Sex-Specific Responses to Tacrolimus and Mycophenolate Mofetil in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design I
2.3. Experimental Design II
2.4. Blood Pressure Measurement
2.5. Urinary Protein, Albumin, Sodium, and Nitrate Excretion
2.6. Two-Color Flowcytometry
2.7. Tissue Digestion for Lymphocyte Preparation
2.8. Statistical Analyses
3. Results
3.1. Body Weights
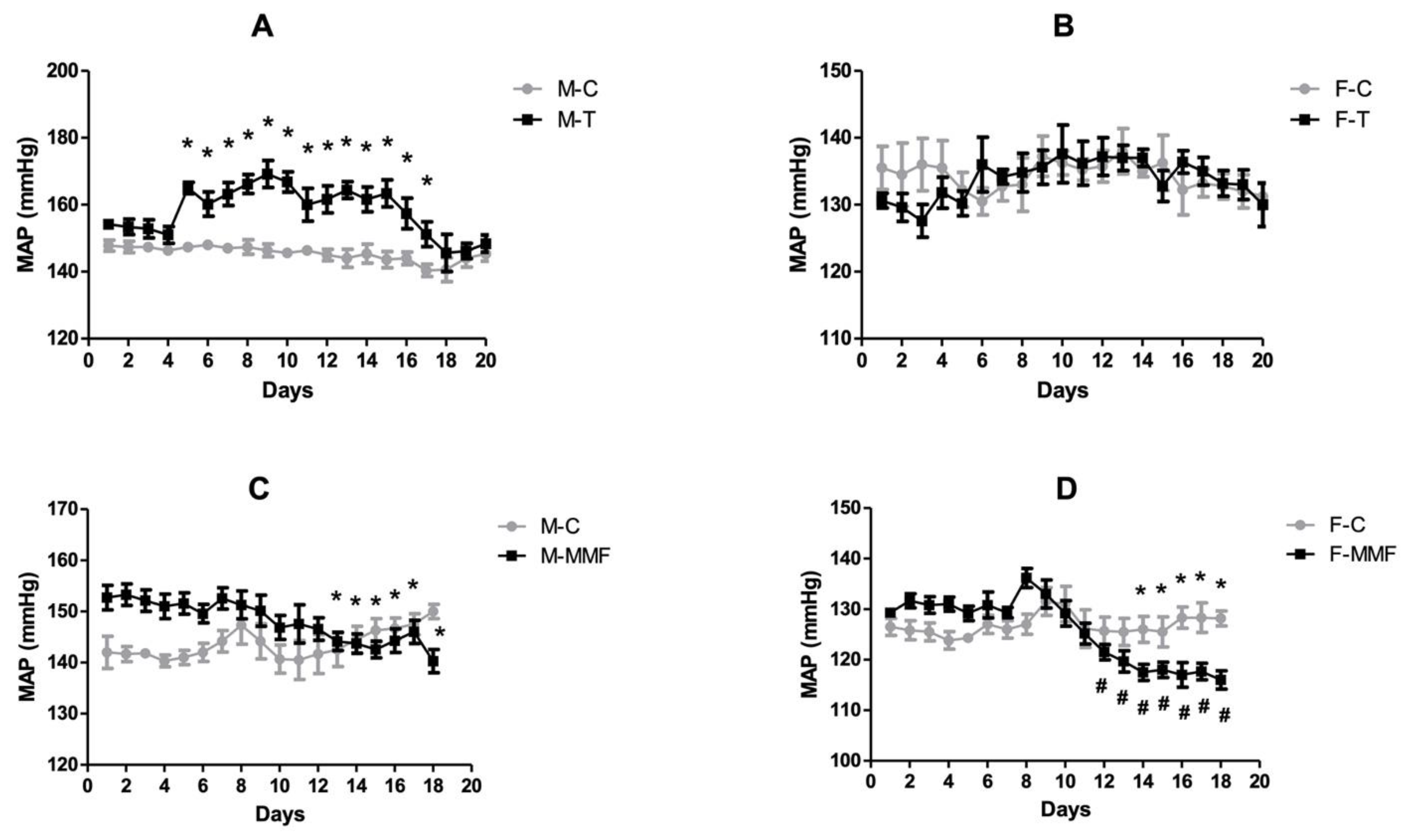
3.2. Mean Arterial Blood Pressure
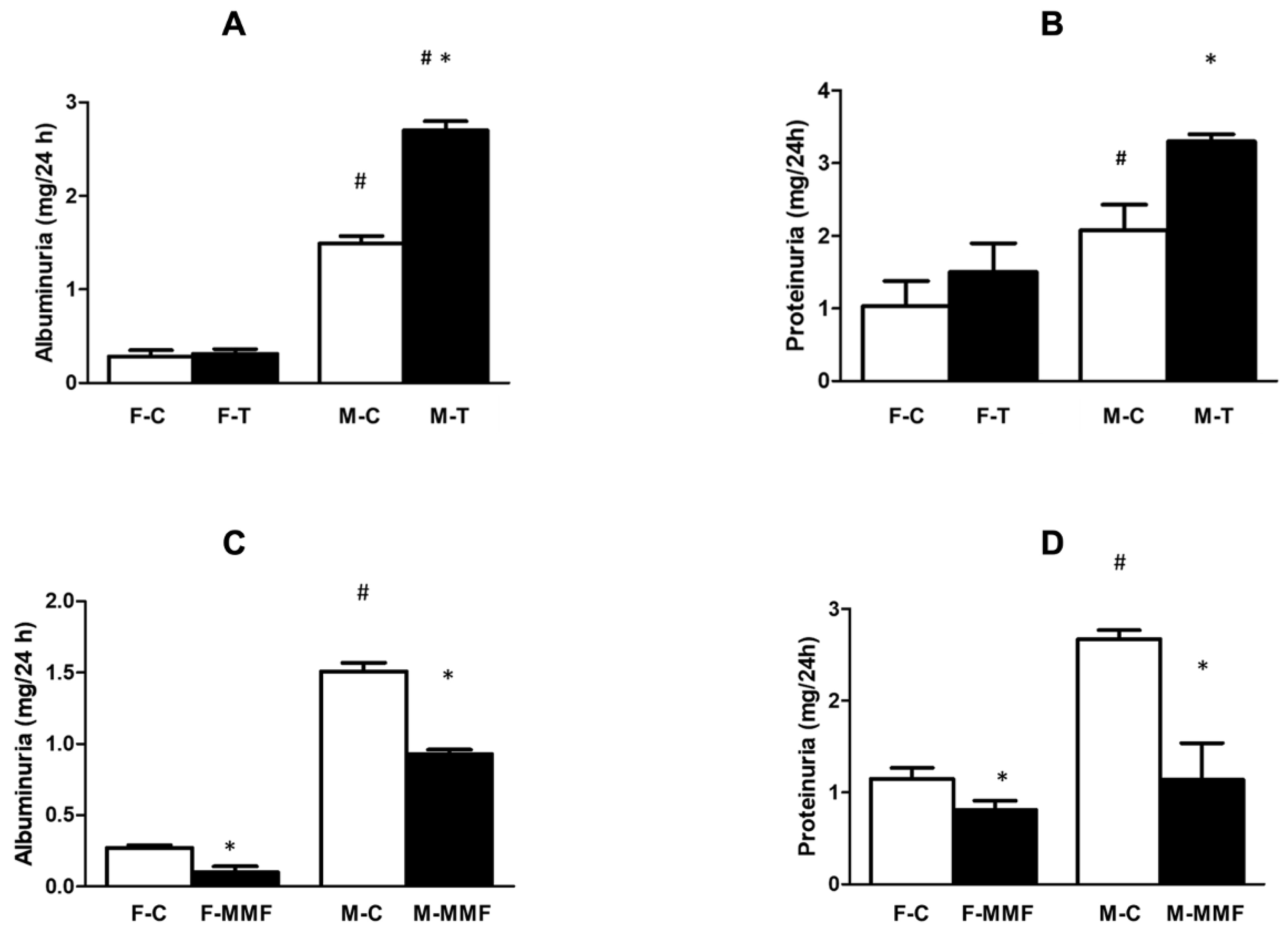
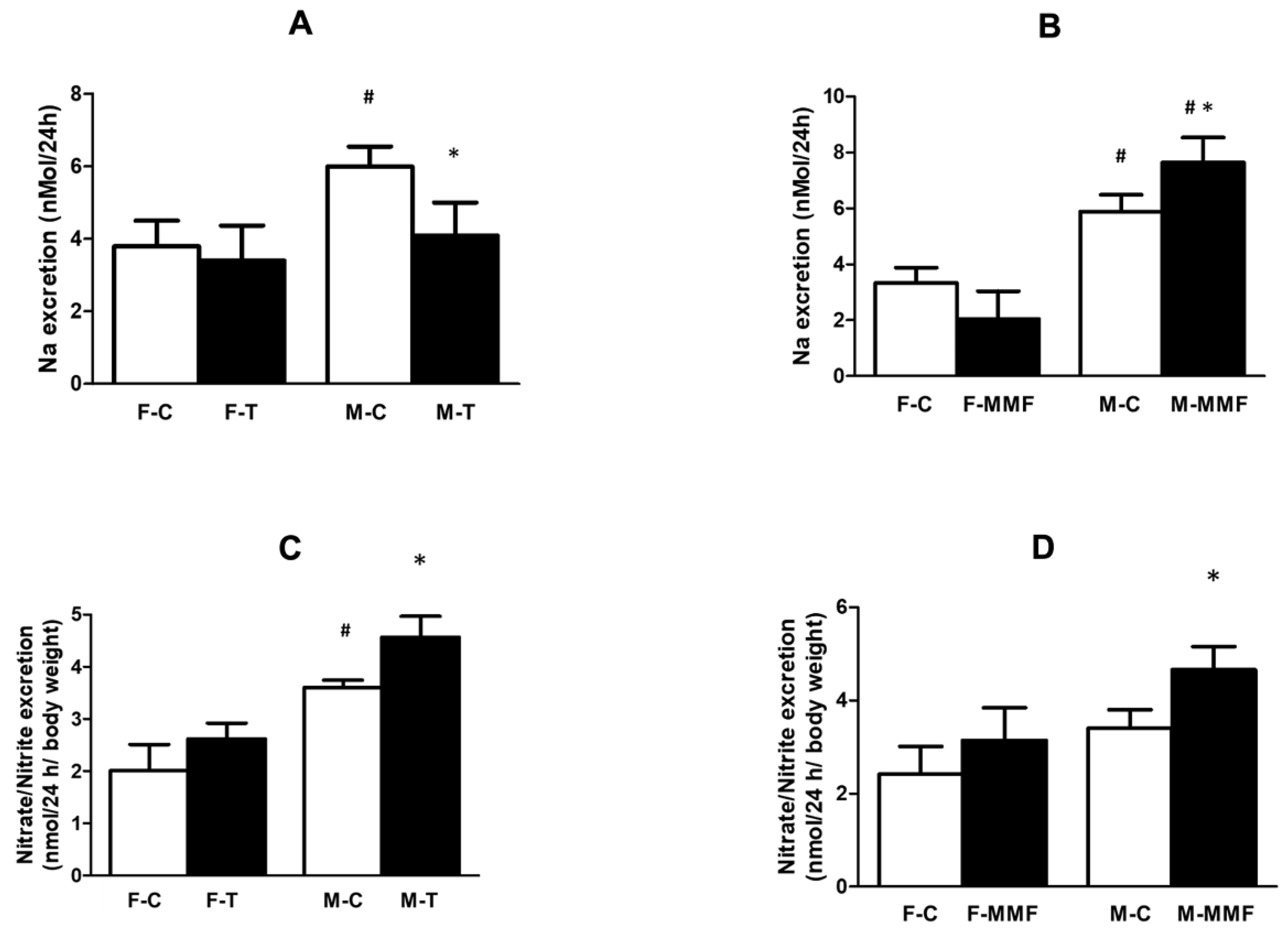
3.3. Renal Function
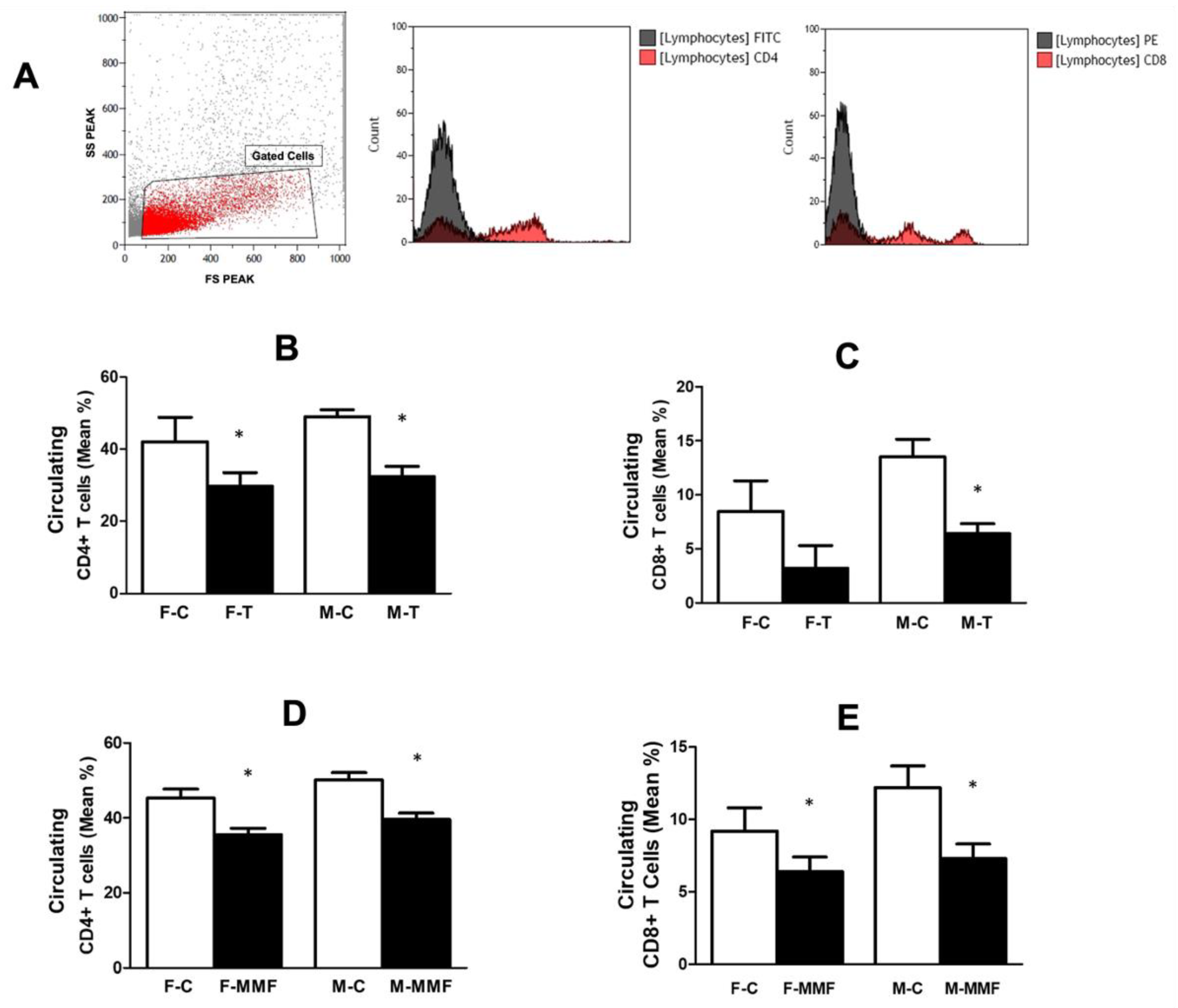
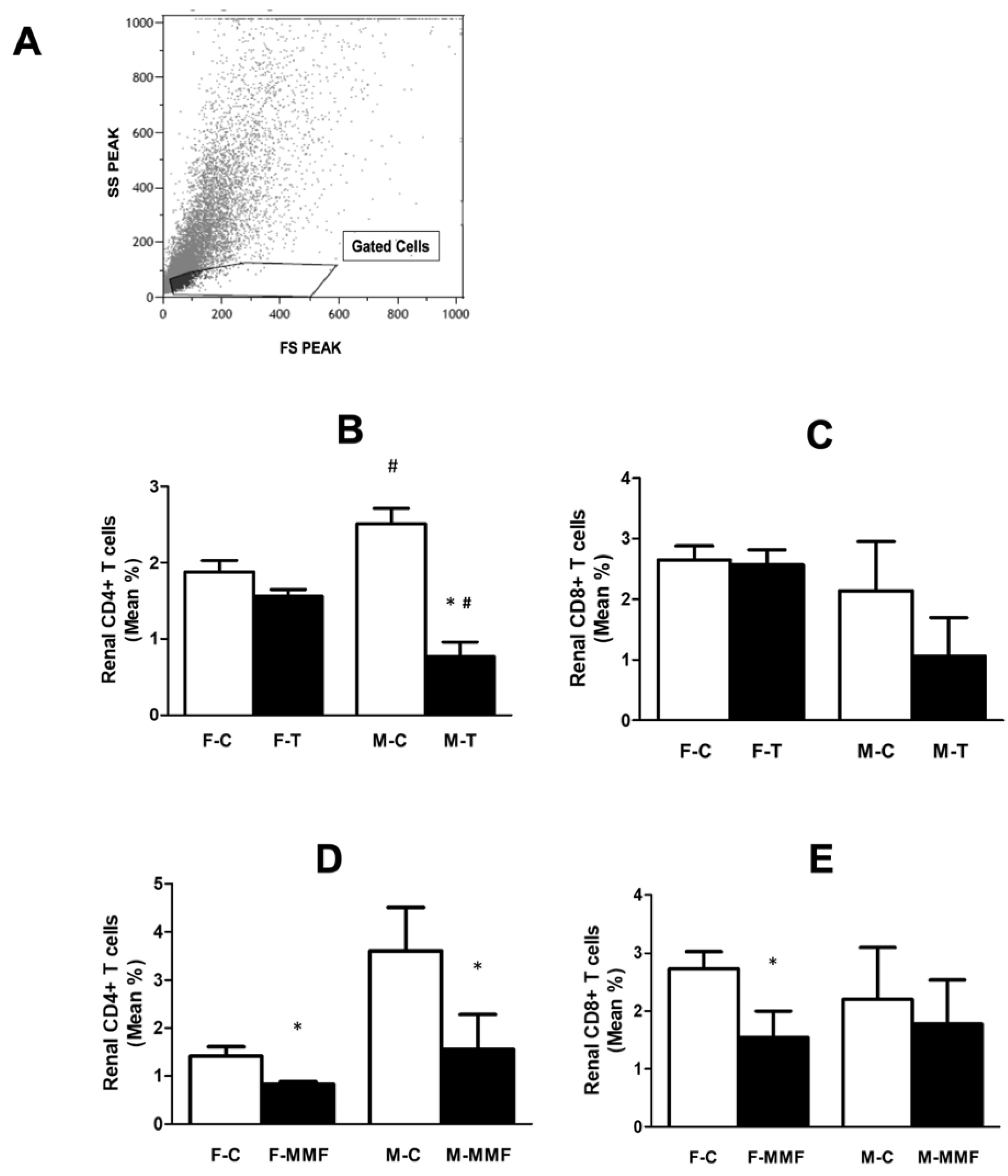
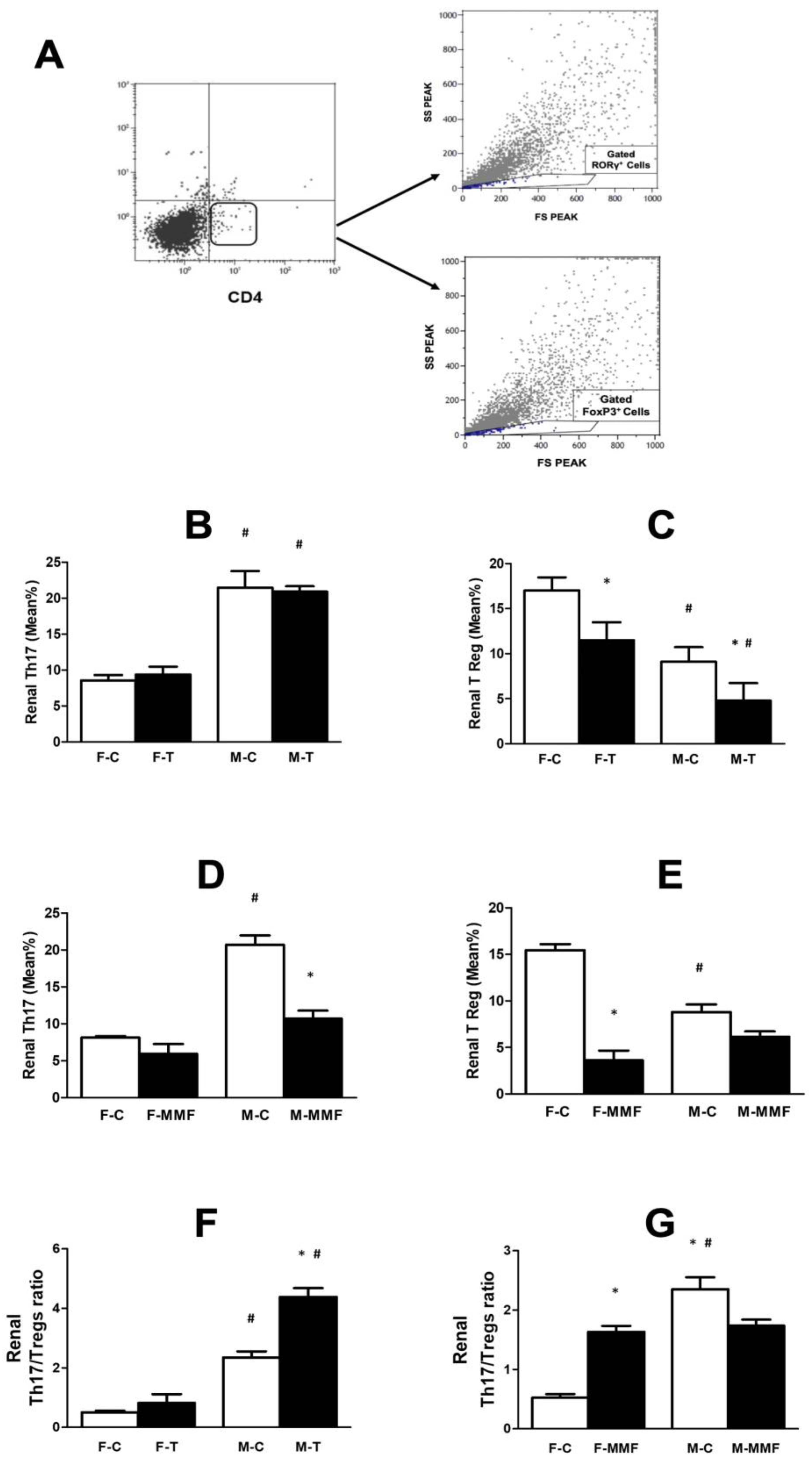
3.4. Circulatory and Renal T Cell Subsets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hypertension Fact Sheet. September 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 1 August 2023).
- Harrison, D.G. The immune system in hypertension. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 130–138. [Google Scholar] [PubMed]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.F.; Waki, H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci. Biobehav. Rev. 2009, 33, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zubcevic, J.; Waki, H.; Raizada, M.K.; Paton, J.F. Autonomic-immune-vascular interaction: An emerging concept for neurogenic hypertension. Hypertension 2011, 57, 1026–1033. [Google Scholar] [CrossRef]
