Abstract
Breast cancer is a complex disease for which pharmacological treatment does not guarantee success or cure. In addition, current pharmacological therapies induce unwanted side effects due to their lack of specificity or selectivity. Therefore, it is necessary to develop new therapeutic options to improve these aspects. Currently, phytochemicals with antineoplastic properties have been identified from a wide variety of plant sources, and new therapeutic options have been developed based on the conjugation of drugs with polymeric matrices, resulting in nanoparticles or hydrogels with improved properties. Some antineoplastic drugs have been conjugated with antibodies to improve their selectivity and specificity. One of the most important advances in the treatment of breast cancer has been the development of cyclin inhibitors and gene therapy. This review provides an overview of drugs derived from medicinal plants and polymeric matrices with high potential for use in the treatment of breast cancer. We also highlight the clinical evidence for the use of anti-HER2 monoclonal antibodies and cyclin inhibitors in breast cancer, as well as the advantages of using conjugated antibodies. Finally, we mention some considerations that should be taken into account in the search for new therapeutic agents from phytochemicals, polymers, antibodies, cyclin inhibitors, and gene therapy focused on the treatment of breast cancer.
1. Introduction
Breast cancer is the most common malignancy worldwide and is the leading cause of death in women. In 2020, an estimated 19.3 million new cases of cancer were diagnosed, of which 11.7% were classified as breast cancer. Unfortunately, the mortality rate for this type of cancer was 6.9%. Although breast cancer mortality has decreased over the past 50 years due to improvements in diagnosis and treatment, it remains a global public health problem, particularly in developed countries [1]. The etiology of the disease is multifactorial and includes biological sex, age, level of economic development of the country of residence, hormonal status, genetic factors such as breast cancer gene dysfunction (BRCA1/2), consumption of processed foods, and obesity, among others [2,3].
The pharmacological treatment of breast cancer tumors depends largely on the type of tumor diagnosed [4,5,6]. However, their classification is far from simple. The classification of breast cancer tumors requires the following: (1) histopathologic analysis to determine the malignancy or degree of infiltration, the tumor architecture, and cytologic characteristics of the tumor, and (2) molecular analysis of characteristic markers, such as estrogen receptors (ERs), progesterone receptors (PRs), human epidermal growth factor receptor 2 (HER2), and (3) cell proliferation markers such as Ki67. These data are essential for determining tumor behavior and response to a given drug therapy [2,7,8,9].
Once the type of tumor has been determined, treatment usually involves a combination of different procedures, depending on the extent and severity of each patient’s breast cancer. There are local treatments (surgery and radiation therapy) and systemic treatments (chemotherapy, hormone therapy, and immunotherapy) [4,5]. Systemic treatments, such as chemotherapy, are designed to reduce the growth of highly proliferating cells that have spread throughout the patient’s body. The chemotherapeutic agents used for breast cancer can be divided into three antineoplastic classes: (1) those that act on DNA (alkylating agents such as cyclophosphamide; cytotoxic antibiotics such as doxorubicin; and antimetabolites such as 5-fluorouracil and camptothecin derivatives), (2) those that do not act on DNA but on extracellular cell division factors such as paclitaxel (taxane), and (3) bisphosphonates in the adjuvant treatment of breast cancer [10].
It is important to note that a large number and variety of compounds derived from medicinal plants have antineoplastic activity, such as vinca alkaloids, taxanes, epipodophyllotoxins, and camptothecin derivatives, among others. Antineoplastic drugs derived from plants are mostly characterized as antimitotic agents, which induce the death of cancer cells while they are in mitosis [11,12]. For this reason, they also affect healthy cells and cause several unwanted side effects in most chemotherapies. However, their cytotoxic effects are also associated with mechanisms such as free radical scavenging, the reduction of tumor angiogenesis, and activation of multiple signaling pathways mediated by membrane receptors, kinases, transcription factors, microRNAs, cyclins, and caspases [13,14].
Hormonal therapy is another form of systemic therapy that is indicated as the treatment of choice for hormone-dependent (estrogen and/or progesterone receptor) breast cancer [2]. It is given over a long period of time, which increases the risk of significant side effects. Immunotherapy differs from other treatments that not only destroy malignant cells but also significantly affect healthy tissue [15]. The goal of immunotherapy is to stimulate the patient’s immune system to recognize and selectively destroy malignant tumor cells. It is usually combined with other therapies to achieve better results, as it provides proven benefits without increasing side effects [16]. Currently, there are several forms of immunotherapy: (a) active immunotherapy (vaccines), in which the tumor proteins are injected into the patient; (b) adoptive cell transfer, in which cells from the patient’s immune system are used to expand them and help them respond; (c) regulatory agents, such as cytokines, which are injected into the patient to enhance the immune response; and (d) monoclonal antibodies, which are injected to bind to specific receptors in the patient’s body [15,17].
One of the major problems in the pharmacological treatment of breast cancer, whether chemotherapy, hormone therapy, or immunotherapy, is the lack of specificity of the treatments for diseased or healthy tissues. One way to reduce the non-specificity of drug treatments on certain tissues is to couple them to controlled and specific release systems, among which we can highlight nanoparticles and hydrogels composed of polymeric matrices and the conjugation of two or more elements in a therapeutic option whose objective is to increase their safety [18,19,20]. On the other hand, the fact that genetic alterations occurring in tumor cells or in their microenvironment affect the efficacy of drug therapy makes gene therapy an increasingly considered a relevant tool for this disease [21].
This review provides an overview of drugs derived from medicinal plants and polymeric matrices with high potential for use in the treatment of breast cancer. We also highlight the clinical evidence for the use of anti-HER2 monoclonal antibodies and cyclin inhibitors in breast cancer, as well as the advantages of using conjugated antibodies. Finally, we mention some considerations that should be taken into account in the development of gene therapies focused on the treatment of breast cancer.
2. Herbal Treatments for Breast Cancer
Many therapies are used to fight and eradicate breast cancer: surgery, radiation therapy, chemotherapy, and hormone therapy [10]. However, aggressive treatments such as radiation and chemotherapy can have adverse effects on the body because they do not only target tumor cells and can negatively affect patients. One of these side effects, which significantly influences the success of the treatment, is the reduction of the immune system activity, leading to immunosuppression, which promotes the development and progression of tumors [22].
It is important to note that some types of breast cancer are resistant to conventional therapies, which poses a significant challenge in eradicating malignant cells [22]. Due to the aforementioned drawbacks and similar concerns, it has become necessary to explore new therapeutic options to treat breast cancer effectively and, at the same time to be less aggressive to the body, causing minimal or no side effects, less toxicity, and less likelihood of inducing treatment resistance to improve the patient’s quality of life [22,23].
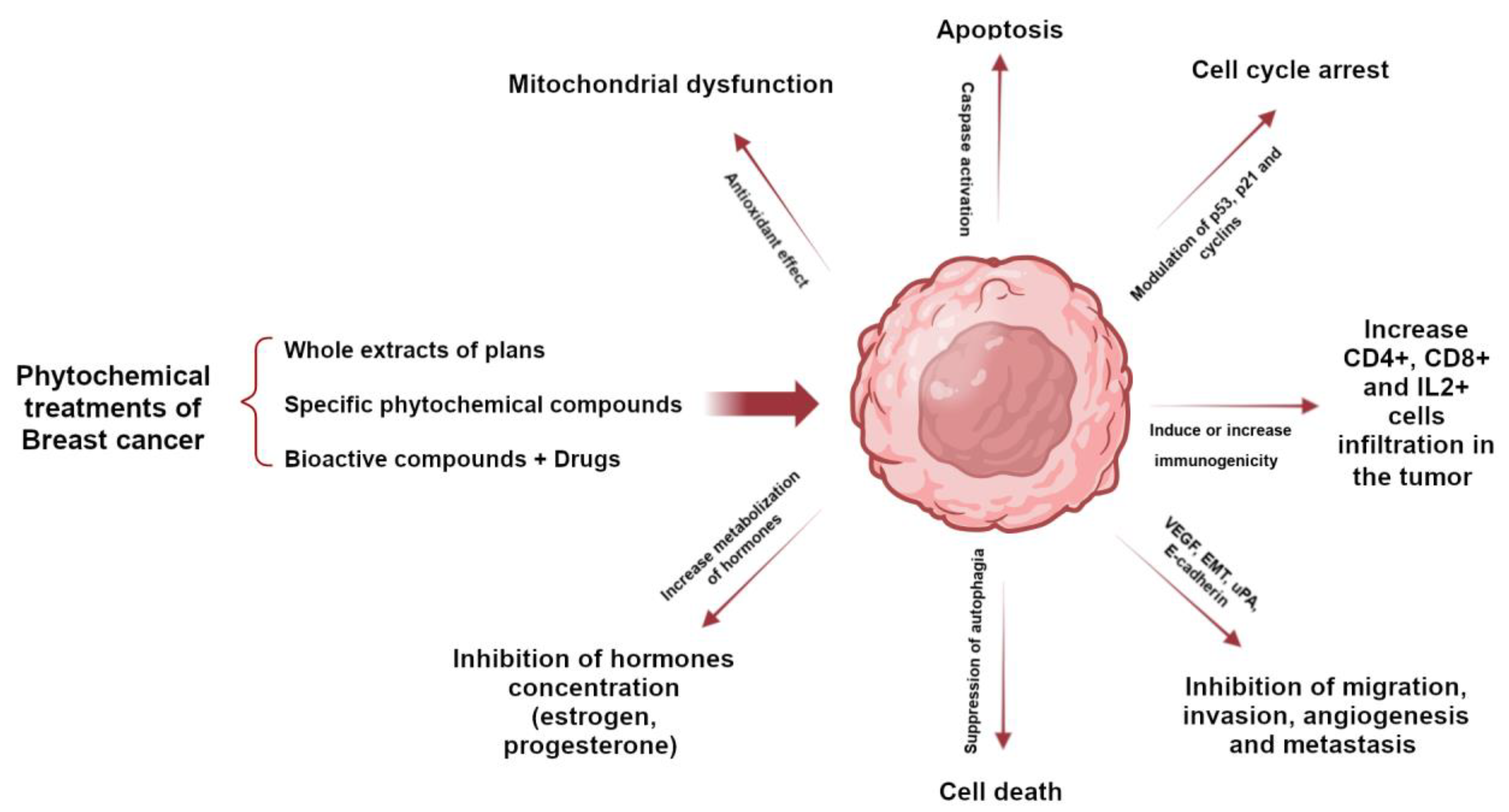
The phytochemical components of plants are a therapeutic option whose properties have been known for a long time; however, with the appearance of synthesized chemical compounds with anti-cancer activity, the use of plants decreased. A few years ago, the use of natural remedies to treat various diseases became relevant again due to their preventive and therapeutic properties. Plants possess phytochemical components with antioxidant and anti-inflammatory properties, mainly coumarins, flavonoids, alkaloids, and terpenoids. Several studies have demonstrated that bioactive compounds from plants can exert anti-tumor activity through various signaling pathways, such as apoptosis, autophagy, modification of the tumor microenvironment, cell arrest, and the suppression of angiogenesis. Selected plants are included in Table 1 because they have many in vitro studies on different types of breast cancer cell lines and in vivo studies in different mouse models of breast tumors. Studies have shown that the structural parts of plants, such as leaves, fruits, and roots, contain chemical compounds with great potential. These compounds have been extracted using various methods and used as whole extracts or fractions to test their effectiveness in inhibiting the growth of various types of breast cancer that affect women worldwide. Certain compounds have been extensively studied and exhibit anti-tumor properties, as they have both anti-proliferative and anti-apoptotic effects. In addition, they can modulate the immune system by activating immune cells and cytokines that can attack or inhibit tumor cell growth (Figure 1) [24,25,26,27,28].

