Antioxidant Potential and Known Secondary Metabolites of Rare or Underutilized Plants of Yucatan Region
Abstract
1. Introduction
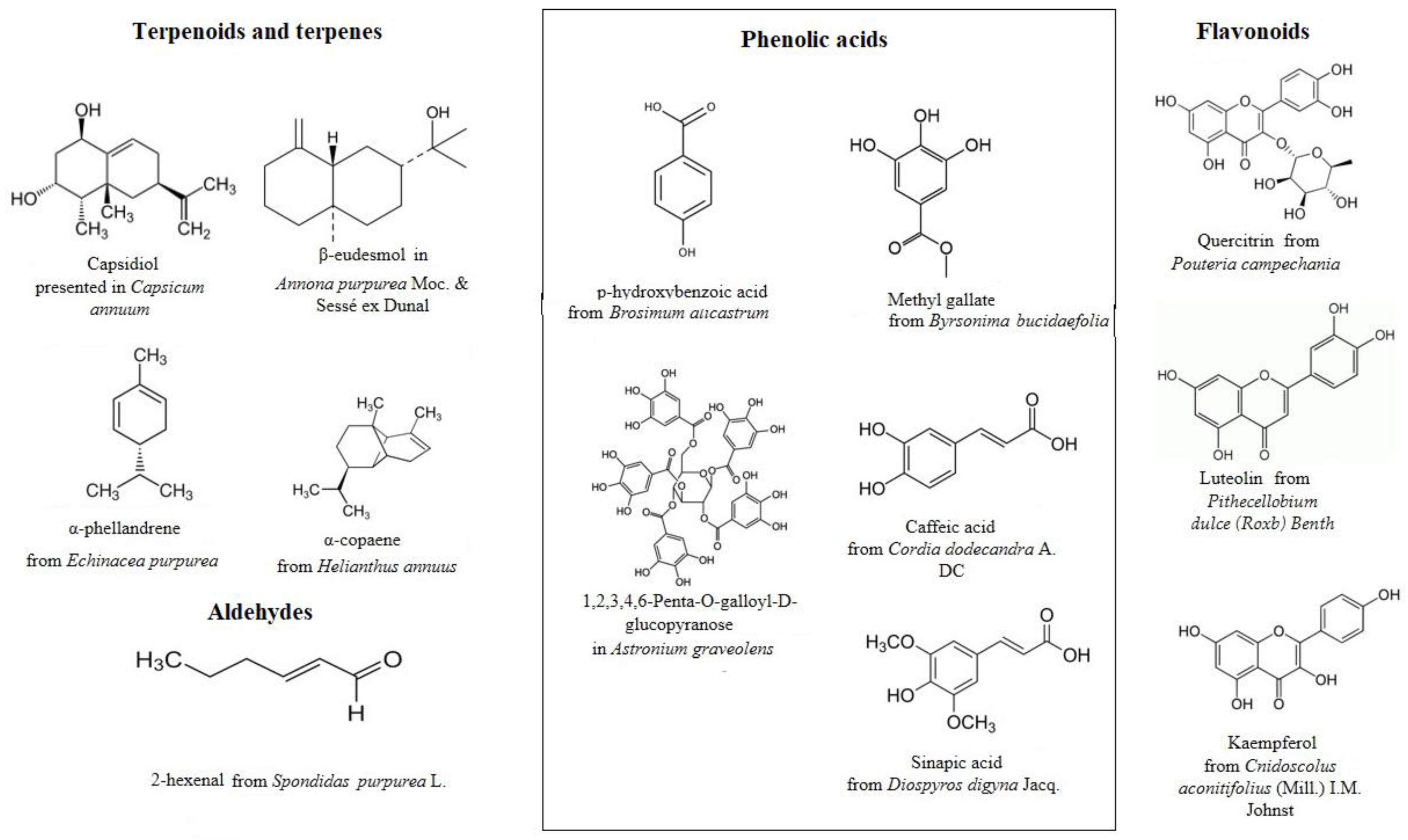
2. Plants and Plant-Derived Compounds with Antioxidant Potential from Mayan Region
| Plant Species | Family | Plant Part | Terpenes Terpenoids | Phenolic Acids | Alkaloids | Flavonoids Flavones | Coumarins | References |
|---|---|---|---|---|---|---|---|---|
| Achras sapota (Sapodilla) | Sapidaceae | stem, leaves, fruit | present (not specified) | present (not specified) | present (not specified) | dihydromyricetin, quercitrin, myricitrin, catechin, epicatechin, gallocatechin | present (not specified) | [16,17,18,19] |
| Annona purpurea Moc. & Sessé ex Dunal (Sancoya) | Annonaceae | leaves, pulp, seeds | β-eudesmol, α-eudesmol | present (not specified) | Norpurpureine 7-formyl-dehydrothalicsimidine 8-7-hydroxy-dehydrothalicsimidine N-methyllaurotetanine N-methylasimilobine Lirinidine Thalicsimidine Purpureine 3-hydroxyglaucine Annomontine Annopurpuricins A-D | present (not specified) | present (not specified) | [20,21,22] |
| Astronium graveolens (Gateado) | Anacardiaceae | leaves | lupeol | 3-O-caffeoylquinic, 5-sinapoylquinic, 1,2,3,4,6-Penta-O-galloyl-D-glucopyranose | quercetin 3-O-glucoside, quercetin 3-O-rhamnoside | - | [23] | |
| Brosimum alicastrum swartz (Ramon) | Moraceae | leaves, seeds, bark | - | Gallic, chloro genic, vanillic, sinapic, ferulic, t-cinnamic, coumaric, caffeic acid, p-hydroxibenzoic, m-hydroxybenzoic acids | - | Quercetin, catechin, epicatechin, catechin gallate, syringetin, Kaempferol−O−dihexoside, Isoquercetin | Xanthyletin, luvangetin, 8-hydroxyxanthyletin | [24,25,26] |
| Byrsonima bucidaefolia (Sak Pah) | Malpighiaceae | leaves | - | Methyl gallate, methyl-m-trigallate | - | - | - | [27] |