- Tipton, A.J.; Sullivan, J.C. Sex Differences in T Cells in Hypertension. Am. J. Phys. Regul. Integr. Comp. Phys. 2012, 303, R359–R367. [Google Scholar] [CrossRef]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [Google Scholar] [CrossRef]
- Rodríguez-Iturbe, B.; Quiroz, Y.; Nava, M.; Bonet, L.; Chávez, M.; Herrera-Acosta, J.; Johnson, R.J.; Pons, H.A. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am. J. Physiol.-Renal Physiol. 2002, 282, F191–F201. [Google Scholar] [CrossRef]
- Morales, J.M.; Domínguez-Gil, B. Impact of tacrolimus and mycophenolate mofetil combination on cardiovascular risk profile after kidney transplantation. J. Am. Soc. Nephrol. 2006, 17, S296–S303. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Walsh, S.B.; McCormick, J.A.; Fürstenberg, A.; Yang, C.L.; Roeschel, T.; Paliege, A.; Howie, A.J.; Conley, J.; Bachmann, S.; et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat. Med. 2011, 17, 1304–1309. [Google Scholar] [CrossRef]
- Chiasson, V.L.; Talreja, D.; Young, K.J.; Chatterjee, P.; Banes-Berceli, A.K.; Mitchell, B.M. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension 2011, 57, 1167–1175. [Google Scholar] [CrossRef]
- De Miguel, C.; Guo, C.; Lund, H.; Feng, D.; Mattson, D.L. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am. J. Physiol.-Renal Physiol. 2011, 300, F734–F742. [Google Scholar] [CrossRef] [PubMed]