Table 1.
Anti-cancer effects of plants and extracts on breast cancer cell lines and experimental models. LD, lethal dose 50; mg, milligram; kg, kilogram; µg, microgram; ml, milliliter; Rg3, ginsenoside Rg3; SD, Sprague Dawley.
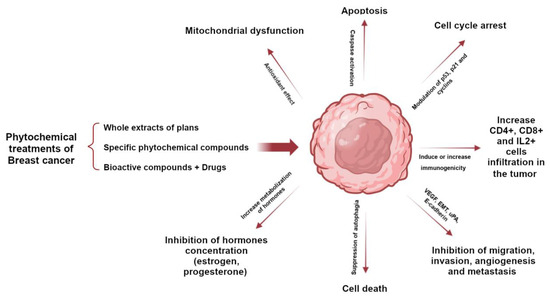
Figure 1.
Role of phytochemicals in breast cancer to induce tumor cell death through activation or inhibition of signaling pathways.
Most of the active ingredients of phytopharmaceuticals are poorly soluble in water, which limits their bioavailability, their ability to cross biological membranes, and thus their effective use in the treatment of various diseases, including cancer. The stability of phytopharmaceuticals and their low specificity are also relevant aspects in their clinical application [71].
On the other hand, the techniques chosen for the extraction of active compounds from plant species (phytopharmaceuticals) depend on the chemical nature and physicochemical properties of these compounds, in addition to the characteristics of the plant material to be worked with. It is important to consider that these compounds are immersed in a complex matrix that includes plant structures that make them difficult to obtain, in addition to biomolecules with different functional groups and polarities [72].
Once the extraction process is complete, the compound of interest will most likely be found as part of a mixture of different compounds that were also extracted with it. It is possible to perform a bioassay-guided fractionation of the extract by separating its fractions. The resulting fractions are evaluated by a biological assay to identify those that induce the desired biological activity. This fractionation–bioassay process is repeated until a compound is obtained that is as pure as possible or until a biological activity is obtained at low concentrations of the evaluated fractions with a sufficient yield to perform all required assays [71,72].
The potential of phytopharmaceuticals has already been widely demonstrated in the clinic. Medicinal plants, or products derived from medicinal plants, including pure molecules, extracts, or fractions of extracts, have historically been the major source of anti-cancer drugs approved for use in humans; phytopharmacology has resulted in highly successful drugs in the clinic. Phytochemicals synthesized by plants with anti-tumor activity in breast cancer have a wider margin of safety. They can act synergistically with chemotherapeutic drugs to increase the efficacy of treatment and sensitize tumor cells to chemotherapy with the advantages of reducing the dose of the chemotherapy and reducing the adverse and toxic effects of chemotherapy (Table 2) [73,74], either by interacting pharmacologically (pure compounds administered separately) or by targeting and delivering of both compounds via nanocarriers.

Table 2.
Research on the potential benefits of combining herbal treatments with established chemotherapy drugs for breast cancer.
3. Polymer-Based Therapies for Breast Cancer
Polymers are macromolecular compounds composed of multiple repeating units, called monomers, linked in sequence by chains or branches [92]. The length of their structure and their side chains determine the general molecular interactions of the polymer and will affect its functions [93]. The properties of these materials can be modified by manipulating the polymer chain length, branching, and side chains to suit a wide variety of applications. This shows great versatility, making polymers one of the most widely used materials in the field of biomedicine [94].
3.1. Nanoparticles and Nanocarriers
In breast cancer treatment, the use of nanotechnology to develop drug delivery systems has demonstrated advantages over conventional drug delivery. This is because nanoparticles allow drugs to be transported to the desired site of action, minimizing side effects on healthy tissue, and reducing the dose required for drug action. These systems protect the drug from rapid degradation and elimination, improving efficacy and biodistribution. Among nanoparticulate drug delivery systems, polymer-based formulations have improved stability in electrolyte solutions and biological fluids, as well as possessing good storability and bioavailability. Nanoparticulate polymeric drug delivery systems can be designed to carry one or more drugs of different chemical nature and allow for the development of personalized therapies through changes in surface chemistry or through conjugation of biological molecules, which promotes the efficient delivery of therapeutic agents in a controlled, targeted, and reengineered delivery of therapeutic agents. The main obstacles to be considered in the development of nanoparticles (NPs) for the effective treatment of breast cancer are the non-specific distribution of administered anti-tumor drugs in the organism, the inability to achieve sufficiently high concentrations at the tumor site, and the resistance developed by cancer cells to various types of chemotherapy [18,20].
NPs have properties such as small size, high specific surface area value, high surface reactivity, unique physicochemical properties, high loading capacity, and biocompatibility [18,20]. These properties make them ideal for use as nanocarriers. The improvement of properties such as pharmacokinetics and bioavailability, the customization of drug release, modification of therapeutic approach through active or passive orientation, reduction of toxicity, and solubility and stability, in addition to the controlled release and delivery of site-specific therapeutic agents (acting on cancer cells), are some of the features that nanocarriers can incorporate into drug delivery systems [95]. These tools have been studied to minimize the side effects of chemotherapeutic drugs on healthy tissues. Among the most widely used synthetic polymers are polylactic acid (PLA), polyglycolic acid (PGA), polylactic-co-glycolic acid (PLGA), polyethylene glycol (PEG), polycaprolactone (PCL), copolymer of N-(2-hydroxypropyl) methacrylamide (HPMA), polyaspartic acid (PAA), and polyglutamic acid. On the other hand, among the most commonly used natural polymers include albumin, alginate, chitosan, collagen, dextran, gelatin, and heparin [95].
Among the studies carried out with the support of nanoparticles are those reported by Guo et al., using the polymer composition 2,3-dimethylmaleicoanhydro-PEG-ε-polylysine; this pH-responsive nanoparticle system was obtained to co-deliver the small molecule chemotherapeutics doxorubicin (DOX) and lapatinib (LAP) to breast cancer cells, specifically to the MCF-7 cell line, reporting a half-maximal inhibitory concentration (IC50) with a decrease of 0.62 at concentrations of 0.5 µg/mL and greater toxicity at pH 6.8 [96]. Shakeran et al. studied NPs composed of SiO2-chitosan functionalized with APTES (3-aminopropyl) triethoxysilane) to deliver MTX (methotrexate); the cytotoxic potential to kill breast cancer cells (MCF-7) was reported, and a positive effect was demonstrated at a low dose of MTX (0.5 mΜ), with a loading efficiency (LE) of 13.9% at pH 7.4 [97]. Another study conducted by Xiao et al. reported that zoledronic acid encapsulated in nanoparticles of the PLGA-b-PEG polymer showed favorable results in in vitro tests on MCF-7 cells; the drug dose required to achieve the IC50 was approximately 37.5 µM lower than the zoledronic acid-loaded charged polymers [98]. Also, Bobde et al. worked on the development of DOX-loaded NPs for the treatment of breast cancer, formed with N-(2-hydroxypropyl) methacrylamide, a system of about 150 nm, with controlled release by internal stimuli (pH); their IC50 after 72 h of treatment were established at 1406 μg/mL for 4T1 cells, 1946 μg/mL for the MCF-7 cell line, and 0.477 μg/mL for MDA-MB-231 cells [99]. Recently, Gayathri et al. synthesized polyvinylpyrrolidone NPs with copper sulfide (PVPP-CuS) to deliver DOX as a treatment for breast cancer; viability levels were measured with NIR laser in MCF-7 cell line, 30 ± 5.8%, and 10.7 ± 3.2% survival was obtained with the drugs loaded on the polymer [100].
In general, advances in nanomaterial technology have led to NPs being considered as an important alternative in the biomedical field, particularly in the treatment of cancer [20]. No significant adverse complications have been reported or attributed to NPs compared to conventional treatments [18]. Nowadays, this type of option is still under investigation to discover the optimal load of the administered drug, as well as its controlled and optimal release, in addition to its safety and specificity [20]. It has been suggested that the combination of NPs with chemotherapy could be a new approach to prolong the survival of breast cancer patients. However, in the search for a treatment with minimal side effects, an improvement in the conjugation of these nanocarriers could be considered. One of the major challenges in this field is to adapt these particles to the new generation of drugs. Obstacles such as the difficulty of crossing the cell membrane and the narrow therapeutic window of drugs, as well as the challenge of improving the interaction of surface-bound ligands and receptors on cells and tissues, need to be overcome [95].
3.2. Hydrogels
Hydrogels are a system of three-dimensional networks of polymer chains and water that fill the space between macromolecules [101]. These hydrogels are formed by physical or chemical cross-linking, and their functional groups are located along the polymer chain, making them sensitive to stimuli [102]. They can absorb water, form localized deposits in the body that swell in the presence of fluids, and can encapsulate active ingredients [92,102]. In addition, they can respond to changes in factors such as temperature, pH, solvent concentration, and others [103]. In this way, they can absorb, retain and release organic solutions under controlled conditions. This, together with properties such as soft, elastic texture, water content, and low surface tension, make hydrogels very suitable materials for biomedical applications, as they interact well with living tissues [92]. Currently, it is desirable to deliver chemotherapeutic agents directly to the tumor site. This approach aims to achieve better localization and efficacy of the drug while trying to minimize systemic side effects [103].