| Capsicum annuum (Bell pepper) | Solanaceae | fruit | capsidiol | cinnamic acid | capsaicinoids | quercetin, luteolin, caffeoyl, cinnamoyl glycosides, apigenin | coumaric acid coumaroyl | [28,29,30] |
| Cnidosfolus aconitifolius IM. Jonst (Chaya) | leaves | α y β amyrin, borneol, hederaginin, oleanolic acid, squalene, lupeol acetate | ellagic, ferulic, p-coumaric, caffeic, protocacheuic, vainillic, chlorogenic, caftaric, p-hidroxibenzoico, coutaric, syringic, synaptic acids | choline, trigonelline, nicotinic acid, palmatine, sitsirikine, Dihydrositsirikine, vinblastine, vindoline, catharanthine, vinleurosine | kaempferol, quercetin, rutin, catechin, hesperidin, narigenin, kaempferol, procuanidin B1, catechin, procyanidin B2, rutin, gallocatechin gallate, epigallocatechin gallate, epicatechin-3-O-gallate, quercetin-3-O-galactoside, quercetin-3-O-glycoside, quercetin-3-O-rhamnoside, trans-resveratrol | - | [31,32,33,34] | |
| Cordia dodecandra DC. (Circote) | Sapidaceae | fruit | - | caffeic acid, rosmarinic acid, caffeoyl hexoside | - | Rutin, Quercetin 3-O-rutinoside, lutein | - | [35,36,37] |
| Diospyros digyna Jacq. (Black sapote) | Ebenaceae | fruit, pulp, peel, seeds | - | Cinnamic, p-hydroxybenzoic, Caffeic, Sinapic, Ferulic, O-Coumaric, Protocatechuic, Chlorogenic, Isochlorogenic | - | Catechin Epicatechin Myricetin Gallocatechol Epigallocatechin, rutin, Myricetrin, Isohermetin, Kaempherol-4′-glucoside, Quercetin, Dihydromyricetin, Cynaroside | - | [38,39] |
| Echinacea purpurea (Purple coneflower) | Asteraceae | whole plant | α-phellandrene, camphene, limonene | present (not specified) | pyrrolizidine alkaloids tussilagine and isotussilagine | quercetin, kaempferol, isorhamnetin | Coumaric acid | [40,41,42,43,44] |