- Boesen, E.I.; Williams, D.L.; Pollock, J.S.; Pollock, D.M. Immunosuppression with mycophenolate mofetil attenuates the development of hypertension and albuminuria in deoxycorticosterone acetate-salt hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L.; James, L.; Berdan, E.A.; Meister, C.J. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 2006, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hošková, L.; Málek, I.; Kautzner, J.; Honsová, E.; van Dokkum, R.P.; Husková, Z.; Vojtíšková, A.; Varcabová, Š.; Červenka, L.; Kopkan, L. Tacrolimus-induced hypertension and nephrotoxicity in Fawn-Hooded rats are attenuated by dual inhibition of renin-angiotensin system. Hypertens. Res. 2014, 37, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Moulana, M.; Maranon, R.O. Regulation of blood pressure is influenced by gender: A study in obese Zucker rats. Life Sci. 2018, 209, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Zhang, H.; Granger, J.P. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 1998, 31, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ruiz, A.F.; Iliescu, R.; Reckelhoff, J.F. Refractory blood pressure in female SHR to increased oxidative stress is not mediated by NO or by upregulation of renal antioxidant enzymes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R266–R271. [Google Scholar] [CrossRef][Green Version]
- Moulana, M. Immunophenotypic profile of leukocytes in hyperandrogenemic female rat an animal model of polycystic ovary syndrome. Life Sci. 2019, 220, 44–49. [Google Scholar] [CrossRef]