In studies conducted by Fathi et al., DOX-loaded pH- and temperature-sensitive hydrogels were developed as a potential therapy for breast cancer. The hydrogels were designed by mixing synthesized poly(n-isopropylacrylamide-co-itaconic acid) (PNIAAm-co-IA) with chitosan (CS); the in vitro tests for cytotoxicity were performed on MCF-7 cells and determined 90% viability in cells treated with the polymers after 24 and 48 h of treatment without being under their release conditions (temperature and pH), demonstrating their cytocompatibility of the prepared hydrogels. DAPI staining showed that the number of cells decreased significantly after treatment with the drug-loaded hydrogel, confirming its anti-proliferative effect [104]. Cimen et al. [105] developed hydrogels with hydrazide (Gel-ADH) and aldehyde-functionalized PEG polymer (diBA-PEG) loaded with DOX, whose release was pH sensitive; the hydrogels showed good biocompatibility, with more than 85% cell viability after 48 h of incubation. Subsequently, both polymers were tested after 72 h of incubation, and 50.6 and 58.3% viability was found for MCF-7 and MDA-MB-231, respectively. The cytotoxic effect of the DOX-loaded hybrid hydrogels showed that 55.2 and 45.79% cell death was achieved in MCF-7 and MDA-MB-231 cells, respectively, after 72 h of treatment [105], suggesting a better effect with longer incubation time.
On the other hand, Sabino et al. synthesized injectable hydrogels based on cross-linked chitosan incorporated with DOX-loaded PEG nanoparticles; the hydrogels were chemo-photosensitive and their effect on neoplastic breast cancer cells was studied in MCF-7 cells incubated with the hydrogel and exposed to near infrared (NIR), which experienced a reduction in their viability of approximately 35% and also demonstrated a high specificity by reducing the viability of cancer cells by 85% when the polymer was not irradiated [106]. In addition, Zhu et al. reported a study on the application of pH- and glutathione-sensitive peptide-based hydrogels as a delivery material for the anti-tumor drug paclitaxel (PTX) and demonstrated its effect on two types of breast cancer cells, MCF-7 and 4T1; the IC50 of the modified hydrogel was 8.596 μg/mL. Furthermore, cell viability of over 90% was reported after 72 h of incubation with the polymeric base material, indicating that it is safe and non-toxic for anti-tumor drugs [107]. Lima-Sousa et al. developed injectable chitosan and agarose hydrogels incorporated with DOX and ibuprofen, as well as graphene oxide NPs for use on MCF-7 cells, resulting in a potential chemophotothermal therapy; the first modification of the hydrogel (thermogel-rGo) decreased cell viability by 60%, while the second (thermogel-Go) obtained a reduction of 73%. The cytocompatibility test based on cell viability remained above 90% [108].
The implementation of hydrogels as drug delivery systems has shown to be an exceptional contribution to the field of biomedicine, as they can be prepared with different polymeric materials and used as highly biocompatible drug delivery agents; they are now considered a promising option to improve the control of drug release in the body [109]. The interest in hydrogels has increased due to the availability of a wide range of polymeric structures with customizable and versatile properties; therefore, many of them are used in the pharmaceutical and biotechnological field, an example of their application is as drug delivery vehicles for chemotherapy and radiotherapy. Injectable hydrogels are systems suitable for local and systemic release that stabilize and solubilize poorly soluble drugs. This system can be administered intramuscularly, intravenously, and subcutaneously, allowing for controlled and targeted drug release and reduced toxicity. Injectable hydrogels have several advantages over conventional hydrogels because they can fill voids, they can be administered to the body by direct injection into the affected area or through a catheter, and, because they are biodegradable, the products derived from the gel are eliminated from the body without the need for surgical removal. Stimuli-sensitive injectable hydrogels are capable of undergoing physical or mechanical transitions in response to different stimuli. To be used in clinical trials, they must have superior mechanical and viscoelastic properties in order to withstand the possible deformations that may occur in the body [110].
3.3. Polymers without Drug Conjugation
Nowadays, the application of polymers without conjugation with drugs is also being studied. Torres-Rodríguez et al. reported the anti-proliferative effect of polymers derived from polyaspartate (PAspNa) with different quaternary ammonium salts derived from tertiary amines (pyridine, N,N-dimethylbenzylamine, and quinoline) in in vitro tests against different cancerous and normal cell lines (HeLa, 4T1, HepG2, and 3T3). The results showed a decrease in the proliferation of HeLa cells of more than 75% in all the concentrations used, while the polymer derivatives did not increase the anti-proliferative activity in 4T1 and HepG2. All chemical modifications affected the proliferation of the 3T3 cell line only at the concentration of 200 μg/mL. The same study reported a low level of hemolysis, demonstrating its hemocompatibility and preliminarily suggesting the safety of this polymer for anti-cancer therapy [111]. Velazco de la Garza et al. used derivatives of polysuccinimide (PSI) with different quaternary ammonium salts synthesized from tertiary amines (quinoline and N, N-dimethylbenzylamine) with an active terminal group; this polymer was shown to have mild hemolytic effects in human erythrocytes and in cell lines of murine fibroblasts (3T3); however, these PSI derivatives showed a decrease in cell proliferation in HeLa and 4T1 cell lines by about 10% [112]. It is interesting to note that the authors of this study recommended further investigation to determine cytotoxicity to better determine biocompatibility with other cells and future applications.
3.4. Perspectives on the Development of Polymers for the Treatment of Breast Cancer
The next generation of nanotechnology is expected to focus on combination therapies, targeted breast drug delivery, gene therapy, and new approaches in immunotherapy, radiation, and multimodal therapies [113]. Since tumor heterogeneity and drug resistance are the main factors that conventional chemotherapy has to deal with, it is feasible that the development of NPs with multi-drug loading will increase the efficacy of therapy and at the same time reduce the dosage by utilizing targeted delivery. After reviewing the current literature on polymeric treatments with a focus on breast cancer, we believe that it is essential to homologate the methodologies used to evaluate cytotoxicity (i.e., viability tests, cell line, cell density, etc.). While the utility of the methods used for it is not questioned, it is necessary that the results be standardized and comparable.
4. Monoclonal Antibody-Based Therapies
One of the greatest advances in breast cancer treatment over the past decade has been the development of monoclonal antibody immunotherapy, which is considered an important component of cancer treatment along with chemotherapy, radiation, and surgery. Monoclonal antibodies have a variety of clinically relevant mechanisms of action. In addition to directly attacking cancer cells, antibodies can also promote the generation of long-lasting anti-cancer immune responses. The diverse properties of antibodies as a therapeutic platform have led to the development of effective therapeutic strategies that will have a major impact on the treatment of breast cancer.
4.1. Monoclonal Antibodies
Monoclonal antibodies (mAbs) have been used in the development of immunotherapies for a wide variety of diseases, such as breast cancer, due to their ability to selectively recognize a specific antigen, resulting in minimal damage to healthy tissue surrounding the tumor [114]. mAbs have a very important role in immunization, as they have great potential as adjuncts to immune responses when the organism cannot produce them on its own, and confer unique specificity by recognizing and targeting specific antigens. In addition, there are different types of mAbs (murine, chimeric, and humanized) that, when used alone, are also combined with radiation, chemotherapy, and hormone therapy, which enhances their therapeutic properties [115].
Although all tumors that occur in the breast are called breast cancer, the disease can be divided into subtypes based on the status of the ER, PR, and HER2 [116]. Each breast cancer subtype has its own characteristics, such as age of onset, disease progression, and even treatment strategy. HER2-positive breast cancer accounts for 25% of all breast cancers [117]. The clinical classification of breast cancer is based on the presence of transmembrane receptors such as estrogen and progesterone, along with the amplification or overexpression of the HER2 protein or oncogene. In approximately one in five women with breast cancer, the cancer cells have too much growth-promoting protein (HER2) on their surface. These cancers, called HER2-positive breast tumors, tend to grow and spread more aggressively [118]. Therefore, the use of monoclonal antibodies in breast cancer restores or enhances the function of the immune system [119]. Because they block a specific target outside the cancer cells, these targets can also be found in the area around tumor cells/tissues [120].
Anti-HER2 monoclonal antibodies, such as trastuzumab and pertuzumab, are effective in all stages of HER2-positive breast cancer. Trastuzumab and pertuzumab are recombinant humanized anti-HER2 IgG1 mAbs designed to block HER2 transmembrane signaling to relevant intracellular molecules. In addition, pertuzumab avoids interactions with other HER2 family receptors that promote cell proliferation by binding to a different epitope of the HER2 dimerization domain than trastuzumab [117]. The anti-tumor activity of HER2 mAbs is largely due to their direct inhibitory effect on the extracellular domain of HER2. Interactions between anti-HER2 monoclonal antibodies and the immune system have been observed, resulting in antibody-dependent cytotoxicity.
Unfortunately, despite the apparent success of this therapy, approximately 70% of eligible patients fail to respond to treatment or develop resistance to treatment within a relatively short period of time. Studies in both experimental animals and patients support the notion that this resistance is due to four main mechanisms: (a) the expression of incomplete forms of HER2, preventing proper binding of the antibody; (b) the aberrant activation of members of the signaling cascade initiated by HER2 dimerization, allowing the induction of cell proliferation even when the receptors are inactivated; (c) the parallel expression of other proliferation-inducing receptors by the tumor cell; and (d) the steric hindrance of antibody binding caused by the presence of carbohydrates and bulky glycoproteins on the tumor cell surface [121].
4.2. Antibody–Drug Conjugates (ADCs)
In recent decades, a new class of drugs has been developed that exploit the affinity of monoclonal antibodies for their receptors to deliver potent cytotoxic molecules to cancer cells; these drugs are known as antibody–drug conjugates (ADCs). ADCs consist of three elements: a monoclonal antibody, a cytotoxic drug (payload), and a chemical linker that binds them together to deliver the cytotoxic drug to the target cell. The ADC binds to a protein or receptor on the surface of the tumor cell, and the cytotoxic drug is released there to destroy it [19].
ADCs are an attractive treatment option for HER2-positive breast cancer. Anti-HER2 therapies selectively target cancer cells that express HER2, of which trastuzumab was the first HER2-targeting mAb to achieve successful results, making it a mainstay of anti-HER2 therapies [117]. However, the drug-to-antibody ratio must also be considered when designing an ADC. A higher charge per unit of antibody confers greater anti-cancer efficacy, but also increased toxicity. Trastuzumab drug conjugates (T-DCs) are used as selective antibodies to target cytotoxic drugs or phytochemicals to cancer cells. For example, deruxtecan is a topoisomerase I inhibitor [122] and emtansine is a phytochemical inhibitor [123], both of which have been conjugated to trastuzumab and labeled as T-DXd and T-DM1, respectively. However, they differ in their mechanism of action and in their ability to cross the cell membrane when released from the ADC complex. The latter property is critical because it gives ADCs the ability to reach and kill neighboring tumor cells [122]. Evidence to date suggests that they represent an entirely new class of effective cancer therapies [124]. In fact, T-Dxd (Enhertu) is recommended for patients with unresectable or metastatic HER2-positive breast cancer [125], and disease progression is lower compared with other ADCs [124,126,127] or conventional chemotherapy [128]. However, early clinical trials have shown a significant risk of adverse events [129].
