Abstract
Oral candidiasis is an opportunistic infection usually related to predisposing factors. Oral manifestations in patients affected by COVID-19 have been reported, as the oral mucosa is the gateway to this viral infection. Xerostomia, as well as other oral symptoms, are predisposing factors for the emergence of oral candidiasis after the COVID-19 pandemic. It is a common pathology, but fatal if left untreated. Nystatin (NYS) is the drug of first choice in the treatment of oral candidiasis. Herein, we reviewed the epidemiology of oral candidiasis and its treatments, focusing on the mechanism of action, dosage forms, and NYS efficacy. NYS is an effective drug against oral candidiasis and belongs to Class IV of the biopharmaceutical classification system; however, its low solubility and low permeability may compromise its availability in the oral cavity and, consequently, its pharmacological action. Future perspectives to overcome drug limitations were also addressed and discussed in our review.
1. Introduction
Candidiasis is a fungal infection caused by the genus Candida and may also be known as candidosis. The term moniliasis is older and considered inappropriate since it refers to the archaic denomination Monilia albicans [1,2]. Its spectrum is very extensive, from mucosal colonization to invasive systemic conditions [2]. In healthy individuals, the genus Candida is found in the oral cavity, belonging to the oral microbiota. Candida spp. infection only arises when an opportunity occurs, thus being known as an opportunistic infection [3,4]. Table 1 shows the various clinical forms of candidiasis.
Among the various forms of candidiasis, there is the oral one, the most common fungal oral infection in the oropharyngeal tract, which manifests itself when related to predisposing factors. In general, there are specific and non-specific defense mechanisms present in saliva and oral mucosa, in addition to the presence of the competitive oral microbiota itself, formed by other microorganisms that restrict the growth of Candida spp. [3,5]. There are several factors that can cause oral candidiasis, and this pathology is closely linked to other disorders or conditions that are considered risk factors for its emergence [3,6], as depicted in Table 2. Therefore, oral candidiasis may be an indication of problems that affect defense mechanisms, whether local or systemic alteration of the oral microbiota or even neglected oral hygiene [5,6].
To cause infection, Candida species have an exceptional ability to adhere to surfaces such as mucosa, teeth, dental fillings, dentures, orthodontic implants, tongue piercings, and even endotracheal tubes present in the oral cavity that are not properly sanitized and have not been replaced [5,6].

Table 1.
Clinical forms of candidiasis (adapted from [7]).
Table 1.
Clinical forms of candidiasis (adapted from [7]).
| Clinical Forms of Candidiasis | Sites/Tissues | Comments |
|---|---|---|
| Oral | Oral cavity, oral mucosa | Superficial candidiasis affecting patients with local and systemic changes |
| Esophageal | Esophageal mucosa | Considered a semi-invasive type of candidiasis |
| Vulvovaginal | Vulva and vagina | High incidence in women during the fertile period |
| Urinary | Urinary tract | Frequent candiduria, but not always followed by symptoms |
| Dialysis-related peritoneal | Peritoneal region | Related to peritoneal dialysis |
| Postoperative peritoneal | Peritoneal region | It occurs frequently in hospitalized patients and is related to cases of secondary or tertiary peritonitis |
| Respiratory tract | Respiratory tract | Uncommon and poorly documented clinical manifestation, with a higher incidence in neutropenic patients with hematologic malignancy or undergoing lung transplantation |
| Hematogenous/Candidemia | Blood | A broad spectrum of episodes, including isolated cases of Candida spp. or in conjunction with other fungi in the bloodstream that spreads to more organs |

Table 2.
Predisposing factors for oral candidiasis [2,3,7,8].
Table 2.
Predisposing factors for oral candidiasis [2,3,7,8].
| Mechanism | Factors |
|---|---|
| Impaired local defense mechanisms | Low saliva production/xerostomia |
| Tabagism | |
| Oral mucosal diseases | |
| Topical use of corticoids | |
| Radiation therapy | |
| Impaired systemic defense mechanisms | Poorly controlled diabetes |
| Immunodeficiencies | |
| Use of immunosuppressors | |
| Malnutrition | |
| Neoplasms | |
| Sarcoidosis | |
| Cirrhosis | |
| Sjögren’s syndrome | |
| Hypoparathyroidism | |
| Hypoadrenalism | |
| Disruption of the oral microbiota | Use of wide-spectrum antibiotics |
| High alcohol consumption | |
| Reflux, low pH | |
| Carbohydrates-rich diet | |
| Dental prosthesis | |
| Oral hygiene | Mixed biofilm over nonrenewable surfaces |
| Neglected oral hygiene | |
| Groups | Children (immaturity of the immune system) |
| Premature newborn | |
| Lactating | |
| Elderly |
Oral candidiasis is not a lethal disease; however, if not properly treated, it can progress to chronic clinical symptoms, invading other tissues and even causing a systemic infection [8]. More recently, the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), led to an increase in cases of oropharyngeal symptoms and oral problems associated with soft tissue and saliva production (dry mouth), even after the recovery from COVID-19. These manifestations in patients who have or had COVID-19 were also predisposing to the onset of oral candidiasis. Case studies have been presented to evaluate the relationship between oral manifestations in patients with a history of COVID-19 [9,10,11,12].