- Maranon, R.; Reckelhoff, J.F. Sex and Gender Differences in Control of Blood Pressure. Clin. Sci. 2013, 125, 311–318. [Google Scholar] [CrossRef]
- Cutler, J.A.; Sorlie, P.D.; Wolz, M.; Thom, T.; Fields, L.E.; Roccella, E.J. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008, 52, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, K.; Ji, H. Sex differences in primary hypertension. Biol. Sex. Differ. 2012, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Grollman, A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex. Rep. Biol. Med. 1967, 25, 257–264. [Google Scholar] [PubMed]
- White, F.N.; Grollman, A. Autoimmune Factors Associated with Infarction of the Kidney. Nephron 1964, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Olsen, F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol. Microbiol. Scand. Sect. C Immunol. 1980, 88C, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pollow, D.P.; Uhrlaub, J.; Romero-Aleshire, M.J.; Sandberg, K.; Nikolich-Zugich, J.; Brooks, H.L.; Hay, M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 2014, 64, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Fontana, V.; De Faria, A.P.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W.; et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef]
- Kvakan, H.; Luft, F.C.; Muller, D.N. Role of the immune system in hypertensive target organ damage. Trends Cardiovasc. Med. 2009, 19, 242–246. [Google Scholar] [CrossRef]
- Trott, D.W.; Thabet, S.R.; Kirabo, A.; Saleh, M.A.; Itani, H.; Norlander, A.E.; Wu, J.; Goldstein, A.; Arendshorst, W.J.; Madhur, M.S.; et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 2014, 64, 1108–1115. [Google Scholar] [CrossRef]
- Lee, B.-W.; Yap, H.-K.; Chew, F.-T.; Quah, T.-C.; Prabhakaran, K.; Chan, G.S.H.; Wong, S.-C.; Seah, C.-C. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: From birth to adulthood. Cytometry 1996, 26, 8–15. [Google Scholar] [CrossRef]
- Uppal, S.S.; Verma, S.; Dhot, P.S. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytom. B Clin. Cytom. 2003, 52, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Tipton, A.J.; Baban, B.; Sullivan, J.C. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R359–R367. [Google Scholar] [CrossRef] [PubMed]
- Tipton, A.J.; Baban, B.; Sullivan, J.C. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 2014, 64, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, V.L.; Pakanati, A.R.; Hernandez, M.; Young, K.J.; Bounds, K.R.; Mitchell, B.M. Regulatory T-Cell Augmentation or Interleukin-17 Inhibition Prevents Calcineurin Inhibitor–Induced Hypertension in Mice. Hypertension 2017, 70, 83–191. [Google Scholar] [CrossRef] [PubMed]
