Sexual Development. Proc. Natl. Acad. Sci. USA 2015, 112, 12824–12829. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Cui, H.; Jiang, Y.; Yang, Z.; Wang, X.; Gao, H.; Liu, C.; Zhang, S.; Su, X.Z.; et al. Systematic CRISPR-Cas9-Mediated Modifications of Plasmodium Yoelii ApiAP2 Genes Reveal Functional Insights into Parasite Development. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Shen, S.; Tang, J.; He, X.; Zhao, Y.; Wang, C.; He, X.; Guo, G.; Liu, M.; Wang, L.; et al. A Cascade of Transcriptional Repression Determines Sexual Commitment and Development in Plasmodium falciparum. Nucleic Acids Res. 2021, 49, 9264–9279. [Google Scholar] [CrossRef]
- Zanghì, G.; Vembar, S.S.; Baumgarten, S.; Ding, S.; Guizetti, J.; Bryant, J.M.; Mattei, D.; Jensen, A.T.R.; Rénia, L.; Goh, Y.S.; et al. A Specific PfEMP1 Is Expressed in P. Falciparum Sporozoites and Plays a Role in Hepatocyte Infection. Cell Rep. 2018, 22, 2951–2963. [Google Scholar] [CrossRef]
- Josling, G.A.; Petter, M.; Oehring, S.C.; Gupta, A.P.; Dietz, O.; Wilson, D.W.; Schubert, T.; Längst, G.; Gilson, P.R.; Crabb, B.S.; et al. A Plasmodium falciparum Bromodomain Protein Regulates Invasion Gene Expression. Cell Host Microbe 2015, 17, 741–751. [Google Scholar] [CrossRef]
- Oladejo, D.O.; Duselu, G.O.; Dokunmu, T.M.; Isewon, I.; Oyelade, J.; Okafor, E.; Iweala, E.E.; Adebiyi, E. In Silico Structure Prediction, Molecular Docking, and Dynamic Simulation of Plasmodium falciparum AP2-I Transcription Factor. Bioinform. Biol. Insights 2023, 17, 11779322221149616. [Google Scholar] [CrossRef] [PubMed]
- Bushell, E.; Gomes, A.R.; Sanderson, T.; Anar, B.; Girling, G.; Herd, C.; Metcalf, T.; Modrzynska, K.; Schwach, F.; Martin, R.E.; et al. Functional Profiling of a Plasmodium Genome Reveals an Abundance of Essential Genes. Cell 2017, 170, 260–272.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the Essential Genes of the Human Malaria Parasite Plasmodium falciparum by Saturation Mutagenesis. Science 2018, 360, eaap7847. [Google Scholar] [CrossRef]
- Carrington, E.; Cooijmans, R.H.M.; Keller, D.; Toenhake, C.G.; Bártfai, R.; Voss, T.S. The ApiAP2 Factor PfAP2-HC Is an Integral Component of Heterochromatin in the Malaria Parasite Plasmodium falciparum. iScience 2021, 24, 102444. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Toledo, K.; Rojas-Meza, A.P.; Mancio-Silva, L.; Hernández-Cuevas, N.A.; Delgadillo, D.M.; Vargas, M.; Martínez-Calvillo, S.; Scherf, A.; Hernandez-Rivas, R. Plasmodium falciparum Heterochromatin Protein 1 Binds to Tri-Methylated Histone 3 Lysine 9 and Is Linked to Mutually Exclusive Expression of Var Genes. Nucleic Acids Res. 2009, 37, 2596–2606. [Google Scholar] [CrossRef]
- Shang, X.; Wang, C.; Fan, Y.; Guo, G.; Wang, F.; Zhao, Y.; Sheng, F.; Tang, J.; He, X.; Yu, X.; et al. Genome-Wide Landscape of ApiAP2 Transcription Factors Reveals a Heterochromatin-Associated Regulatory Network during Plasmodium falciparum Blood-Stage Development. Nucleic Acids Res. 2022, 50, 3413–3431. [Google Scholar] [CrossRef]
- Baruch, D.I.; Pasloske, B.L.; Singh, H.B.; Bi, X.; Ma, X.C.; Feldman, M.; Taraschi, T.F.; Howard, R.J. Cloning the P. Falciparum Gene Encoding PfEMP1, a Malarial Variant Antigen and Adherence Receptor on the Surface of Parasitized Human Erythrocytes. Cell 1995, 82, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Quansah, E.; Pappoe, F.; Shen, J.; Liu, M.; Yang, S.; Yu, L.; Zhang, C. ApiAP2 Gene-Network Regulates Gametocytogenesis in Plasmodium Parasites. Cell Microbiol. 2022, 2022, 5796578. [Google Scholar] [CrossRef]