Among the species, Candida albicans is the main cause of oral candidiasis. Other species can also be pathogenic, such as C. glabrata, C. parapsilosis, C. krusei, C. pseudotropicallis, C. guillermondi, and C. tropicalis. However, all Candida species can cause mucositis like this infection [5,8,13]. To cause infection, there are fundamental properties for biofilm formation described as virulence factors, such as adhesion to host surfaces, morphological transformation, more invasive forms (hyphae), and the production of enzymes in the extracellular environment [6,13]. Oral candidiasis is seen in several different clinical findings, acute or chronic, and in a wide spectrum of severity, which makes its diagnosis difficult. Among them, we can mention pseudomembranous candidiasis, atrophic erythematous candidiasis, chronic hyperplastic candidiasis, angular cheilitis, and central papillary atrophy, among others [8,14].
Patients with oral candidiasis may be asymptomatic or manifest various symptoms, depending on their clinical form (Table 3). In general, burning sensations, the presence of metallic taste, pain, and dysphagia are reported [5,8,14].

Table 3.
Clinical manifestations of oral candidiasis (adapted from [8]).
2. Oral Candidiasis
2.1. Pseudomembranous Candidiasis
The form of pseudomembranous oral candidiasis is popularly recognized as “thrush” and usually presents in the form of yellow or white plaques adhering to the oral mucosa, with an inflammatory basis. The plaques are easily removable by scraping with a tongue depressor or gauze pad and are characterized by a disordered set of hyphae, yeasts, epithelial cells, and fragments of necrotic tissue, distributed in the jugal mucosa (cheek region), palate, dorsum of the tongue, the gums, and the oral floor [2,5,15]. After the removal of the plaques, the region has an erythematous, ulcerated, and sensitive surface [14].
The pseudomembranous form can manifest itself in patients who make use of broad-spectrum antibiotics, which leads to a decrease in the competing bacteria present in the oral cavity. It can also affect immunosuppressed patients, either through diseases such as carriers of the human immunodeficiency virus (HIV) and leukemias or by using immunosuppressive drugs [1,2,15]. Young children up to 2 years old can be affected due to their poorly developed immune systems [2,15,16]. The symptoms are burning sensation and burning, itching, taste alteration, being described as bitter or salty, and even dysphagia (difficulty swallowing) [2,8,15].
2.2. Erythematosus Candidiasis
This form is related to the use of medications (corticosteroids and broad-spectrum antibiotics), prolonged use of removable prostheses, and xerostomia [2,14]. It commonly appears as red spots with the absence of white plaques, affecting the dorsum of the tongue, palate, or any superficial part of the oral mucosa [2,8].
Erythematous candidiasis is the most common oral candidiasis; however, in many cases, it is clinically neglected. It can be found in several different forms, depending on the cause or location [2,14]. According to Gianni and Shetty (2011) [2], erythematous candidiasis is categorized into some subtypes, such as acute atrophic, chronic atrophic, angular cheilitis, median rhomboid glossitis (also known as central papillary atrophy), and chronic multifocal.
Central papillary atrophy is clinically presented with a well-defined erythematous region in the midline of the posterior region of the lingual dorsum, which is due to the loss of filiform papillae. It is presented in the form of a red or red-whitish spot and is flat or raised [2,14]. Some carriers may also present the infection in other parts of the oral cavity, and thus this type of candidiasis is called chronic multifocal. In this case, in addition to the dorsum of the tongue, they may arise at the junction between the hard palate and the soft palate and the labial commissures [2,6,8]. The lesion in the labial commissures, recognized as angular cheilitis, is characterized by erythema, pain, fissures, and desquamation, which may be associated with chronic multifocal candidiasis or arise alone. In these cases of cheilitis, saliva usually accumulates in the commissures, which leads to a moist region that favors fungal growth. This occurs in patients who have decreased vertical occlusion dimension and accentuated grooves and who also make use of dental prostheses [2,17]. In unusual conditions, habits such as licking the lips or sucking the fingers can create a pattern of moistening of the perioral skin, causing cheilocandidiasis [18].
Acute atrophic candidiasis, also known as “antibiotic sore mouth,” is clearly caused by antibiotic use and in cases of uncontrolled diabetes. Its clinical presentation occurs in the form of erythema, involving atrophy of the papillae of the dorsum of the tongue. Patients complain of burning sensations in the mouth due to the diffuse loss of the filiform papillae of the dorsal region of the tongue. Pruritus, xerostomia, and taste changes are also reported [2,8].
Finally, chronic atrophic candidiasis is commonly referred to as prosthetic stomatitis and is related to the prolonged use of removable prostheses or in cases that are poorly fitted, of inadequate design, or have poor oral and denture hygiene [2,8,19]. Prothesis hygiene must be carried out to remove microorganisms, including Candida spp. The involved methods are chemical cleaners and/or mechanical removal. It has been reported that biofilm formation depends on the types of denture resin, the kind of cleanser, and, also, the cleanser concentration. Furthermore, the prothesis roughness can lead to microbial colonization [19]. Patients are usually asymptomatic, and clinically, they can present with erythema and hemorrhagic petechiae. Clinical manifestations occur in the palate near the prosthesis, where the salivary flow is restricted [2,8].