- Pollow, D.P.; Uhlorn, J.A.; Sylvester, M.A.; Romero-Aleshire, M.J.; Uhrlaub, J.L.; Lindsey, M.L.; Nikolich-Zugich, J.; Brooks, H.L. Menopause and FOXP3+ Treg cell depletion eliminate female protection against T cell-mediated angiotensin II hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Brinson, K.N.; Elmarakby, A.A.; Tipton, A.J.; Crislip, G.R.; Yamamoto, T.; Baban, B.; Sullivan, J.C. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am. J. Phys. Regul. Integr. Comp. Phys. 2013, 305, R701–R710. [Google Scholar] [CrossRef] [PubMed]
- Fourtounas, C.; Dousdampanis, P.; Sakellaraki, P.; Rodi, M.; Georgakopoulos, T.; Vlachojannis, J.G.; Mouzaki, A. Different immunosuppressive combinations on T-cell regulation in renal transplant recipients. Am. J. Nephrol. 2010, 32, 1–9. [Google Scholar] [CrossRef]
- Sandberg, K.; Ji, H.; Hay, M. Sex-specific immune modulation of primary hypertension. Cell Immunol. 2015, 294, 95–101. [Google Scholar] [CrossRef]
- Manzi, S.; Meilahn, E.N.; Rairie, J.E.; Conte, C.G.; Medsger, T.A., Jr.; Jansen-McWilliams, L.; D’Agostino, R.B.; Kuller, L.H. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham Study. Am. J. Epidemiol. 1997, 145, 408–415. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Baban, B.; Tipton, A.J.; O’Connor, P.M.; Sullivan, J.C. Chronic ANG II infusion induces sex-specific increases in renal T cells in Sprague-Dawley rats. Am. J. Physiol. Renal Physiol. 2015, 308, F706–F712. [Google Scholar] [CrossRef]