Some criteria to be considered in their development are that the ADCs should enter the systemic circulation, bind to the tumor cell antigen, internalize in the target cell in the same manner as the monoclonal antibody, and also allow the release of the cytotoxic agent and its interaction with the target sites of the cytotoxic agent in the tumor cell. A key element in the success of ADCs is the chemical linker, which must be stable at physiological pH (7.4), because once the ADC is internalized, the chemical linker must be cleaved by the pH of the medium or by proteolytic enzymes of the tumor cells. These can be divided into chemically labile agents and enzyme-sensitive agents [19].
ADCs are generally well-tolerated; however, some expected adverse reactions require careful monitoring and prompt intervention. Neutropenia, nausea and vomiting, alopecia, diarrhea, left ventricular failure, and interstitial lung disease/pneumonitis are examples of the toxicities that may occur during breast cancer with this treatment [127,130]. Severe hematologic adverse events, if not managed effectively, can lead to problems such as bleeding, febrile neutropenia, and likely subsequent infection leading to sepsis. Across all studies, the incidence of any grade of neutropenia in patients taking trastuzumab emtansine (T-DM1) ranged from 5% to 11% [127,131]. The incidence of severe anemia in patients receiving T-DM1 was as low as 2.7% [132].
Cardiotoxicity is a well-documented side effect of HER2-targeting drugs. HER2 receptors regulate cell development, homeostasis, and oxidative stress on cardiac myocytes. They also play an important role in fetal heart development. Many preclinical studies have elucidated the mechanisms underlying the cardiotoxicity of HER2-targeted drugs. HER2-targeted anti-cancer drugs can induce both irreversible and reversible cardiac damage. Arrhythmias, myocardial ischemia, hypertension, left ventricular dysfunction (LVD), and heart failure (HF) are all possible side effects [130].
ADCs were developed as a platform for delivering cytotoxic chemicals directly into cancer cells. The goal was to increase their activity while reducing side effects and treatment-related problems. In fact, most approved ADCs have a better overall safety profile compared to chemotherapy. However, ADCs can be hazardous in a variety of ways, depending on the chemical properties of the payload (i.e., hydrophilicity), the drug-to-antibody ratio (DAR), the stability of the linker (cleavable or not), and the expression of the target in non-cancerous tissues. In this sense, gastrointestinal toxicity is common in breast cancer patients treated with ADCs, and is largely caused by the effects of the cytotoxic payload on mucosal cells. Nausea and vomiting have a significant impact on patient quality of life and compliance. They can also cause systemic problems such as metabolic imbalances and nutrient depletion [128].
5. Cyclin-Dependent Kinase (CDK) 4/6 Inhibitors for Breast Cancer
Cyclin-dependent kinases (CDKs) are important enzymes in cell regulation [133]. The binding of CDK 4/6 to D1 cyclins, stimulated by the activation of estrogen receptors, phosphorylates the retinoblastoma tumor protein, leading to the deregulation of the CDK-RB1-E2F pathway, providing a signal to initiate cell division [134]. The use of cyclin-dependent kinase (CDK) 4/6 inhibitors as a targeted therapy to bypass cell cycle activation mechanisms and disrupt malignant cell proliferation in high-risk early-stage hormone receptor-positive and HER2-negative breast cancer has recently been explored [133].
There are three approved CDK 4/6 inhibitors: palbociclib, ribociclib, and abemaciclib. They are a class of pyrimidine derivatives that is usually used in combination with endocrine therapy (aromatase inhibitors or fulvestrant), and have shown good results in HR-positive, HER2-negative advanced breast cancer and have changed the first- and second-line treatment of advanced metastatic breast cancer disease [135].
Palbociclib was approved by the FDA in 2015 as an oral drug under the name of IBRANCE®. This inhibitor has been studied in combination with letrozole (PALOMA-2); this study started treatment with a concentration of 125 mg/day for palbociclib and letrozole 2.5 mg/day. This combination significantly prolonged progression-free survival (PFS) to 19.8 months compared to 13.8 months with letrozole plus placebo [136,137]. However, this combination was associated with any grade of adverse events in 36% of the 444 participants, and 9.7% discontinued treatment due to adverse events, the most common of which were neutropenia, leukopenia, anemia, diarrhea, asthenia, vomiting, and infections [137]. In the PALOMA-3 study, palbociclib plus fulvestrant increased progression-free survival to 34.9 months compared to 28 months for placebo plus fulvestrant. A total of 85 of the 347 patients discontinued due to disease progression, of which 2.6% was due to adverse events including neutropenia, leukopenia, fatigue, and nausea [138].
Following the approval of palbociclib between 2015 and 2016, a data review study was conducted in patients with metastatic breast cancer, aged between 24 and 91 years. This study was conducted to characterize the dose modifications of palbociclib due to its toxicity. The initial dose of palbociclib (125 mg/day) was modified in 38 patients, with 25 patients receiving a final dose of 100 mg/day, 4 patients receiving 75 mg/day, and the remaining patients receiving the initial dose. The most common dose-modification-related adverse event was grade 3–4 neutropenia, which occurred in 54.8% of patients [139].
KISQALI® (ribociclib) oral tablets (600 mg/day) were evaluated in the same way in combination with letrozole 2.5 mg/day; the MONALEESSA-2 study conducted the analysis in 668 patients with a follow-up of 15.3 months from the for which the PFS rate for the combination of ribociclib and letrozole was 63% compared to 42.2% for placebo. Disease progression was the reason for discontinuation in 87 of 334 patients and 146 of 334 patients receiving placebo. One of the most common adverse events was neutropenia, which occurred in 104 patients [140]. The results showed that ribociclib in combination with letrozole prolonged progression-free survival by 20.8% compared to placebo in HR+ and (HER2) advanced breast cancer, making it a first-line treatment option [141]. In a total of 43 patients treated with ribociclib and endocrine therapy, the dose of ribociclib was reduced; 58.1% of adverse events were neutropenia and leukopenia, which resolved and were well tolerated after their discontinuation and dose reduction [142].
Both VERZENIO® (abemaciclib) and KISQALI® (ribociclib) were approved by the FDA in 2017. VERZENIO® is available as an oral tablet for adjuvant use in combination with endocrine therapy (ET). In the phase III MonarchE study, the drug was evaluated in 5637 patients for 5 years on ET and 2 years on abemaciclib. The dose of VERZENIO® was 150 mg, while the dose of endocrine therapy depended on the physician. Ki-67 is a marker of cell proliferation that is prognostic outcome in breast cancer and helps predict the response to neoadjuvant chemotherapy or endocrine therapy [143]. Abemaciclib in combination with ET has been shown to reduce the expression of Ki-67 expression, making CDK 4/6 inhibition effective in high-risk tumors. This combination also performed well in terms of invasive-disease-free survival (IDFS) of 25% compared to the hormone therapy drug alone [144]. The most common side effects in this study in combination with abemaciclib plus ET were diarrhea, neutropenia, and fatigue. The MonarchE study evaluated the safety of abemaciclib in combination with endocrine therapy in 2791 patients receiving it. In this study, 61.7% of patients received a dose change due to adverse events, of which 43.4% were related to diarrhea and neutropenia. Dose changes were made before the six-month mark and were reduced by the end of the 24-month period, which helped improve tolerability and treatment adherence. Abemaciclib was discontinued in 25.8% of patients, of which 18.55% discontinued due to adverse events and continued on endocrine therapy alone and only 6.5% discontinued abemaciclib and ET. The presence of diarrhea as an adverse event at baseline allows for appropriate measures to be taken to manage diarrhea and for antidiarrheal medications to be initiated, allowing for more effective treatment [145]. The results of these trials show that the use of cyclin-dependent kinase (CDK) 4/6 inhibitors significantly improves progression-free survival compared with aromatase inhibitors or fulvestrant alone, and that the toxicity of the combination is tolerable compared with other more invasive treatments such as chemotherapy [146]. Knowledge of the safety profile helps to avoid the side effects of treatments and to make treatments more effective.
Currently, palbociclib, ribociclib, and abemaciclib are prescribed based on each patient’s clinical history and physician experience, although recent studies of the inhibitors in high-risk, early-stage hormone-receptor-negative human epidermal growth factor receptor 2 breast cancer are opening investigations into their use in other types of breast cancer. Despite the good results and the improvement of CDK 4/6 inhibitors in breast cancer therapy, there is one natural feature that has not been addressed: resistance to hormonal therapies due to cyclin overexpression D1. Early resistance has been studied with new combinations, such as PI3K inhibitors, that help reduce G1-S regulators; investigating these resistance factors in breast cancer would offer conclusive and relevant data for a better benefit in patients with breast cancer. Future research on this type of drug helps to have a more effective and specific treatment scheme according to the clinical history of each patient [147].
6. Gene Therapy
One of the most promising treatments for a wide range of diseases is gene therapy. Gene therapy is the transfer of genetic elements with the goal of treating a disease or improving a patient’s clinical condition [148]. The study of the molecular basis of breast cancer has led to the emergence of gene therapy as a viable treatment option for this disease. Gene therapy involves the introduction of genetic material into target cells via a vector, followed by gene correction, addition, or suppression. In this strategy, it is essential to target tumor cells while avoiding normal cells [148,149].
Since genetic alterations and gene expression profiles play various roles in the development and progression of breast cancer, gene therapy has greatly improved the treatment of this type of disease [150]. Gene correction, suicide gene therapy, gene suppression and silencing, targeting of transcription factors by decoy oligodeoxynucleotides, targeting of miRNA, targeting of breast cancer cells by aptamers, and vaccination by DNA or RNA are all gene therapy strategies used to treat breast cancer [149]. All of these strategies focus on the following: (1) increasing the anti-tumor activity of immune cells by means of cytokines (interleukins, tumor necrosis factors, colony stimulating factors, or interferons), (2) increasing the immunogenicity of tumors by introducing foreign antigens (“tumor vaccines”), (3) introducing a “suicide” gene or increased sensitivity to certain drugs, (4) blocking the expression of oncogenes by antisense therapy, (5) introducing tumor suppressor genes such as p53 into the target cell, (6) eliminating tumor cells using oncolytic adenoviruses, (7) transferring genes with anti-angiogenic effects to inhibit the formation of blood vessels induced by the tumor itself, and (8) introducing drug resistance genes to reduce the toxicity of chemotherapy, particularly to the bone marrow [149,151,152,153].