2.3. Hyperplastic Candidiasis
Hyperplastic candidiasis is known as chronic hyperplastic candidiasis or Candida leukoplakia and presents as non-removable white or red plaques with elevated lesions ranging in size from small to nodular to palpable [2,8]. The frequent localization is on the surface of the dorsum of the tongue, the palate, and the jugal mucosa. This is the rarest type of oral candidiasis, but its malignant transformation potential is still controversial [8,20].
2.4. Mucocutaneous Candidiasis
Mucocutaneous candidiasis is a chronic, persistent form and is considered the most severe form of the disease. In this scenario, a systemic distribution and the most extensive degree of impairment of the host’s immune system are seen. It initially presents in pseudomembranous or hyperplastic form but then involves the skin, esophageal mucosa, and nails [2,8,21]. It is also associated with various endocrinopathies, such as Addison’s syndrome, hypothyroidism, hypoparathyroydism, and diabetes mellitus [2,22]. Often, endocrine disruption occurs months or years after Candida spp. infection [2].
In some patients with this manifestation, candidiasis has been related to mutations in the autoimmunity regulatory gene, leading to the formation of antibodies against the host’s own tissues. Normally, the immune disorder is evident early in the life of patients who have oral candidiasis, nails, skin, and other mucous membranes [2,7]. Patients may still develop endocrine abnormalities such as endocrine-candidiasis syndrome and autoimmune polyendocrinopathy-candidiasis dystrophy syndrome (APECED) [2,23].
2.5. The Genus Candida
Of the fungi of medical interest, the genus Candida is the most important due to its high frequency of colonization and infecting the human host [7,13]. The oral prevalence of this fungus is variable, but, according to Colombo et al. (2013) [7], it is found between 20 and 40% in the gastrointestinal tract of healthy adults, and approximately 20 to 30% of women have colonization by Candida spp. in the vaginal tract. In immunocompromised patients, Candida spp. colonization can reach 60% of cases [24]. These microorganisms are considered commensal and become pathogenic when changes in the host’s defense mechanism occur, secondary anatomical barriers are compromised, or invasive medical processes are made. Hence the clinical interest in Candida spp. infection, more specifically oral candidiasis, due to the significant number of complications that intersect the various medical specialties [7,13].
The morphology of the genus Candida is the autochthonous form of yeasts that colonize the oral cavity and the gastrointestinal tract, belonging to the microbiota. Among the approximately 20 Candida species of medical interest, Candida albicans has the highest prevalence in the oral cavity. If there is a disruption of local defense mechanisms, metabolic dysfunction, or even the presence of diseases associated with immunosuppression, colonization can lead to infection and pathology [7]. Thus, oral candidiasis is the most common opportunistic infection found in patients with acquired immunodeficiency syndrome (AIDS) and is considered a marker of the progression of immune deterioration in these patients [3].
The genus Candida can also be seen in the form of pseudohyphae and hyphae, in addition to the form of yeasts. This characteristic is recognized as dimorphism and is associated with the virulence of this microorganism because, in this form, it becomes invasive, penetrating the tissue, which leads to an infectious picture. The pseudohyphae and hyphae are the morphological forms that adhere to the oral cavity surface for the colonization of Candida species, resulting in biofilm development and penetration into the tissue. Candida spp. and bacteria can be found in the biofilm [13]. Such interaction occurs through commensalism; however, studies are necessary to investigate the complex interactions between fungi and bacteria [25]. At the site, a state of hypersensitivity and the production of toxins occur [3,13].
Candida albicans exhibits different forms of growth and can reproduce by multilateral budding in the form of yeast [7,13]. However, they can also be found in elongated and filamentous forms, called pseudohyphae and hyphae [13].
2.6. Oral Candidiasis and COVID-19
COVID-19 is a recent infection, and there is not enough information to relate directly to oral candidiasis. Although oral candidiasis in patients with COVID-19 was reported [10,11,26,27,28,29]. The issue is that the presence of Candida species in the oral cavity can be a reservoir for infection in other parts of the body [13].
COVID-19 patients can experience oral manifestations like lesions, ulcers, erythematous plaque, dysgeusia, and dysphagia. It is not clear whether these manifestations are caused by a viral infection, opportunist infections (e.g., oral candidiasis), immunosuppression, or COVID-19 agonist treatment [11,27,29,30]. There are some cases in which RT-PCR was performed on patients confirming a diagnosis of COVID-19; however, the oral candidiasis was confirmed only by a clinical diagnosis [11,27,28,29]. In fact, the diagnosis of all oral candidiasis manifestations is substantially clinical and is based on the observation of lesions without performing a biopsy (except in cases of candida leukoplakia) [8].