| Helianthus annuus (Common sunflower) | Asteraceae | seeds | α-copaene, bornyl acetate, β-elemene, β-selinene, germacrene-D | present (not specified) | present (not specified) | kaempferol, apigenin, dihydroflavonol, daidzein, biochanin A, formononetin, luteolin, quercetin | p-coumaroyl | [45,46,47] |
| Parmentiera aculeata (H.B. & K.) Seeman (Cucumber kat) | Bignoniaceae | fruit | Lactucin-8-O-methylacrylate | present (not specified) | present (not specified) | - | present (not specified) | [48,49] |
| Pouteria campechiana (H.B. & K) Baehni (Canisté) | Sapidaceae | fruit, leaves, seeds, bark | Corsolic acid, euscaphic acid, fatty acid ester of betulinic acid, fatty acid ester of oleanolic acid, maslinic acid, ursolic acid, lucumic acid A, lucimic acid B, 4(R),23-epoxy-2α,3α,19α-trihydroxy-24-norurs-12-en- 28-oic acid, 2α,3α,19α,23-tetrahydroxy-13α,27-cyclours-11-en-28- oic acid, 2α, 3α, 19α, 23 tetrahydroxyursolic acid, 2α,3β,19α-trihydroxy-24-norursa-4(23),12-dien-28-O-ic acid, 3β, 28- dihydroxy-olean-12-enyl fatty acid ester | Caffeic, ferulic, gallic, protocatechuic, vanillic, p-coumaric acid | - | Apigenin, catechin, epicatechin, gallocatechin, kaempferol, luteolon, myricetin, myricitin, myricetin-3-O-α-L-rhamnoside, myricetin-3-O-β-galactoside, quercetin, rutin, myricetin 3-O-α-rhamnopyranoside, quercetin 3-O-α-rhamnopyranoside, quercetin 3-O-β-rhamnopyranoside, quercetin 3-O-β-arabinopyranoside, taxifolin 3-O-arabinofuranoside, taxifolin 3-O-α, rhamnopyranoside | - | [50] |
| Pithecellobium dulce (Roxb) Benth (Dziuche) | Leguminosae | seeds | (−)-19β-D-glucopyranosyl-6,7-dihydroxykaurenoate | Caffeic acid, chlorogenic acid, ferulic acid, gallic acid, p-coumaric acid, protocatechuic acid, | - | apigenin, catechin, daidzein, kaemferol, luteolin, quercetin, myricetin, naringin and rutin | - | [51,52] |
| Spondias purpurea L. | Anacardiaceae | leaves | spathulenol, linolenic acid, t-caryophyllene, α-muurolene | caffeic acid | - | epigallocatechin | - | [53,54] |