- Chan, C.T.; Sobey, C.G.; Lieu, M.; Ferens, D.; Kett, M.M.; Diep, H.; Kim, H.A.; Krishnan, S.M.; Lewis, C.V.; Salimova, E.; et al. Obligatory Role for B Cells in the Development of Angiotensin II-Dependent Hypertension. Hypertension 2015, 66, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, L.S.; Shikatani, E.A.; Besla, R.; Levy, A.S.; Dinh, D.D.; Momen, A.; Zhang, H.; Afroze, T.; Chen, M.B.; Chiu, F.; et al. B-cell deficiency lowers blood pressure in mice. Hypertension 2019, 73, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Kresovich, J.K.; Xu, Z.; O’brien, K.M.; Parks, C.G.; Weinberg, C.R.; Sandler, D.P.; Taylor, J.A. Peripheral Immune Cell Composition is Altered in Women Before and After a Hypertension Diagnosis. Hypertension 2023, 80, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Velicković-Radovanović, R.; Mikov, M.; Paunović, G.; Djordjević, V.; Stojanović, M.; Cvetković, T.; Djordjević, A.C. Gender differences in pharmacokinetics of tacrolimus and their clinical significance in kidney transplant recipients. Gend. Med. 2011, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- van Hest, R.M.; van Gelder, T.; Vulto, A.G.; Mathot, R.A. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin. Pharmacokinet. 2005, 44, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zheng, Y.-M.; Song, T.; Reyes-García, J.; Wang, C.; Wang, Y.-X. Inhibition of big-conductance Ca2+-activated K+ channels in cerebral artery (vascular) smooth muscle cells is a major novel mechanism for tacrolimus-induced hypertension. Pflügers Arch.