Common risk factors for COVID-19 (for example, immunosuppression, steroid and antibiotic therapies, oral manifestations, and salivary gland damage) can lead to the development of oral candidiasis [3,9,10,11,27,29,30]. In patients with COVID-19, the SARS-CoV-2 virus compromises the immune response since the virus can affect the production, decrease, or release of some mediators: interleukin-6, tumor necrosis factor, interleukin-1α, and interleukin-1β. One of them, salivary histatin-5, is damaged, which inhibits Candida albicans [9].
Other factors also involve the relationship between oral candidiasis and COVID-19. The angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 receptors, proteins that mediate the SARS-CoV-2 virus entry in the host cells, and the spike protein of the SARS-CoV-2 virus are present in the oral mucosa. This suggests the emergence of oral manifestations [29,31].
Virág et al. (2021) [32] investigated the effect of antivirals against the SARS-CoV-2 virus by testing them in Vero E6 cell-based cytopathic assays on Wuhan and British mutant strains. The mechanism of this effect involves the binding of nystatin to cholesterol found in the plasma membrane of the cell host. The viral envelope fusion with the plasma membrane of the cell host occurs for the entry of the virus. Therefore, in the post-pandemic scenario where novel strains of the SARS-CoV-2 virus have been reported, NYS should be investigated as an alternative use [32].
3. Nystatin (NYS)
NYS was discovered in the 1950s by Hazen and Brown [32,33,34,35]. In therapeutics, NYS has been employed for many years, demonstrating its safety and efficacy with good pharmacological action; however, few relevant adverse effects are reported. More recently, NYS has been used more frequently due to the increase in the number of cases of candidiasis in patients with neoplasms, HV, and other systemic disorders, including COVID-19 [9,36,37,38]. Figure 1 shows the chemical structure of NYS.
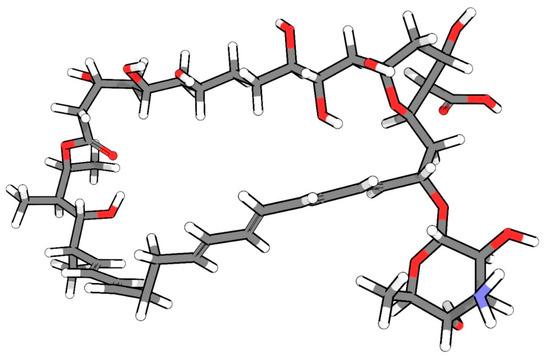
Figure 1.
Chemical structure of NYS in 3D (carbon atoms are in gray, hydrogen atoms in white, oxygen atoms in red, and nitrogen atoms in blue)
3.1. Physicochemical Characteristics
From a chemical point of view, NYS is considered a polyene, that is, belonging to the group of substances formed by carbon atoms with several double bonds. In fact, NYS is a tetraene, which, more specifically, are polyenes that have four unsaturated double bonds in sequence [34,39]. It is still considered a macrolide; that is, it has a large cyclic chain. These chemical characteristics are usually associated with its low permeability, which prevents it from crossing intestinal cells when administered orally. Finally, this molecule has carboxylic and amino groups, which leads to pKa: 3.62 (acidic) and 9.11 (basic) [32,40,41,42].
NYS is considered practically insoluble in water, chloroform, ethyl ether, and ethanol; poorly soluble in methanol; and easily soluble in dimethylformamide and dimethylsufoxide. This drug presents as a hygroscopic powder, yellow, fine, and has a characteristic odor, often reported as cereals (and with an extremely unpleasant taste, according to Souza et al., 2023 [33]). In addition, NYS has sensitivity to heat, light, and the presence of oxygen [32,39].
NYS is defined as a substance or a mixture of two or more substances. This complexity occurs due to its production process. NYS is obtained by fermentation, and three biologically active compounds are produced: NYS A1, NYS A2, and NYS A3 [32,43].
The main component is NYS A1, which has D-mycosamine bound to carbon 19 oxygen. NYS A2 also has D-mycosamine, bound to the same carbon. However, this compound has differences in the stereochemistry of the hydroxyl, methyl, and D-mycosamine groups. And yet, it does not have a hydroxyl on carbon 10, and it has a hydroxyl on carbon 16 (instead of carbonyl). NYS A3 also has the sugar D-mycosamine on carbon 19, but another sugar is found, L-digitoxose on carbon 35. It also presents variations in the stereochemistry in the groups of hydroxyl, methyl, and D-mycosamine groups [32,43].
3.2. Mechanism of Action and Pharmacological Aspects
This antifungal is classified as Class IV of the biopharmaceutical classification system (BCS), presenting low solubility and low permeability. Due to its low permeability, it is weakly absorbed by the skin, mucous membranes, or gastrointestinal tract, and its therapeutic use is restricted to the treatment of fungal infections topically. When administered orally, most of it is found in the stool unchanged [32,44,45,46]. Due to its low aqueous solubility, NYS offers difficulties in the preparation of medications, besides being less available to exert its action topically, especially in the treatment of oral candidiasis, since the oral mucosa is bathed in saliva [44,46].
The NYS molecule has much similarity to amphotericin B, another antifungal polyene, and both have the same mechanism of action. However, amphotericin B is used for the treatment of systemic and local infections, and NYS is restricted to topical application, used in the prophylaxis and treatment of superficial candidiasis of the skin and mucous membranes, as it is effective against most infections caused by Candida species [41,45].