3. Description of Rare or Underutilized Plants of Mayan Region
3.1. Achras sapota L.
3.2. Annona purpurea Moc. & Sessé ex Dunal
3.3. Astronium graveolens Jacq.
3.4. Brosimum alicastrum Swartz
3.5. Byrsonima bucidaefolia Standl
3.6. Capsicum annuum
3.7. Cnidoscolus aconitifolius (Mill.) I.M. Johnst
3.8. Cordia dodecandra
3.9. Diospyros digyna Jacq.
3.10. Echinacea purpurea (Purple coneflower)
3.11. Helianthus annuus
3.12. Parmentiera aculeata
3.13. Pouteria campechiana (H.B. & K) Baehni
3.14. Pithecellobium dulce (Roxb) Benth
3.15. Spondias purpurea L.
4. Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hufnagel, L.; Mics, F.; Hufnagel, L.; Mics, F. Introductory Chapter: Biodiversity of Mexico. In Natural History and Ecology of Mexico and Central America; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Goettsch, B.; Urquiza-Haas, T.; Koleff, P.; Acevedo Gasman, F.; Aguilar-Meléndez, A.; Alavez, V.; Alejandre-Iturbide, G.; Aragón Cuevas, F.; Azurdia Pérez, C.; Carr, J.A.; et al. Extinction Risk of Mesoamerican Crop Wild Relatives. Plants People Planet 2021, 3, 775–795. [Google Scholar] [CrossRef]
- Castañeda, R.; Cáceres, A.; Velásquez, D.; Rodríguez, C.; Morales, D.; Castillo, A. Medicinal Plants Used in Traditional Mayan Medicine for the Treatment of Central Nervous System Disorders: An Overview. J. Ethnopharmacol. 2022, 283, 114746. [Google Scholar] [CrossRef]
- Rodríguez-García, C.M.; Ruiz-Ruiz, J.C.; Peraza-Echeverría, L.; Peraza-Sánchez, S.R.; Torres-Tapia, L.W.; Pérez-Brito, D.; Tapia-Tussell, R.; Herrera-Chalé, F.G.; Segura-Campos, M.R.; Quijano-Ramayo, A.; et al. Antioxidant, Antihypertensive, Anti-Hyperglycemic, and Antimicrobial Activity of Aqueous Extracts from Twelve Native Plants of the Yucatan Coast. PLoS ONE 2019, 14, e0213493. [Google Scholar] [CrossRef]
- Lentz, D.L.; Pohl, M.D.L.; Alvarado, J.L.; Tarighat, S.; Bye, R. Sunflower (Helianthus annuus L.) as a Pre-Columbian Domesticate in Mexico. Proc. Natl. Acad. Sci. USA 2008, 105, 6232–6237. [Google Scholar] [PubMed]
- Sytar, O.; Brestic, M.; Hajihashemi, S.; Skalicky, M.; Kubeš, J.; Lamilla-Tamayo, L.; Ibrahimova, U.; Ibadullayeva, S.; Landi, M. COVID-19 Prophylaxis Efforts Based on Natural Antiviral Plant Extracts and Their Compounds. Molecules 2021, 26, 727. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Martínez, E.M.M.; Sandate-Flores, L.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Parra-Arroyo, L.; Antunes-Ricardo, M.; Serna-Saldívar, S.O.; Iqbal, H.M.N.; Parra-Saldívar, R. Underutilized Mexican Plants: Screening of Antioxidant and Antiproliferative Properties of Mexican Cactus Fruit Juices. Plants 2021, 10, 368. [Google Scholar] [CrossRef]
- Soto, K.M.; Pérez Bueno, J.d.J.; Mendoza López, M.L.; Apátiga-Castro, M.; López-Romero, J.M.; Mendoza, S.; Manzano-Ramírez, A. Antioxidants in Traditional Mexican Medicine and Their Applications as Antitumor Treatments. Pharmaceuticals 2023, 16, 482. [Google Scholar] [CrossRef]
- Mendez, M.; Durán, R.; Campos, S.; Dorantes, A. Flora Medicinal. Biodivers. Y Desarro. Hum. En Yucatán 2010, 349–352. [Google Scholar]
- EncicloVida es una Plataforma de Consulta Creada por la Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Available online: https://enciclovida.mx (accessed on 23 March 2023).