Gene therapy using DNA therapy and RNA therapy (siRNA, miRNA, ribozyme, or cyclic RNA) is promising for the treatment of breast cancer [154]. In this case, there is a direct selective destruction of cancer cells, induction of an immune response against cancer antigens, or inhibition of their growth [155]. Free nucleic acid gene therapy can strategically target genes that are specifically activated in cancer. However, therapeutic efficacy is limited by poor cellular uptake, low potency, unstable circulation, immune response to foreign bodies, off-target toxicity, and tumor-causing insertional mutations. The bioavailability of nucleic acid-based therapies has been greatly improved by advances in synthetic biology and nanotechnology [154].
The possibility of inducing the expression of a therapeutic protein (by inserting a functional gene) or, conversely, of suppressing the aberrant expression of a protein (by inhibiting the expression of a defective gene) when this is at the origin of breast cancer, opens up countless possibilities for revolutionizing clinical practice. As in other cases, nanotechnology offers interesting possibilities to protect the genetic material from degradation and, above all, to achieve its selective release at the intracellular level. To date, two types of systems have been developed for the transfer of genetic material, viral and non-viral or synthetic in nature. Synthetic systems are the ones mainly addressed by nanotechnology through the appropriate combination of biomaterials, which can be of natural or synthetic origin. The application of nanotechnology to vaccine development offers interesting possibilities by allowing the design of nanosystems that promote antigen uptake by antigen-presenting cells. In addition, nanosystems allow the incorporation of adjuvants that, when released together with the antigen, increase its potency, or modulate the immune response and induce cellular responses. Cancer vaccines offer several advantages over traditional therapies, mainly due to increased specificity, reduced toxicity, and the long-term effect produced by immunological memory. Such vaccines can be developed as a prophylactic or therapeutic strategy, in both cases aiming at selective biodistribution to cancer cells [156]. However, factors such as the inability to identify a single gene that works in anti-tumor therapy, the lack of tumor selectivity, the short duration of therapeutic gene expression, the difficulty of transfecting the entire tumor mass, and the strong antiviral immune response generated in the host are the main limitations for the application of gene therapy [21].
7. Conclusions and Perspectives
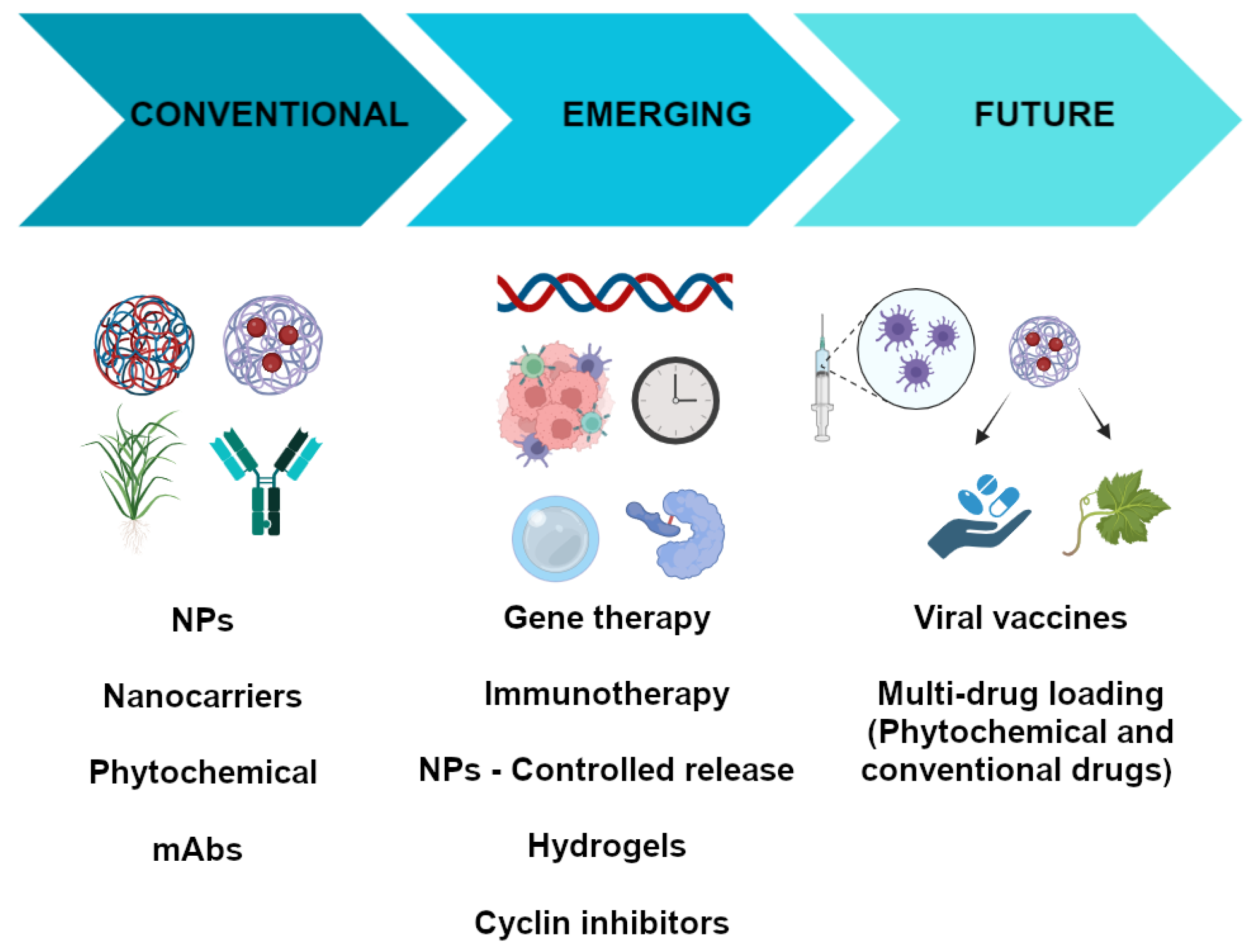
The pharmacological treatment of breast cancer is one of the most complex in human history because it is highly dependent on the nature of the tumor. Although there are well-established guidelines to help physicians make the best choice of pharmacologic agent based on the morphology, histology, and the expression of certain molecular markers in the tumor, the success of such therapy is not guaranteed. However, the use of antineoplastic therapy may be associated with the occurrence of adverse effects that compromise compliance with pharmacological therapy. Therefore, research is needed to develop new therapeutic options that are more effective and safer than those currently available. One way to achieve this goal is to focus efforts on identifying chemotherapeutic compounds from medicinal plants, as has been done in the past, but also through a more refined approach. For example, future research could utilize the in silico analysis of compounds that modulate signaling pathways involved in tumor growth and prototypical compounds obtained through the genetic modulation of plants. With respect to classical phytochemicals, there are also several aspects that need to be improved, such as the yield of the compounds obtained and their purity and solubility. One way to improve the solubility of these compounds is to incorporate them into polymeric matrices that facilitate their delivery to breast tumors. This can be achieved by using nanoparticles or hydrogels composed of polymers that have the ability to bind to the phytopharmaceutical or other types of compounds and that are released in the tumor or its microenvironment of influence avoiding their release in the rest of the body. One way to target the specific release of these types of drugs is to conjugate them to a molecular tag expressed by the tumor cells. Currently, there are two types of conjugates that could be improved: (1) the antibody/nanoparticle conjugate and (2) the antibody/drug conjugate (phytochemicals/cyclins). Both have unique advantages: in the first case, a greater accumulation of antibodies directed against molecular markers that favor cell proliferation is favored, while, in the second case, a more efficient and selective release of the drug into the cells is favored, and inflammatory and proliferative signals can be downregulated. In addition, these therapeutic options could be combined with the inhibition/gene expression (viral vaccines) of critical elements involved in tumor cell proliferation (Figure 2). However, translating in vitro advances in genetic modification to a more complex model, such as the mouse, is currently challenging. For example, the efficiency of molecular target expression or silencing is reduced and the modification is not maintained over time. However, some gene therapy drugs are already on the market.
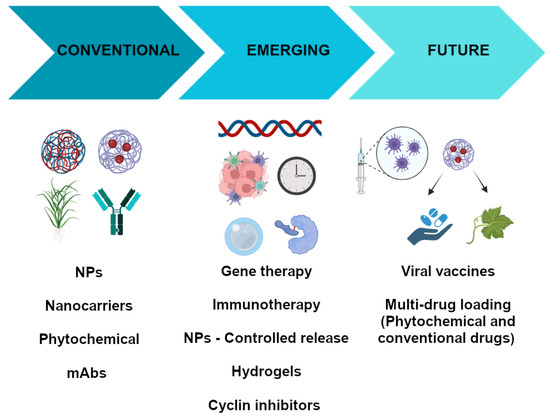
Figure 2.
Breast cancer therapies: conventional, emerging, and future therapies.