- Droll, D.; Wei, G.; Guo, G.; Fan, Y.; Baumgarten, S.; Zhou, Y.; Xiao, Y.; Scherf, A.; Zhang, Q. Disruption of the RNA Exosome Reveals the Hidden Face of the Malaria Parasite Transcriptome. RNA Biol. 2018, 15, 1206–1214. [Google Scholar] [CrossRef]
- Bancells, C.; Llorà-Batlle, O.; Poran, A.; Nötzel, C.; Rovira-Graells, N.; Elemento, O.; Kafsack, B.F.C.; Cortés, A. Revisiting the Initial Steps of Sexual Development in the Malaria Parasite Plasmodium falciparum. Nat. Microbiol. 2019, 4, 144–154. [Google Scholar] [CrossRef]
- Brancucci, N.M.B.; Bertschi, N.L.; Zhu, L.; Niederwieser, I.; Chin, W.H.; Wampfler, R.; Freymond, C.; Rottmann, M.; Felger, I.; Bozdech, Z.; et al. Heterochromatin Protein 1 Secures Survival and Transmission of Malaria Parasites. Cell Host Microbe 2014, 16, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Yuda, M.; Kaneko, I.; Murata, Y.; Iwanaga, S.; Nishi, T. Mechanisms of Triggering Malaria Gametocytogenesis by AP2-G. Parasitol. Int. 2021, 84, 102403. [Google Scholar] [CrossRef]
- Josling, G.A.; Russell, T.J.; Venezia, J.; Orchard, L.; van Biljon, R.; Painter, H.J.; Llinás, M. Dissecting the Role of PfAP2-G in Malaria Gametocytogenesis. Nat. Commun. 2020, 11, 1503. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Santos, J.M.; Orchard, L.M.; Yamada, N.; van Biljon, R.; Painter, H.J.; Mahony, S.; Llinás, M. The PfAP2-G2 Transcription Factor Is a Critical Regulator of Gametocyte Maturation. Mol. Microbiol. 2021, 115, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qiao, D.; Wen, Y.; Bi, Y.; Chen, Y.; Huang, Z.; Cui, L.; Guo, J.; Cao, Y. PfAP2-G2 Is Associated to Production and Maturation of Gametocytes in Plasmodium falciparum via Regulating the Expression of PfMDV-1. Front. Microbiol. 2021, 11, 631444. [Google Scholar] [CrossRef]
- Usui, M.; Prajapati, S.K.; Ayanful-Torgby, R.; Acquah, F.K.; Cudjoe, E.; Kakaney, C.; Amponsah, J.A.; Obboh, E.K.; Reddy, D.K.; Barbeau, M.C.; et al. Plasmodium falciparum Sexual Differentiation in Malaria Patients Is Associated with Host Factors and GDV1-Dependent Genes. Nat. Commun. 2019, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Yuda, M.; Kaneko, I.; Iwanaga, S.; Murata, Y.; Kato, T. Female-Specific Gene Regulation in Malaria Parasites by an AP2-Family Transcription Factor. Mol. Microbiol. 2020, 113, 40–51. [Google Scholar] [CrossRef]
- Miao, J.; Wang, C.; Lucky, A.B.; Liang, X.; Min, H.; Adapa, S.R.; Jiang, R.; Kim, K.; Cui, L. A Unique GCN5 Histone Acetyltransferase Complex Controls Erythrocyte Invasion and Virulence in the Malaria Parasite Plasmodium falciparum. PLoS Pathog. 2021, 17, e1009351. [Google Scholar] [CrossRef]
- Subudhi, A.K.; Green, J.L.; Satyam, R.; Salunke, R.P.; Lenz, T.; Shuaib, M.; Isaioglou, I.; Abel, S.; Gupta, M.; Esau, L.; et al. DNA-Binding Protein PfAP2-P Regulates Parasite Pathogenesis during Malaria Parasite Blood Stages. Nat. Microbiol. 2023. [Google Scholar] [CrossRef]
- Akkaya, M.; Bansal, A.; Sheehan, P.W.; Pena, M.; Molina-Cruz, A.; Orchard, L.M.; Cimperman, C.K.; Qi, C.F.; Ross, P.; Yazew, T.; et al. A Single-Nucleotide Polymorphism in a Plasmodium berghei ApiAP2 Transcription Factor Alters the Development of Host Immunity. Sci. Adv. 2020, 6, eaaw6957. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, H.; Guan, J.; Liu, C.; Yang, Z.; Yuan, J. Plasmodium Transcription Repressor AP2-O3 Regulates Sex-specific Identity of Gene Expression in Female Gametocytes. EMBO Rep. 2021, 22, e51660. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Maria Hoeijmakers, W.A.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-Defined Endocytosis Pathway Mediates Artemisinin Resistance in Malaria Parasites. Science 2020, 367, 51–59. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).