- Türkez, H.; Çelik, K.; Toğar, B. Effects of Copaene, a Tricyclic Sesquiterpene, on Human Lymphocytes Cells in Vitro. Cytotechnology 2014, 66, 597–603. [Google Scholar] [CrossRef]
- Leite, D.O.D.; de F. A. Nonato, C.; Camilo, C.J.; de Carvalho, N.K.G.; da Nobrega, M.G.L.A.; Pereira, R.C.; da Costa, J.G.M. Annona Genus: Traditional Uses, Phytochemistry and Biological Activities. Curr. Pharm. Des. 2020, 26, 4056–4091. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure–Antioxidant Activity Relationships of o-Hydroxyl, o-Methoxy, and Alkyl Ester Derivatives of p-Hydroxybenzoic Acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, A.; Lu, X.; Liu, M.; Wang, H.; Song, H.; Wei, C.; Wang, Y.; Duan, X. P-Hydroxybenzoic Acid Alleviates Inflammatory Responses and Intestinal Mucosal Damage in DSS-Induced Colitis by Activating ERβ Signaling. J. Funct. Foods 2021, 87, 104835. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Sharma, N.; Kaur, H.; Kaur, M.; Sandhu, K.S.; Maqsood, S.; Ozogul, F. A Review of Sapodilla (Manilkara zapota) in Human Nutrition, Health, and Industrial Applications. Trends Food Sci. Technol. 2022, 127, 319–334. [Google Scholar] [CrossRef]
- Ma, J.; Luo, X.D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive Novel Polyphenols from the Fruit of Manilkara zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef]
- Jadhav, S.S.; Swami, S.B.; Pujari, K.H. Study the physico-chemical properties of Sapota (Achras sapota L.). Trends Technol. Sci. Res. 2018, 3, 555605. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Saravana, P.; Payal, H.; Mohammed, S.P.; Binnie, W. Antioxidant Activity, Total Phenolic and Flavonoid Contents of Artocarpus Heterophyllus and Manilkara zapota Seeds and Its Reduction Potential. Int. J. Pharm. Pharm. Sci. 2011, 3, 256–260. [Google Scholar]
- Muñoz-Acevedo, A.; Aristizábal-Córdoba, S.; Rodríguez, J.D.; Torres, E.A.; Molina, A.M.; Gutiérrez, R.G.; Kouznetsov, V.V. Citotoxicidad/Capacidad Antiradicalaria in-Vitro y Caracterización Estructural Por GC-MS/1H-13C-RMN de Los Aceites Esenciales de Hojas de Árboles Joven/Adulto de Annona purpurea Moc. & Sessé Ex Dunal de Repelón (Atlántico, Colombia). Boletín Latinoam. Y Del Caribe Plantas Med. Y Aromáticas 2016, 15, 99–111. [Google Scholar]
- Anaya-Esparza, L.M.; de Lourdes Garcia-Magana, M.; Domínguez-Ávila, J.A.; Yahia, E.M.; Salazar-Lopez, N.J.; Gonzalez-Aguilar, G.A.; Montalvo-González, E. Annonas: Underutilized Species as a Potential Source of Bioactive Compounds. Food Res. Int. 2020, 138, 963–9969. [Google Scholar] [CrossRef]
- Toledo-González, K.A.; Riley-Saldaña, C.A.; Salas-Lizana, R.; De-la-Cruz-Chacón, I.; González-Esquinca, A.R. Alkaloidal Variation in Seedlings of Annona purpurea Moc. & Sessé Ex Dunal Infected with Colletotrichum gloeosporioides (Penz.) Penz. and Sacc. Biochem. Syst. Ecol. 2023, 107, 104611. [Google Scholar] [CrossRef]
- Hernández, V.; Malafronte, N.; Mora, F.; Pesca, M.S.; Aquino, R.P.; Mencherini, T. Antioxidant and Antiangiogenic Activity of Astronium graveolens Jacq. Leaves. Nat. Prod. Res. 2014, 28, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Mendoza, L.; Sotelo-García, D.M.; Via, L.D.; Parra-Delgado, H. Biological Properties of Aqueous Extract and Pyranocoumarins Obtained from the Bark of Brosimum alicastrum Tree. J. Ethnopharmacol. 2022, 290, 115128. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Canto-Pinto, J.C.; Cuevas-Glory, L.F.; Sauri-Duch, E.; Pérez-Pacheco, E.; Betancur-Ancona, D. Effect of Extraction Solvent on the Phenolic Compounds Content and Antioxidant Activity of Ramon Nut (Brosimum alicastrum). Chem. Pap. 2019, 73, 1647–1657. [Google Scholar] [CrossRef]