Author Contributions
Conceptualization, Methodology, Formal Analysis, Investigation; Writing—Original Draft, Visualization, Validation, I.C.-T., L.B., J.P., M.d.C.R.-S. and H.A.-M.; Conceptualization, Investigation, Formal Analysis, Supervision, Writing—Review and Editing, Project Administration, L.E.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the authors of the reviewed publications for their contributions to the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Wang, Y.; Minden, A. Current Molecular Combination Therapies Used for the Treatment of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 11046. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Y.; Zhou, X.; Yu, H.; Tan, Y.; Du, Y.; Zhang, Q.; Wu, Y. Current landscape of personalized clinical treatments for triple-negative breast cancer. Front. Pharmacol. 2022, 13, 977660. [Google Scholar] [CrossRef]
- Jacobs, A.T.; Martinez Castaneda-Cruz, D.; Rose, M.M.; Connelly, L. Targeted therapy for breast cancer: An overview of drug classes and outcomes. Biochem. Pharmacol. 2022, 204, 115209. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An update on the pathological classification of breast cancer. Histopathology 2023, 82, 5–16. [Google Scholar] [CrossRef]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Keshamma, E.; Kumar, A.; Jha, R.; Amle, V.S.; Dudhate, G.S.; Patel, D.; Saha, P.; Kumar, R. Breast Cancer Treatment Relying on Herbal Bioactive Components. J. Res. Appl. Sci. Biotechnol. 2022, 1, 105–115. [Google Scholar] [CrossRef]
- Khan, M.I.; Bouyahya, A.; Hachlafi, N.E.L.; Menyiy, N.E.; Akram, M.; Sultana, S.; Zengin, G.; Ponomareva, L.; Shariati, M.A.; Ojo, O.A.; et al. Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: A review on recent investigations. Environ. Sci. Pollut. Res. 2022, 29, 24411–24444. [Google Scholar] [CrossRef]
- Ashraf, M.A. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. Biomed. Res. Int. 2020, 2020, 8602879. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Mezni, E.; Behi, K.; Gonçalves, A. Immunotherapy and breast cancer: An overview. Curr. Opin. Oncol. 2022, 34, 587–594. [Google Scholar] [CrossRef]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.-S.; Kumar, A.; Vishakha; Behl, T.; Jha, S.K.; Tang, H. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol. Cancer 2023, 22, 105. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Martínez, L.M.; Cruz, N.; Cómbita, A.L. Terapia génica para el tratamiento del cáncer. Rev. Colomb. Cancerol. 2014, 18, 27–40. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer 2020, 12, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, D.O.; Dembitsky, V.M. Anti-breast Cancer Agents Derived from Plants. Nat. Prod. Bioprospect 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. Biomed. Res. Int. 2017, 2017, 7207983. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Miller, F.G.; Nwokocha, C.R.; Anderson, M.S.; Wilson-Clarke, C.; Vaz, K.; Anderson-Jackson, L.; Brown, J. Medicinal Herbs Used in Traditional Management of Breast Cancer: Mechanisms of Action. Medicines 2020, 7, 47. [Google Scholar] [CrossRef]
- Shareef, M.; Ashraf, M.A.; Sarfraz, M. Natural cures for breast cancer treatment. Saudi Pharm. J. 2016, 24, 233–240. [Google Scholar] [CrossRef]
- Shrihastini, V.; Muthuramalingam, P.; Adarshan, S.; Sujitha, M.; Chen, J.T.; Shin, H.; Ramesh, M. Plant Derived Bioactive Compounds, Their Anti-Cancer Effects and In Silico Approaches as an Alternative Target Treatment Strategy for Breast Cancer: An Updated Overview. Cancers 2021, 13, 6222. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, I.; Jung, Y.S.; Chon, S.J.; Yun, B.H.; Seo, S.K. Korean red ginseng induces extrinsic and intrinsic apoptotic pathways in MCF-7 breast cancer cells and MCF-10A non-malignant breast cells. J. Obstet. Gynaecol. Res. 2021, 47, 2758–2766. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Lin, M.; Gao, Y.; Liu, Y.; Yang, S.; Zhou, X.; Zhang, Y.; Hu, Y.; Wang, H.; et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 2020, 203, 112627. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Li, G.; Wang, Z.; Yang, J.; Li, Y.; Wang, H.; Jin, H.; Qiao, J.; Wang, H.; et al. Acute and repeated dose 26-week oral toxicity study of 20(S)-ginsenoside Rg3 in Kunming mice and Sprague–Dawley rats. J. Ginseng Res. 2020, 44, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Marni, R.; Kundrapu, D.B.; Chakraborti, A.; Malla, R. Insight into drug sensitizing effect of diallyl disulfide and diallyl trisulfide from Allium sativum L. on paclitaxel-resistant triple-negative breast cancer cells. J. Ethnopharmacol. 2022, 296, 115452. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.; Prakash, A.; Agrawala, P.K.; Dutta, A. Investigation on Oral Toxicity of Diallyl Sulfide A Principle Organosulfur Compound Derived from Allium Sativum Garlic in Mice. Def. Life Sci. J. 2022, 1, 3–10. [Google Scholar] [CrossRef]
- Hao, M.; Chu, Y.; Lei, J.; Yao, Z.; Wang, P.; Chen, Z.; Wang, K.; Sang, X.; Han, X.; Wang, L.; et al. Pharmacological Mechanisms and Clinical Applications of Curcumin: Update. Aging Dis. 2023, 14, 716–749. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, F.; Rajabi, M.R.; Mazoochi, T.; Taghizadeh, M.; Nikzad, H.; Atlasi, M.A.; Taherian, A. Comparing Apoptosis and Necrosis Effects of Arctium Lappa Root Extract and Doxorubicin on MCF7 and MDA-MB-231 Cell Lines. Asian Pac. J. Cancer Prev. 2017, 18, 795–802. [Google Scholar] [CrossRef]
- Taleb Agha, M.; Baharetha, H.M.; Al-Mansoub, M.A.; Tabana, Y.M.; Kaz Abdul Aziz, N.H.; Yam, M.F.; Abdul Majid, A.M.S. Proapoptotic and Antiangiogenic Activities of Arctium Lappa L. on Breast Cancer Cell Lines. Scientifica 2020, 2020, 7286053. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Mousavi, Z.; Rastegar, T.; Amin, G. Safety Assessment of Arctium lappa L. Fruit Extract in Female Wistar Rats: Acute and Repeated Oral Toxicity Studies. Res. J. Pharmacogn. 2019, 6, 39–48. [Google Scholar] [CrossRef]
- Korak, T.; Ergül, E.; Sazci, A. Nigella sativa and Cancer: A Review Focusing on Breast Cancer, Inhibition of Metastasis and Enhancement of Natural Killer Cell Cytotoxicity. Curr. Pharm. Biotechnol. 2020, 21, 1176–1185. [Google Scholar] [CrossRef]
- Linjawi, S.A.; Khalil, W.K.; Hassanane, M.M.; Ahmed, E.S. Evaluation of the protective effect of Nigella sativa extract and its primary active component thymoquinone against DMBA-induced breast cancer in female rats. Arch. Med. Sci. 2015, 11, 220–229. [Google Scholar] [CrossRef]
- Al-Ali, A.; Alkhawajah, A.A.; Randhawa, M.A.; Shaikh, N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J. Ayub Med. Coll. Abbottabad 2008, 20, 25–27. [Google Scholar] [PubMed]
- Mbuthia, K.S.; Mireji, P.O.; Ngure, R.M.; Stomeo, F.; Kyallo, M.; Muoki, C.; Wachira, F.N. Tea (Camellia sinensis) infusions ameliorate cancer in 4TI metastatic breast cancer model. BMC Complement. Altern. Med. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.; Andrade, E.D.S.; Monteiro, M.; Fialho, E.; Silva, J.L.; Daleprane, J.B.; Ferraz da Costa, D.C. Green Tea (Camellia sinensis) Extract Induces p53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells. Foods 2021, 10, 3154. [Google Scholar] [CrossRef] [PubMed]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Gong, L.; Lvzi, X.; Qiu, K.; Zhang, Z.; Wan, L. Echinacoside inhibits breast cancer cells by suppressing the Wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2020, 526, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Shouman, S.A.; Elkhoely, A.; Attia, Y.M.; Elsesy, M.S.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A.; et al. Anticancer potentiality of lignan rich fraction of six Flaxseed cultivars. Sci. Rep. 2018, 8, 544. [Google Scholar] [CrossRef]
- Hu, T.; Linghu, K.; Huang, S.; Battino, M.; Georgiev, M.I.; Zengin, G.; Li, D.; Deng, Y.; Wang, Y.T.; Cao, H. Flaxseed extract induces apoptosis in human breast cancer MCF-7 cells. Food Chem. Toxicol. 2019, 127, 188–196. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Power, K.; Chen, J.; Thompson, L.U. Flaxseed attenuates the tumor growth stimulating effect of soy protein in ovariectomized athymic mice with MCF-7 human breast cancer xenografts. Int. J. Cancer 2006, 119, 925–931. [Google Scholar] [CrossRef]
- Wei, C.-K.; Ni, Z.-J.; Thakur, K.; Liao, A.-M.; Hu, F.; Huang, J.-H.; Wei, Z.-J. Acute, genetic and sub-chronic toxicities of flaxseed derived Maillard reaction products. Food Chem. Toxicol. 2019, 131, 110580. [Google Scholar] [CrossRef]
- Adebayo, I.A.; Arsad, H.; Samian, M.R. Antiproliferative Effect on Breast Cancer (MCF7) of Moringa Oleifera Seed Extracts. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules 2022, 27, 4430. [Google Scholar] [CrossRef] [PubMed]
- de Barros, M.C.; Silva, A.G.B.; Souza, T.; Chagas, C.A.; Machado, J.C.B.; Ferreira, M.R.A.; Soares, L.A.L.; Xavier, V.L.; de Araújo, L.C.C.; Borba, E.F.O.; et al. Evaluation of acute toxicity, 28-day repeated dose toxicity, and genotoxicity of Moringa oleifera leaves infusion and powder. J. Ethnopharmacol. 2022, 296, 115504. [Google Scholar] [CrossRef] [PubMed]
- Baraya, Y.S.; Wee, C.L.; Mustapha, Z.; Wong, K.K.; Yaacob, N.S. Strobilanthes crispus elicits anti-tumor immunogenicity in in vitro and in vivo metastatic breast carcinoma. PLoS ONE 2022, 17, e0271203. [Google Scholar] [CrossRef]
- Baraya, Y.S.; Wong, K.K.; Yaacob, N.S. Strobilanthes crispus inhibits migration, invasion and metastasis in breast cancer. J. Ethnopharmacol. 2019, 233, 13–21. [Google Scholar] [CrossRef]
- Muhammad, S.N.H.; Yaacob, N.S.; Safuwan, N.A.M.; Fauzi, A.N. Antiglycolytic Activities of Strobilanthes crispus Active Fraction and its Bioactive Components on Triple-Negative Breast Cancer Cells In Vitro. Anticancer. Agents Med. Chem. 2022, 22, 1363–1369. [Google Scholar] [CrossRef]
- Baraya, Y.S.; Yankuzo, H.M.; Wong, K.K.; Yaacob, N.S. Strobilanthes crispus bioactive subfraction inhibits tumor progression and improves hematological and morphological parameters in mouse mammary carcinoma model. J. Ethnopharmacol. 2021, 267, 113522. [Google Scholar] [CrossRef]