-Eur. J. Physiol. 2021, 473, 53–66. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, S.; Fei, L.; Dong, F.; Xie, L.; Qiu, X.; Lei, Y.; Guo, J.; Zhong, M.; Ren, X.; et al. Tacrolimus Causes Hypertension by Increasing Vascular Contractility via RhoA (Ras Homolog Family Member A)/ROCK (Rho-Associated Protein Kinase) Pathway in Mice. Hypertension 2022, 79, 2228–2238. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marañón, R.O.; Moulana, M. Sex-Specific Responses to Tacrolimus and Mycophenolate Mofetil in Spontaneously Hypertensive Rats. Future Pharmacol. 2023, 3, 862-876. https://doi.org/10.3390/futurepharmacol3040052
Marañón RO, Moulana M. Sex-Specific Responses to Tacrolimus and Mycophenolate Mofetil in Spontaneously Hypertensive Rats. Future Pharmacology. 2023; 3(4):862-876. https://doi.org/10.3390/futurepharmacol3040052
Chicago/Turabian StyleMarañón, Rodrigo Oscar, and Mohadetheh Moulana. 2023. "Sex-Specific Responses to Tacrolimus and Mycophenolate Mofetil in Spontaneously Hypertensive Rats" Future Pharmacology 3, no. 4: 862-876. https://doi.org/10.3390/futurepharmacol3040052
APA StyleMarañón, R. O., & Moulana, M. (2023). Sex-Specific Responses to Tacrolimus and Mycophenolate Mofetil in Spontaneously Hypertensive Rats. Future Pharmacology, 3(4), 862-876. https://doi.org/10.3390/futurepharmacol3040052