- Pech-Cohuo, S.C.; Martín-López, H.; Uribe-Calderón, J.; González-Canché, N.G.; Salgado-Tránsito, I.; May-Pat, A.; Cue-vas-Bernardino, J.C.; Ayora-Talavera, T.; Cervantes-Uc, J.M.; CPacheco, N. Physicochemical, Mechanical, and Structural Properties of Bio-Active Films Based on Biological-Chemical Chitosan, a Novel Ramon (Brosimum alicastrum) Starch, and Quercetin. Polymers 2022, 14, 1346. [Google Scholar] [CrossRef]
- Castillo-Avila, G.M.; García-Sosa, K.; Peña-Rodríguez, L.M. Antioxidants from the Leaf Extract of Byrsonima Bucidaefolia. Nat. Prod. Commun. 2009, 4, 83–86. [Google Scholar] [CrossRef]
- Howard, L.R.; Wildman, R. Antioxidant Vitamin and Phytochemical Content of Fresh and Processed Pepper Fruit (Capsicum annuum). In Handbook of Nutraceuticals and Functional Foods; CRC Press: Boca Ratón, FL, USA, 2007; pp. 165–191. [Google Scholar]
- Maldonado-Bonilla, L.D.; Betancourt-Jiménez, M.; Lozoya-Gloria, E. Local and Systemic Gene Expression of Sesquiterpene Phytoalexin Biosynthetic Enzymes in Plant Leaves. Eur. J. Plant Pathol. 2008, 121, 439–449. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, C.J.; Huh, S.U.; Choi, L.M.; Lee, G.J.; Kim, Y.J.; Paek, K.H. Capsicum annuum WRKYb Transcription Factor That Binds to the CaPR-10 Promoter Functions as a Positive Regulator in Innate Immunity upon TMV Infection. Biochem. Biophys. Res. Commun. 2011, 411, 613–619. [Google Scholar] [CrossRef]
- Hutasingh, N.; Chuntakaruk, H.; Tubtimrattana, A.; Ketngamkum, Y.; Pewlong, P.; Phaonakrop, N.; Roytrakul, S.; Rungrotmongkol, T.; Paemanee, A.; Tansrisawad, N.; et al. Metabolite profiling and identification of novel umami compounds in the chaya leaves of two species using multiplatform metabolomics. Food Chem. 2023, 404, 134564. [Google Scholar] [CrossRef]
- Ramos-Gomez, M.; Figueroa-Pérez, M.G.; Guzman-Maldonado, H.; Loarca-Piña, G.; Mendoza, S.; Quezada-Tristán, T.; Reynoso-Camacho, R. Phytochemical Profile, Antioxidant Properties and Hypoglycemic Effect of Chaya (Cnidoscolus chayamansa) in STZ-Induced Diabetic Rats. J. Food Biochem. 2017, 41, e12281. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Siddique, I.; da Silva, M.; Vitali, L.; Ferreira, S.R.S. Subcritical Water Extraction and Microwave-Assisted Extraction Applied for the Recovery of Bioactive Components from Chaya (Cnidoscolus aconitifolius Mill.). J. Supercrit. Fluids 2020, 165, 104976. [Google Scholar] [CrossRef]
- Quintal Martínez, J.P.; Segura Campos, M.R. Cnidoscolus aconitifolius (Mill.) I.M. Johnst.: A Food Proposal Against Thromboembolic Diseases. Food Rev. Int. 2021, 39, 1377–1410. [Google Scholar] [CrossRef]
- Sánchez-Recillas, A.; Rivero-Medina, L.; Ortiz-Andrade, R.; Araujo-León, J.A.; Flores-Guido, J.S. Airway Smooth Muscle Relaxant Activity of Cordia dodecandra A. DC. Mainly by CAMP Increase and Calcium Channel Blockade. J. Ethnopharmacol. 2019, 229, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, K.; Castañeda-Pérez, E.; Herrera-Pool, E.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pech-Cohuo, S.C.; Pacheco, N. Ultrasound-Assisted Extraction of Phenolic Compounds from Different Maturity Stages and Fruit Parts of Cordia dodecandra A. DC.: Quantification and Identification by UPLC-DAD-ESI-MS/MS. Agriculture 2022, 12, 2127. [Google Scholar] [CrossRef]