- Ng, M.G.; Ng, C.H.; Ng, K.Y.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Anticancer Properties of Strobilanthes crispus: A Review. Processes 2021, 9, 1370. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, S.; Ali, A.; Gupta, A.C.; Shanker, K.; Bawankule, D.U.; Luqman, S. Microwave-assisted Single Step Cinnamic Acid Derivatization and Evaluation for Cytotoxic Potential. Curr. Pharm. Biotechnol. 2020, 21, 236–243. [Google Scholar] [CrossRef]
- Thangam, R.; Gokul, S.; Sathuvan, M.; Suresh, V.; Sivasubramanian, S. A novel antioxidant rich compound 2-hydoxy 4-methylbenzaldehyde from Decalepis arayalpathra induces apoptosis in breast cancer cells. Biocatal. Agric. Biotechnol. 2019, 21, 101339. [Google Scholar] [CrossRef]
- Nemec, M.J.; Kim, H.; Marciante, A.B.; Barnes, R.C.; Hendrick, E.D.; Bisson, W.H.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolics from mango (Mangifera indica L.) suppress breast cancer ductal carcinoma in situ proliferation through activation of AMPK pathway and suppression of mTOR in athymic nude mice. J. Nutr. Biochem. 2017, 41, 12–19. [Google Scholar] [CrossRef]
- Yap, K.M.; Sekar, M.; Seow, L.J.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Lum, P.T.; Subramaniyan, V.; Wu, Y.S.; Fuloria, N.K.; et al. Mangifera indica (Mango): A Promising Medicinal Plant for Breast Cancer Therapy and Understanding Its Potential Mechanisms of Action. Breast Cancer 2021, 13, 471–503. [Google Scholar] [CrossRef]
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A Toxicological Evaluation of Mango Leaf Extract (Mangifera indica) Containing 60% Mangiferin. J. Toxicol. 2019, 2019, 4763015. [Google Scholar] [CrossRef] [PubMed]
- Villas-Boas, G.R.; Paes, M.M.; Gubert, P.; Oesterreich, S.A. Evaluation of the toxic potential of the aqueous extract from Mangifera indica Linn. (Anacardiaceae) in rats submitted to experimental models of acute and subacute oral toxicity. J. Ethnopharmacol. 2021, 275, 114100. [Google Scholar] [CrossRef] [PubMed]
- Lendzion, K.; Gornowicz, A.; Strawa, J.W.; Bielawska, K.; Czarnomysy, R.; Popławska, B.; Bielawski, K.; Tomczyk, M.; Miltyk, W.; Bielawska, A. LC-PDA-MS and GC-MS Analysis of Scorzonera hispanica Seeds and Their Effects on Human Breast Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 11584. [Google Scholar] [CrossRef]
- Lendzion, K.; Gornowicz, A.; Bielawski, K.; Bielawska, A. Phytochemical Composition and Biological Activities of Scorzonera Species. Int. J. Mol. Sci. 2021, 22, 5128. [Google Scholar] [CrossRef] [PubMed]
- Olayoku, F.R.; Verhoog, N.J.D.; Louw, A. Cyclopia extracts act as selective estrogen receptor subtype downregulators in estrogen receptor positive breast cancer cell lines: Comparison to standard of care breast cancer endocrine therapies and a selective estrogen receptor agonist and antagonist. Front. Pharmacol. 2023, 14, 1122031. [Google Scholar] [CrossRef] [PubMed]
- Cele, N.D.; Mthimunye, N.E.; Mkhwanazi, Q.B.; Nxumalo, S.; Tshabuse, F.; Pooe, O.J.; Chellan, N.; Mthembu, M.S.; Opoku, A.R. In Vitro Antidiabetic, Antioxidant, and Cytotoxic Evaluation of Honeybush Tea (Cyclopia genistoides) Extracts. J. Food Biochem. 2023, 2023, 8774094. [Google Scholar] [CrossRef]
- Triyasa, K.S.; Diantini, A.; Barliana, M.I. A Review of Herbal Medicine-Based Phytochemical of Garcinia as Molecular Therapy for Breast Cancer. Drug Des. Devel Ther. 2022, 16, 3573–3588. [Google Scholar] [CrossRef] [PubMed]
- Pachare, A.; Garge, V.N. Acute Oral Toxicity of Ethanolic Leaf Extract of Garcinia indica in Albino Rats. Int. J. Pharm. Res. Appl. 2022, 7, 502–506. [Google Scholar]
- Singh, V.K.; Arora, D.; Ansari, M.I.; Sharma, P.K. Phytochemicals based chemopreventive and chemotherapeutic strategies and modern technologies to overcome limitations for better clinical applications. Phytother. Res. 2019, 33, 3064–3089. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options. Molecules 2022, 27, 3209. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Chowdhury, N.; Singh, M. Noscapine chemosensitization enhances docetaxel anticancer activity and nanocarrier uptake in triple negative breast cancer. Exp. Cell Res. 2016, 346, 65–73. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Chowdhury, N.; Singh, M. Reversal of drug-resistance by noscapine chemo-sensitization in docetaxel resistant triple negative breast cancer. Sci. Rep. 2017, 7, 15824. [Google Scholar] [CrossRef]
- Ganji-Harsini, S.; Khazaei, M.; Rashidi, Z.; Ghanbari, A. Thymoquinone Could Increase the Efficacy of Tamoxifen Induced Apoptosis in Human Breast Cancer Cells: An In Vitro Study. Cell J. 2016, 18, 245–254. [Google Scholar] [CrossRef]
- Ibiyeye, K.M.; Nordin, N.; Ajat, M.; Zuki, A.B.Z. Ultrastructural Changes and Antitumor Effects of Doxorubicin/Thymoquinone-Loaded CaCO3 Nanoparticles on Breast Cancer Cell Line. Front. Oncol. 2019, 9, 599. [Google Scholar] [CrossRef]
- Khan, A.; Aldebasi, Y.H.; Alsuhaibani, S.A.; Khan, M.A. Thymoquinone Augments Cyclophosphamide-Mediated Inhibition of Cell Proliferation in Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2019, 20, 1153–1160. [Google Scholar] [CrossRef]
- Şakalar, Ç.; İzgi, K.; İskender, B.; Sezen, S.; Aksu, H.; Çakır, M.; Kurt, B.; Turan, A.; Canatan, H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. 2016, 37, 4467–4477. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X.N.; Chen, J.; Wang, W.X.; Lu, Z.F. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp. Ther. Med. 2014, 7, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination Therapy using Co-encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef] [PubMed]
- Mirzapur, P.; Khazaei, M.R.; Moradi, M.T.; Khazaei, M. Apoptosis induction in human breast cancer cell lines by synergic effect of raloxifene and resveratrol through increasing proapoptotic genes. Life Sci. 2018, 205, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Ponce-Cusi, R.; Carrión, F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018, 40, 2381–2388. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. Curcumin as an Enhancer of Therapeutic Efficiency of Chemotherapy Drugs in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2144. [Google Scholar] [CrossRef]
- Ferguson, J.E.; Orlando, R.A. Curcumin reduces cytotoxicity of 5-Fluorouracil treatment in human breast cancer cells. J. Med. Food 2015, 18, 497–502. [Google Scholar] [CrossRef]
- Baptista Moreno Martin, A.C.; Tomasin, R.; Luna-Dulcey, L.; Graminha, A.E.; Araújo Naves, M.; Teles, R.H.G.; da Silva, V.D.; da Silva, J.A.; Vieira, P.C.; Annabi, B.; et al. [10]-Gingerol improves doxorubicin anticancer activity and decreases its side effects in triple negative breast cancer models. Cell Oncol. 2020, 43, 915–929. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, H.; Zhu, X.; Liu, X.; Li, J. Ginsenoside Rg3 promotes cytotoxicity of Paclitaxel through inhibiting NF-κB signaling and regulating Bax/Bcl-2 expression on triple-negative breast cancer. Biomed. Pharmacother. 2017, 89, 227–232. [Google Scholar] [CrossRef]
- Farabegoli, F.; Papi, A.; Bartolini, G.; Ostan, R.; Orlandi, M. (-)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine 2010, 17, 356–362. [Google Scholar] [CrossRef]
- Espinosa-Paredes, D.A.; Cornejo-Garrido, J.; Moreno-Eutimio, M.A.; Martínez-Rodríguez, O.P.; Jaramillo-Flores, M.E.; Ordaz-Pichardo, C. Echinacea Angustifolia DC Extract Induces Apoptosis and Cell Cycle Arrest and Synergizes with Paclitaxel in the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines. Nutr. Cancer 2021, 73, 2287–2305. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, M.-G.; Kwon, Y.-S.; Nam, K.-S. Arctigenin Enhances the Cytotoxic Effect of Doxorubicin in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2997. [Google Scholar] [CrossRef] [PubMed]
- Weichold, O. Introduction to Polymer Chemistry. In Encyclopedia of Glass Science, Technology, History, and Culture; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1043–1055. [Google Scholar]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Saqib, S.; Ullah, S.; Sagir, M.; Tahir, M.B.; Mahmood, A.; Al-Sehemi, A.G.; Assiri, M.A.; Ibrahim, M.; Zulfiqar, A. Applications of Polymeric Materials in Biomedical Engineering. In Sustainable Production and Applications of Waterborne Polyurethanes; Inamuddin, Boddula, R., Khan, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 133–142. [Google Scholar]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomedicine 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sui, J.; Ma, M.; Hu, J.; Sun, Y.; Yang, L.; Fan, Y.; Zhang, X. pH-Responsive charge switchable PEGylated ε-poly-l-lysine polymeric nanoparticles-assisted combination therapy for improving breast cancer treatment. J. Control Release 2020, 326, 350–364. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Varshosaz, J.; Sutherland, D.S. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater. Sci. Eng. C 2021, 118, 111526. [Google Scholar] [CrossRef]
- Xiao, M.C.; Chou, Y.H.; Hung, Y.N.; Hu, S.H.; Chiang, W.H. Hybrid polymeric nanoparticles with high zoledronic acid payload and proton sponge-triggered rapid drug release for anticancer applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111277. [Google Scholar] [CrossRef]
- Bobde, Y.; Biswas, S.; Ghosh, B. PEGylated N-(2 hydroxypropyl) methacrylamide-doxorubicin conjugate as pH-responsive polymeric nanoparticles for cancer therapy. React. Funct. Polym. 2020, 151, 104561. [Google Scholar] [CrossRef]