The mechanism of antifungal action of NYS occurs through its interaction with ergosterol, a sterol present in the plasma membrane of fungal cells, causing disorganization in the plastic membrane of the fungus since channels are formed, which leads to a loss of selective permeability [34,47]. Through these channels occurs the outflow of water and ions, essential for cell survival, which culminates in cell damage and then in death [35,36,39,41,47]. Some authors propose a sequence of three events for the action of NYS:
- (A)
- the binding of an antifungal monomer with the plasma membrane of the fungus;
- (B)
- the formation of an oligomonomer;
- (C)
- and its insertion in the lipid bilayer, generating a pore through which the passive flow of molecules through the membrane occurs. Although this explanation is still controversial [32,39,47].
NYS also binds weakly to cholesterol, a sterol present in the plasma membrane of mammalian cells. It is this link that explains its adverse and toxic effects, and therefore NYS should not be administered parenterally [34,41,45]. When administered by this route, it can cause hemolysis, necrosis, and cold abscesses at the injection sites due to its immediate binding with the plasma membranes of red blood cells, interfering with their structure [32,35] Thus, when NYS is administered orally, the desired effect is a superficial effect on the oral mucosa that lines the gastrointestinal tract [45].
In the American and Canadian markets, NYS in the form of liposomes is found for the treatment of invasive intravenous infections. In this case, NYS is not restricted to the treatment of candidiasis but can also be employed to combat Aspergillus spp. infections. This liposomal form of NYS can be used intravenously since it does not present the adverse effects of NYS in the conventional form. This is explained by the presence of lipids in the composition of liposomes, which have a higher affinity for NYS than cholesterol, but NYS’s affinity for ergosterol still remains higher [32,34,39,48].
When administered topically, the adverse effects of NYS are uncommon, and episodes of nausea, vomiting, and diarrhea may occur, as well as allergic reactions [49]. In many cases, nausea is caused by the extremely unpleasant taste of the drug, which can compromise compliance to treatment [49].
3.3. Pharmaceutical Preparations Containing NYS
NYS can be found in several pharmaceutical forms, intended for cutaneous, vaginal, and oral administration, and is found in the national and international markets for the treatment of vaginal, oral, esophageal, intestinal, and cutaneous candidiasis [7,32]. Therefore, NYS can be found in the following pharmaceutical forms: powder, tablets, creams, ointments, pastilles, and suspensions. The type of pharmaceutical form used will depend on the type of candidiasis to be treated [39,40,41,42]. Table 4 presents pharmaceutical forms of NYS.

Table 4.
Dosage forms containing nystatin (NYS) for the treatment of fungal infections and type and site of administration [39,40,41,42].
The NYS aqueous oral suspension is used in the treatment of oral candidiasis, whose concentration is 100,000 IU/mL. The patient is instructed to apply 5 mL of the drug 3 to 4 times a day, performing mouthwash for 5 min, with subsequent swallowing of the product. Treatment lasts from 7 to 14 days [50]. In the Brazilian market, only this pharmaceutical form of NYS is found for the treatment of oral and oropharyngeal candidiasis. However, in other countries, NYS is available in the pharmaceutical form of medicated lozenges and oral tablets. Each tablet contains 200,000 IU of NYS and should be administered 1 to 2 units, 4 to 5 times a day, for 10 to 14 days. Each tablet has 500,000 IU, which should be applied 3 times a day for 7 to 14 days [40]. Nystatin oral suspension is usually administered for mild to moderate conditions, and swallowing the suspension can impact the liver’s function [38].
Aguiar et al. (2010) [51] described a preliminary clinical study with 14 patients who were diagnosed with oral candidiasis. The subjects were divided into 2 groups, where the first received the treatment with the use of 1 NYS oral tablet containing 500,000 IU, 4 times a day, for 7 days. The second group was treated with 5 mL of NYS oral suspension (total dose of 500,000 IU), applied 4 times a day, also for 7 days. The treatment performed with oral tablets proved to be more effective, eradicating the infection more quickly. This data suggest that the increased contact between the drug and the oral mucosa led to a more pronounced therapeutic effect. Thus, the use of dosage forms that allow intimate contact with the mucosa and have higher residual power may provide a more prominent pharmacological action. NYS is known for its remarkable post-antifungal effect (a delay in fungal regrowth that persists after a brief exposure to an antifungal agent). For that reason, a topical pastille of NYS was developed. The benefits of using those in substitution for oral NYS suspension are controversial; however, the combination of the two therapies appears to be more effective in treating oral candidiasis [52].
Topical therapy using NYS is the backbone for oral candidiasis treatment. The main reasons are its increased efficacy, low cost, and fewer side effects when compared with triazoles and echinocandins. To improve nystatin action, pharmaceutical systems have been developed to increase its aqueous solubility [32,34]. Another interesting treatment with high efficacy is photodynamic therapy; however, its high cost reduces its accessibility. For denture stomatitis, NYS suspensions of 100,000 IU and six sessions of photodynamic therapy showed the same results [39]. Regarding the COVID-19 patients, the overconsumption of corticosteroids and antimicrobial therapy was associated with multiple Candida strains; however, NYS suspensions showed good outcomes as treatment [53].