- Pacheco, N.; Méndez-Campos, G.K.; Herrera-Pool, I.E.; Alvarado-López, C.J.; Ramos-Díaz, A.; Ayora-Talavera, T.; Talcott, S.U.; Cuevas-Bernardino, J.C. Physicochemical Composition, Phytochemical Analysis and Biological Activity of Ciricote (Cordia dodecandra A. D.C.) Fruit from Yucatán. Nat. Prod. Res. 2022, 36, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Serio, G.; Bertea, C.M.; Chiarelli, R.; Lauria, A.; Gentile, C. Phytochemical Profile and Antioxidant Properties of the Edible and Non-Edible Portions of Black Sapote (Diospyros Digyna Jacq.). Food Chem. 2022, 380, 132137. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gutierrez-Orozco, F.; de Leon, C.A. Phytochemical and Antioxidant Characterization of the Fruit of Black Sapote (Diospyros Digyna Jacq.). Food Res. Int. 2011, 44, 2210–2216. [Google Scholar] [CrossRef]
- Coeugniet, E.G.; Elek, E. Immunomodulation with Viscum Album and Echinacea purpurea Extracts. Onkologie 1987, 10, 27–33. [Google Scholar] [CrossRef]
- Lim, T.K. Echinacea purpurea. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; pp. 340–371. [Google Scholar] [CrossRef]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, Phytochemistry and Analysis Methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef]
- Classen, B.; Thude, S.; Blaschek, W.; Wack, M.; Bodinet, C. Immunomodulatory Effects of Arabinogalactan-Proteins from Baptisia and Echinacea. Phytomedicine 2006, 13, 688–694. [Google Scholar] [CrossRef]
- Mazza, G.; Cottrell, T. Volatile Components of Roots, Stems, Leaves, and Flowers of Echinacea Species. J. Agric. Food Chem. 1999, 47, 3081–3085. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Z.; Yu, X.; Yan, J.; Zhou, Y.; Cheng, H.; Tai, G. Components of Heat-Treated Helianthus annuus L. Pectin Inhibit Tumor Growth and Promote Immunity in a Mouse CT26 Tumor Model. J. Funct. Foods 2018, 48, 190–199. [Google Scholar] [CrossRef]
- Guo, S.; Ge, Y.; Na Jom, K. A Review of Phytochemistry, Metabolite Changes, and Medicinal Uses of the Common Sunflower Seed and Sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95. [Google Scholar] [PubMed]
- Lokumana, C.R. Collection and Analysis of Volatiles of Various Cultivated Sunflower, Helianthus annuus, (Asteraceae) Germplasm and Investigation of Some Aspects of Host Selection in Adult Red Sunflower Seed Weevil, Smicronyx fulvus L., (Coleoptera Curculionidae); North Dakota State University: Fargo, ND, USA, 2017. [Google Scholar]
- Gómez, C.C.E.; Ordaz, C.P.; San Martín, E.M.; Pérez, N.H.; Pérez, G.I.; Gómez, M.d.C.G. Cytotoxic Effect and Apoptotic Activity of Parmentiera Edulis DC. Hexane Extract on the Breast Cancer Cell Line MDA-MB-231. J. Appl. Pharm. Sci. 2016, 6, 15–22. [Google Scholar] [CrossRef]
- Perez, R.M.; Perez, C.; Zavala, M.A.; Perez, S.; Hernandez, H.; Lagunes, F. Hypoglycemic Effects of Lactucin-8-O-Methylacrylate of Parmentiera Edulis Fruit. J. Ethnopharmacol. 2000, 71, 391–394. [Google Scholar] [CrossRef]
- Do, T.V.T.; Suhartini, W.; Phan, C.U.; Zhang, Z.; Goksen, G.; Lorenzo, J.M. Nutritional Value, Phytochemistry, Health Benefits, and Potential Food Applications of Pouteria campechiana (Kunth) Baehni: A Comprehensive Review. J. Funct. Foods 2023, 103, 105481. [Google Scholar] [CrossRef]
- Mena-Rejón, G.J.; Sansores-Peraza, P.; Brito-Loeza, W.F.; Quijano, L. Chemical Constituents of Pithecellobium Albicans. Fitoterapia 2008, 79, 395–397. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Kuri-García, A.; Vargas-Madriz, H.; Chávez-Servín, J.L.; Ferriz-Martínez, R.A.; Hernández-Sandoval, L.G.; Guzmán-Maldonado, S.H. Phenolic Profile and Antioxidant Capacity of Pithecellobium dulce (Roxb) Benth: A Review. J. Food Sci. Technol. 2020, 57, 4316–4336. [Google Scholar] [CrossRef]