- Gayathri, R.; Rajalakshmi, P.S.; Aswathi, T.; Rengan, A.K. Doxorubicin loaded polyvinylpyrrolidone-copper sulfide nanoparticles enabling mucoadhesiveness and chemo-photothermal synergism for effective killing of breast cancer cells. Materialia 2021, 19, 101195. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Scie Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Bozoğlan, B.K.; Duman, O.; Tunç, S. Preparation and characterization of thermosensitive chitosan/carboxymethylcellulose/scleroglucan nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 162, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Cimen, Z.; Babadag, S.; Odabas, S.; Altuntas, S.; Demirel, G.; Demirel, G.B. Injectable and Self-Healable pH-Responsive Gelatin–PEG/Laponite Hybrid Hydrogels as Long-Acting Implants for Local Cancer Treatment. ACS Appl. Polym. Mater. 2021, 3, 3504–3518. [Google Scholar] [CrossRef]
- Sabino, I.J.; Lima-Sousa, R.; Alves, C.G.; Melo, B.L.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Injectable in situ forming hydrogels incorporating dual-nanoparticles for chemo-photothermal therapy of breast cancer cells. Int. J. Pharm. 2021, 600, 120510. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Li, Y.; Huang, Z.; Luo, S.; He, Y.; Han, H.; Raza, F.; Wu, J.; Ge, L. Injectable pH and redox dual responsive hydrogels based on self-assembled peptides for anti-tumor drug delivery. Biomater. Sci. 2020, 8, 5415–5426. [Google Scholar] [CrossRef] [PubMed]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Maters Sci. Eng. C 2020, 117, 111294. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, J.; Sun, H.; Li, J.; Wang, X. Polymer-based hydrogels with local drug release for cancer immunotherapy. Biomed. Pharmacother. 2021, 137, 111333. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade Del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Torres-Rodriguez, A.; Avérous, L.; Pollet, E.; de Jesús Sosa-Santillán, G.; Zugasti-Cruz, A.; Sierra-Rivera, C.A.; Pérez-Aguilar, N.V.; Garcia-Lobato, M.A.; Oyervides-Muñoz, E. Antimicrobial and anticancer potential of novel polyaspartate derivatives synthesized via quaternary ammonium grafting. J. Appl. Polym. Sci. 2022, 139, e52907. [Google Scholar] [CrossRef]
- Velazco de la Garza, J.; Avérous, L.; Sosa-Santillán, G.d.J.; Pollet, E.; Zugasti-Cruz, A.; Sierra-Rivera, C.A.; Pérez-Aguilar, N.V.; Oyervides-Muñoz, E. Biological properties of novel polysuccinimide derivatives synthesized via quaternary ammonium grafting. Eur. Polym. J. 2020, 131, 109705. [Google Scholar] [CrossRef]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Melkonian, A.L.; Curry, K.C.; Xu, Y.; Tidwell, M.; Liu, M.; Zaky, A.F.; Liu, X. Monoclonal antibody-based cancer therapies. Chin. J. Chem. Eng. 2021, 30, 301–307. [Google Scholar] [CrossRef]
- Waller-Pulido, A.; Jiménez-Pérez, M.I.; Gonzalez-Sanchez, F.A.; Rojo-Gutierrez, P.R.; Torres-Anguiano, E.; Aleman-Aguilar, J.P.; Garcia-Varela, R. Production of monoclonal antibodies for therapeutic purposes: A review. Int. Immunopharmacol. 2023, 120, 110376. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Czerniecki, B.J. Clinical development of immunotherapies for HER2(+) breast cancer: A review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer 2020, 6, 10. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Bayer, V. An Overview of Monoclonal Antibodies. Semin. Oncol. Nurs. 2019, 35, 150927. [Google Scholar] [CrossRef]
- Masoud, V.; Pages, G. Targeted therapies in breast cancer: New challenges to fight against resistance. World J. Clin. Oncol. 2017, 8, 120–134. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019, 4, 34. [Google Scholar] [CrossRef]
- Mosele, M.F.; Lusque, A.; Dieras, V.; Deluche, E.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; Levy, C.; Signolle, N.; et al. LBA1 Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): Biomarker analyses from patients from DAISY trial. Ann. Oncol. 2022, 33, S123. [Google Scholar] [CrossRef]
- Zafar, S.; Armaghan, M.; Khan, K.; Hassan, N.; Sharifi-Rad, J.; Habtemariam, S.; Kieliszek, M.; Butnariu, M.; Bagiu, I.-C.; Bagiu, R.V.; et al. New insights into the anticancer therapeutic potential of maytansine and its derivatives. Biomed. Pharmacother. 2023, 165, 115039. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bianchini, G.; Cortes, J.; Henning, J.W.; Untch, M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open 2022, 7, 100553. [Google Scholar] [CrossRef] [PubMed]
- Sidaway, P. Trastuzumab deruxtecan improves survival. Nat. Rev. Clin. Oncol. 2020, 17, 521. [Google Scholar] [CrossRef]
- Li, Z.; Guo, S.; Xue, H.; Li, L.; Guo, Y.; Duan, S.; Zhu, H. Efficacy and safety of trastuzumab deruxtecan in the treatment of HER2-low/positive advanced breast cancer: A single-arm meta-analysis. Front. Pharmacol. 2023, 14, 1183514. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Tamura, K.; Tsurutani, J.; Takahashi, S.; Iwata, H.; Krop, I.E.; Redfern, C.; Sagara, Y.; Doi, T.; Park, H.; Murthy, R.K.; et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 2019, 20, 816–826. [Google Scholar] [CrossRef]
- D’Arienzo, A.; Verrazzo, A.; Pagliuca, M.; Napolitano, F.; Parola, S.; Viggiani, M.; Caputo, R.; Puglisi, F.; Giuliano, M.; Del Mastro, L.; et al. Toxicity profile of antibody-drug conjugates in breast cancer: Practical considerations. eClinicalMedicine 2023, 62, 102113. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.M.; Smitt, M.; Yu, R.; Leung, A.C.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Elfgen, C.; Bjelic-Radisic, V. Targeted Therapy in HR+ HER2− Metastatic Breast Cancer: Current Clinical Trials and Their Implications for CDK4/6 Inhibitor Therapy and beyond Treatment Options. Cancers 2021, 13, 5994. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Dottorini, L.; Di Menna, G.; Borgonovo, K.; Parati, M.C.; Rea, C.G.; Ghilardi, M.; Ghidini, A.; Luciani, A. The role of CDK4/6 inhibitors in older and younger patients with breast cancer: A systematic review and meta-analysis. Breast 2023, 71, 138–142. [Google Scholar] [CrossRef]
- Takahashi, M.; Masuda, N.; Nishimura, R.; Inoue, K.; Ohno, S.; Iwata, H.; Hashigaki, S.; Muramatsu, Y.; Umeyama, Y.; Toi, M. Palbociclib-letrozole as first-line treatment for advanced breast cancer: Updated results from a Japanese phase 2 study. Cancer Med. 2020, 9, 4929–4940. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Hu, X.; Li, W.; Sun, T.; Shen, K.; Wang, S.; Cheng, Y.; Zhang, Q.; Cui, S.; Tong, Z.; et al. Palbociclib plus letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer: Primary results from PALOMA-4. Eur. J. Cancer 2022, 175, 236–245. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Cho, M.; Yu, K.W.; Waisman, J.; Yuan, Y.; Mortimer, J. A single institution experience with palbociclib toxicity requiring dose modifications. Breast Cancer Res. Treat. 2018, 168, 381–387. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Decker, T.; Lüdtke-Heckenkamp, K.; Melnichuk, L.; Hirmas, N.; Lübbe, K.; Zahn, M.O.; Schmidt, M.; Denkert, C.; Lorenz, R.; Müller, V.; et al. Anti-hormonal maintenance treatment with the CDK4/6 inhibitor ribociclib after 1st line chemotherapy in hormone receptor positive/HER2 negative metastatic breast cancer: A phase II trial (AMICA). Breast 2023, 72, 103575. [Google Scholar] [CrossRef]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.M.; Shao, Z.M.; Fasching, P.A.; Huang, C.S.; Jaliffe, G.G.; Tryakin, A.; Goetz, M.P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021, 32, 1571–1581. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; O’Shaughnessy, J.; Boyle, F.; Toi, M.; Broom, R.; Blancas, I.; Gumus, M.; Yamashita, T.; Im, Y.H.; Rastogi, P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Safety and patient-reported outcomes from the monarchE study. Ann. Oncol. 2022, 33, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Piezzo, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Gioia, G.D.; Lauro, V.D.; Fusco, G.; Martinelli, C.; Nuzzo, F.; Pensabene, M.; et al. Targeting Cell Cycle in Breast Cancer: CDK4/6 Inhibitors. Int. J. Mol. Sci. 2020, 21, 6479. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Abreu, M.T.; Palafox, M.; Asghar, U.; Rivas, M.A.; Cutts, R.J.; Garcia-Murillas, I.; Pearson, A.; Guzman, M.; Rodriguez, O.; Grueso, J.; et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor–Positive Breast Cancer. Cancer Res. 2016, 76, 2301–2313. [Google Scholar] [CrossRef]
- Bulaklak, K.; Gersbach, C.A. The once and future gene therapy. Nat. Commun. 2020, 11, 5820. [Google Scholar] [CrossRef]
- Dastjerd, N.T.; Valibeik, A.; Rahimi Monfared, S.; Goodarzi, G.; Moradi Sarabi, M.; Hajabdollahi, F.; Maniati, M.; Amri, J.; Samavarchi Tehrani, S. Gene therapy: A promising approach for breast cancer treatment. Cell Biochem. Funct. 2022, 40, 28–48. [Google Scholar] [CrossRef]
- Bottai, G.; Truffi, M.; Corsi, F.; Santarpia, L. Progress in nonviral gene therapy for breast cancer and what comes next? Expert. Opin. Biol. Ther. 2017, 17, 595–611. [Google Scholar] [CrossRef]
- El-Tanani, M.; Al Khatib, A.O.; Al-Najjar, B.O.; Shakya, A.K.; El-Tanani, Y.; Lee, Y.-F.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; Aljabali, A.A.; et al. Cellular and molecular basis of therapeutic approaches to breast cancer. Cell Signal 2023, 101, 110492. [Google Scholar] [CrossRef]
- Singh, V.; Khan, N.; Jayandharan, G.R. Vector engineering, strategies and targets in cancer gene therapy. Cancer Gene Ther. 2022, 29, 402–417. [Google Scholar] [CrossRef]
- Tufail, M.; Cui, J.; Wu, C. Breast cancer: Molecular mechanisms of underlying resistance and therapeutic approaches. Am. J. Cancer Res. 2022, 12, 2920–2949. [Google Scholar] [PubMed]
- Mirza, Z.; Karim, S. Nanoparticles-based drug delivery and gene therapy for breast cancer: Recent advancements and future challenges. Semin. Cancer Biol. 2021, 69, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Montaño-Samaniego, M.; Bravo-Estupiñan, D.M.; Méndez-Guerrero, O.; Alarcón-Hernández, E.; Ibáñez-Hernández, M. Strategies for Targeting Gene Therapy in Cancer Cells with Tumor-Specific Promoters. Front. Oncol. 2020, 10, 605380. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.N.; Fatma, H.; Saraf, S.K. Vaccines in Breast Cancer: Challenges and Breakthroughs. Diagnostics 2023, 13, 2175. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).