4. Conclusions
Oral candidiasis is an opportunistic fungal infection that requires an early diagnosis for better treatment and outcomes. The diagnosis is commonly performed by observing lesions in the oral cavity. The COVID-19 pandemic brought a new predisposing factor, contributing to the prevalence of the disease. The mainstay of therapy is NYS. The protocol of the NYS suspension of 1,000,000 IU is an effective drug against oral candidiasis, despite its low solubility and low permeability, which affect its availability in the oral cavity. Other pharmaceutical forms for the improvement of nystatin solubility seem to be an interesting alternative. However, the risk-benefit ratio for the patient must be discussed, especially in an acute COVID-19 scenario. The NYS binding to membrane sterols, specifically cholesterol in human cells, impairs the SAR-CoV-2 virus budding process; however, it cannot be used as an antiviral drug to treat COVID-19. Nonetheless, NYS can be considered a disinfectant for the gastrointestinal tract against the SAR-CoV-2 virus; thus, pharmaceutical technologies that are relevant to improving the aqueous medium solubility/dispersibility to deliver NYS must be studied. Finally, investigation is still needed to better understand the relationship between NYS, COVID-19, and oral candidiasis.
Author Contributions
Conceptualization, C.H.d.R.S. and M.M.G.B.d.A.; methodology, C.H.d.R.S., R.M.M., A.R.B. and M.M.G.B.d.A.; formal analysis, C.H.d.R.S., A.R.B. and M.M.G.B.d.A.; investigation, R.M.M. and M.M.G.B.d.A.; writing—original draft preparation, C.H.d.R.S., R.M.M., A.R.B. and M.M.G.B.d.A.; writing—review and editing, R.M.M. and A.R.B.; supervision, C.H.d.R.S.; project administration, C.H.d.R.S.; funding acquisition, A.R.B. and C.H.d.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Process 303862/2022-0); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES, Finance Code 001); PrInt USP–PAME (call 15/2023); and Provost of Inclusion and Belonging and Provost of Research and Innovation, University of São Paulo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
M.M.G.B.d.A. is highly thankful to the Provost of Inclusion and Belonging (Pró-Reitoria de Inclusão e Pertencimento, PRIP) and Provost of Research and Innovation (Pró-Reitoria de Pesquisa e Inovação), University of São Paulo, for the post-doctoral fellowship (001/2023). R.M.M. acknowledges deeply the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil, CAPES, for the doctoral scholarship. A.R.B. is highly thankful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, for the Research Productivity Scholarship, and to the PrInt USP/CAPES Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- López-Martínez, R. Candidosis, a new challenge. Clin. Dermatol. 2010, 28, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Giannini, P.J.; Shetty, K.V. Diagnosis and management of oral candidiasis. Otolaryngol. Clin. N. Am. 2011, 44, 231–240. [Google Scholar] [CrossRef]
- Rautemaa, R.; Ramage, G. Oral candidosis clinical challenges of a biofilm disease. Crit. Rev. Microbiol. 2011, 37, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.O.; Williams, D.W. Diagnosis and management of oral candidosis. Br. Dent. J. 2017, 223, 675–681. [Google Scholar] [CrossRef]
- Hu, L.; He, C.; Zhao, C.; Chen, X.; Hua, H.; Yan, Z. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb. Pathog. 2019, 134, 103575. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Guimarães, T.; Camargo, L.F.A.; Richtmann, R.; de Queiroz-Telles, F.; Salles, M.J.C.; da Cunha, C.A.; Yasuda, M.A.S.; Moretti, M.L.; Nucci, M. Brazilian guidelines for the management of candidiasis—A joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz. J. Infect. Dis. 2013, 17, 283–312. [Google Scholar] [CrossRef]
- Lu, S.Y. Oral candidosis: Pathophysiology and best practice for diagnosis, classification, and successful management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef]
- Tsai, C.S.; Lee, S.S.J.; Chen, W.C.; Tseng, C.H.; Lee, N.Y.; Chen, P.L.; Li, M.C.; Syue, L.S.; Lo, C.L.; Ko, W.C.; et al. COVID-19-associated candidiasis and the emerging concern of Candida Auris infections. J. Microbiol. Immunol. Infect. 2022, 56, 672–679. [Google Scholar] [CrossRef]
- Corchuelo, J.; Ulloa, F.C. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int. J. Infect. Dis. 2020, 100, 154–157. [Google Scholar] [CrossRef]
- Berlingieri, G.; Alvares, C.M.A.; Serrano, R.V.; Palma, L.F.; Campos, L. Phototherapies for COVID-19-associated opportunistic oral infections. Photodiagnosis Photodyn. Ther. 2022, 37, 102678. [Google Scholar] [CrossRef] [PubMed]
- Segrelles-Calvo, G.; de S Araújo, G.R.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Melean, N.R.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Candida spp. co-infection in COVID-19 patients with severe pneumonia: Prevalence study and associated risk factors. Respir. Y Med. 2021, 188, 106619. [Google Scholar] [CrossRef] [PubMed]
- Ten Cate, J.M.; Klis, F.M.; Pereira-Cenci, T.; Crielaard, W.; De Groot, P.W.J. Molecular and cellular mechanisms that lead to Candida biofilm formation. J. Dent. Res. 2009, 88, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and white manifestations in the oral cavity. Head Neck Pathol. 2019, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral candidiasis: A disease of opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Nwizu, N.; Chiquet, B. Candidiasis in the pediatric population: A case report and review of best practices. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, e53. [Google Scholar] [CrossRef]
- Devcic, M.K.; Simonic-Kocijan, S.; Prpic, J.; Paskovic, I.; Cabov, T.; Kovac, Z.; Glazar, I. Oral candidal colonization in patients with different prosthetic appliances. J. Fungi 2021, 7, 662. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.Y.; Kim, J.-W.; Yu, D.S.; Lee, Y.B. A case of cheilocandidiasis. Ann. Dermatol. 2019, 31, S22–S23. [Google Scholar] [CrossRef]
- Hayran, Y.; Sarikaya, I.; Aydin, A.; Tekin, Y.H. Determination of the effective anticandidal concentration of denture cleanser tablets on some denture base resins. J. Appl. Oral Sci. 2018, 26, 1–10. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Pérez-Jardón, A.; Caponio, V.C.A.; Spirito, F.; Chamorro-Petronacci, C.M.; Álvarez-Calderón-Iglesias, Ó.; Gándara-Vila, P.; Lo Muzio, L.; Pérez-Sayáns, M. Oral chronic hyperplastic candidiasis and its potential risk of malignant transformation: A systematic review and prevalence meta-analysis. J. Fungi 2022, 8, 1093. [Google Scholar] [CrossRef]
- Okada, S.; Puel, A.; Casanova, J.-L.; Kobayashi, M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin. Transl. Immunol. 2016, 5, e114–e130. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, K.; Kanegane, H.; Kashimada, K.; Morio, T. Endocrinopathies in inborn errors of immunity. Prim. Care Clin. Off. Pract. 2021, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Ferré, E.M.N.; Schmitt, M.M.; Lionakis, M.S. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Front. Pediatr. 2021, 9, 723532. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Que, Y.A.; Revelly, J.P.; Pagani, J.L. Preventing invasive candida infections. Where could we do better? J. Hosp. Infect. 2015, 89, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Eichelberger, K.R.; Paul, S.; Peters, B.M.; Cassat, J.E. Candida–bacterial cross-kingdom interactions. Trends Microbiol. 2023, 26, 1–13. [Google Scholar] [CrossRef]
- Santana, L.A.M.; Vieira, W.A.; Gonçalo, R.I.C.; Santos, M.A.L.; Takeshita, W.M.; Miquita, L. Oral mucosa lesion in confirmed and non-vaccinated cases for COVID-19: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e241–e250. [Google Scholar] [CrossRef]
- Santos, J.A.; Normando, A.G.C.; Silva, R.L.C.; De Paula, R.M.; Cembranel, A.C.; Silva, A.R.S.; Guerra, E.N.S. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int. J. Infect. Dis. 2020, 97, 326–328. [Google Scholar] [CrossRef]
- Santos, A.L.; Mota, L.A.S.; Da Costa, G.A.; Gonçalo, R.C.; Barbosa, B.F.; Menezes, L.S.; Cavalcanti, M.G.P. Erythematous candidiasis in a patient with prolonged post-COVID-19 xerostomia: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, e84. [Google Scholar] [CrossRef]
- Villarroel-Dorrego, M.; Chacón, L.; Rosas, R.; Barrios, V.; Pernía, Y.; Vélez, H. Oral Findings in Patients With COVID-19. ACTAS Dermo-Sifiliográficas 2022, 113, T183–T186. [Google Scholar] [CrossRef]
- Bhujel, N.; Zaheer, K.; Singh, R.P. Oral mucosal lesions in patients with COVID-19: A systematic review. Br. J. Oral Maxillofac. Surg. 2021, 59, 1024–1030. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.J.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor-binding domain ofthe viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef] [PubMed]
- Virág, E.; Seffer, D.; Hűvös, A.P.; Varajti, K.; Hegedűs, G.; Jankovics, I.; Pallos, J.P. Repurposed Nystatin to Inhibit SARS-Cov-2 and Mutants in the GI Tract. Biomed. J. Sci. Tech. Res. 2021, 40, 31854–31865. [Google Scholar] [CrossRef]
- Sousa, F.; Nascimento, C.; Ferreira, D.; Reis, S.; Costa, P. Reviving the interest in the versatile drug nystatin: A multitude of strategies to increase its potential as an effective and safe antifungal agente. Adv. Drug Deliv. Rev. 2023, 199, 114969. [Google Scholar] [CrossRef]
- Hac-Wydro, K.; Dynarowicz-Łatka, P. Interaction between nystatin and natural membrane lipids in langmuir monolayers—The role of a phospholipid in the mechanism of polyenes mode of action. Biophys. Chem. 2006, 123, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Groeschke, J.; Solassol, I.; Bressolle, F.; Pinguet, F. Stability of amphotericin B and nystatin in antifungal mouthrinses containing sodium hydrogen carbonate. J. Pharm. Biomed. Anal. 2006, 42, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Dorocka-Bobkowska, B.; Konopka, K.; Düzgüneş, N. Influence of antifungal polyenes on the adhesion of Candida albicans and Candida glabrata to human epithelial cells in vitro. Arch. Oral Biol. 2003, 48, 805–814. [Google Scholar] [CrossRef]
- Sharifi, M.; Badiee, P.; Abastabar, M.; Morovati, H.; Haghani, I.; Noorbakhsh, M.; Mohammadi, R. A 3-year study of Candida infections among patients with malignancy: Etiologic agents and antifungal susceptibility profile. Front. Cell. Infect. Microbiol. 2023, 13, 555. [Google Scholar] [CrossRef]
- Hapid, M.H.; Dewi, T.S. COVID-19 infection as an exacerbated factor of oral candidiasis in HIV/AIDS Patient. Int. Med. Case Rep. J. 2023, 16, 303–310. [Google Scholar] [CrossRef]
- Stefanovic, J.; Jakovljevic, D.; Gojgic-Cvijovic, G.; Lazic, M.; Vrvic, M. Synthesis, characterization, and antifungal activity of nystatin-gum arabic conjugates. J. Appl. Polym. Sci. 2013, 4736–4743. [Google Scholar] [CrossRef]
- Law, V.; Knox, C.; Djoumbou, Y.; Jewison, T.; Guo, A.C.; Liu, Y.; MacIejewski, A.; Arndt, D.; Wilson, M.; Neveu, V.; et al. DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acid Res. 2014, 42, D1091–D1097. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. The effects of pluronic block copolymers on the aggregation state of nystatin. J. Control. Release 2004, 95, 161–171. [Google Scholar] [CrossRef]
- DrugBank 5.1.10. Available online: http://www.drugbank.ca/drugs/DB00646 (accessed on 19 July 2023).
- Brescansin, E.G.; Portilho, M.; Teixeira Pessine, F.B. Physical and chemical analysis of commercial nystatin. Acta Sci. Health Sci. 2013, 35, 216–221. [Google Scholar] [CrossRef]
- Sakeer, K.; Al-Zein, H.; Hassan, I.; Desai, S.; Nokhodchi, A. Enhancement of dissolution of nystatin from buccoadhesive tablets containing various surfactants and a solid dispersion formulation. Arch. Pharmacal Res. 2010, 33, 1771–1779. [Google Scholar] [CrossRef]
- Ship, J.A.; Vissink, A.; Challacombe, S.J. Use of prophylactic antifungals in the immunocompromised host. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 103, S6.e1–S6.e14. [Google Scholar] [CrossRef] [PubMed]
- Spulber, M.; Fifere, A.; Anamaria, D.A.; Fifere, N. Nystatin-Polyethylene Oxide Conjugates with Enhanced Solubility in Water. J. Incl. Phenom. 2011, 71, 87–93. [Google Scholar] [CrossRef]
- Silva, L.; Coutinho, A.; Fedorov, A.; Prieto, M. Nystatin-induced lipid vesicles permeabilization is strongly dependent on sterol structure. Biochim. Et Biophys. Acta Biomembr. 2006, 1758, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.; Efimova, S.; Alexandrov, A.; Omelchuk, O.; Ghazy, E.; Bychkova, E.; Zatonsky, G.; Grammatikova, N.; Dezhenkova, L.; Solovieva, S.; et al. Semisynthetic amides of amphotericin B and nystatin A1: A comparative study of in vitro activity/toxicity ratio in relation to selectivity to ergosterol membranes. Antibiotics 2023, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Quindós, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Y Oral Ciurgíar Bucal 2019, 24, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Misra, S.R.; Panda, S.; Sokolowski, G.; Mishra, L.; Das, R.; Lapinska, B. Nystatin effectiveness in oral candidiasis treatment: A systematic review & meta-analysis of clinical trials. Life 2022, 12, 1677. [Google Scholar]
- De Aguiar, M.M.G.B.; De Albuquerque, R.P.; Marinho, D.S.; Braga, B.R.S.; Dornelas, C.B.; Oliveira, A.; De Sousa, V.P.; Torres, S.R.; Alviano, D.S.; Alviano, C.S.; et al. Oral sustained release nystatin tablets for the treatment of oral candidiasis: Formulation development and validation of UV spectrophotometric analytical methodology for content determination. Drug Dev. Ind. Pharm. 2010, 36, 594–600. [Google Scholar] [CrossRef]
- Lyu, X.; Zhao, C.; Yan, Z.M.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Babamahmoodi, F.; Rezai, M.S.; Ahangarkani, F.; Mohammadi Kali, A.; Alizadeh-Navaei, R.; Alishahi, A.; Najafi, N.; Haddadi, A.; Davoudi, A.; Azargon, L.; et al. Multiple Candida strains causing oral infection in COVID-19 patients under corticosteroids and antibiotic therapy: An observational study. Front. Cell. Infect. Microbiol. 2022, 12, 1103226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).