- De Almeida, C.L.F.; Brito, S.A.; De Santana, T.I.; Costa, H.B.A.; De Carvalho Júnior, C.H.R.; Da Silva, M.V.; De Almeida, L.L.; Rolim, L.A.; Dos Santos, V.L.; Wanderley, A.G.; et al. Spondias purpurea L. (Anacardiaceae): Antioxidant and Antiulcer Activities of the Leaf Hexane Extract. Oxid. Med. Cell. Longev. 2017, 2017, 6593073. [Google Scholar] [CrossRef]
- Marisco, G.; Regineide, X.; Brendel, M.; Pungartnik, C. Antifungal Potential of Terpenes from Spondias purpurea L. Leaf Extract against Moniliophthora Perniciosa That Causes Witches Broom Disease of Theobroma Cacao. Int. J. Complement. Altern. Med. 2017, 7, 00215. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid That Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yılmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kılıç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on In Vivo Pharmacological Effects and Bioavailability Traits. Oxid. Med. Cell. Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef] [PubMed]
- Karle, P.P.; Dhawale, S.C. Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review. Syst. Rev. Pharm. 2019, 10, 11–14. [Google Scholar] [CrossRef]
- Tulloch, A.; Goldson-Barnaby, A.; Bailey, D.; Gupte, S. Manilkara zapota (Naseberry): Medicinal Properties and Food Applications. Int. J. Fruit Sci. 2020, 20, S1–S7. [Google Scholar] [CrossRef]
- Mohd Tamsir, N.; Mohd Esa, N.; Omar, S.N.C.; Shafie, N.H. Manilkara zapota (L.) P. Royen: Potential Source of Natural Antioxidants. Malaysian J. Med. Health Sci. 2020, 16, 196–204. [Google Scholar]
- Rivas-Gastelum, M.F.; Garcia-Amezquita, L.E.; Garcia-Varela, R.; Sánchez-López, A.L. Manilkara zapota "chicozapote" as a fruit source of health-beneficial bioactive compounds and its effects on chronic degenerative and infectious diseases, a review. Front Nutr. 2023, 10, 1194283. [Google Scholar] [CrossRef]
- Uglat, J.; Shivashankar, S.; Chandrashekhar, H.; Kalmesh, G.M. Biochemical Changes in Fruit Pulp and Seed in Relation to Corky Develpment of Sapota Cv Cricket Ball. Environ. Ecol. 2012, 30, 1587–1590. [Google Scholar]
- Kulkarni, A.P.; Policegoudra, R.S.; Aradhya, S.M. Chemical composition and antioxidant activity of sapota (Achras sapota linn.) fruit. J. Food Biochem. 2007, 31, 31–399. [Google Scholar] [CrossRef]
- Aguirre Crespo, F.J.; Pérez, E.C.; Valdovinos Estrella, J.D.G.; Maldonado Velazquez, M.G.; Ortega Morales, B.O.; Zamora Crecencio, P.; Hernández Nuñez, E.; Estrada Soto, S.E. Vasorelaxant and Antioxidant Activity of Some Medicinal Plants from Campeche, Mexico. Pharmacogn. Mag. 2021, 17, 23–30. [Google Scholar]
- Bala, S.; Kumar, J.; Duhan, S. Biochemical Changes in Pulp and Peel of Sapota (Manilkara zapota L.) at Different Stages of Ripening. Res. Crop. 2017, 18, 260–263. [Google Scholar] [CrossRef]
- Sathish Kumar, R.; Sureshkumar, K.; Velraj, R. Optimization of Biodiesel Production from Manilkara zapota (L.) Seed Oil Using Taguchi Method. Fuel 2015, 140, 90–96. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, Bioactive Compounds and Antioxidant Activity of Common Indian Fruits and Vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef] [PubMed]
- Topete-Corona, C.; Cuevas-Guzmán, R.; Sánchez-Rodríguez, E.V.; Moreno-Hernández, A.; Morales-Arias, J.G.; Núñez-López, N.M. Estructura Poblacional y Hábitat de Un Árbol Tropical Con Frutos Comestibles, Annona purpurea (Annonaceae), En El Occidente de México. Rev. Biol. Trop. 2020, 68, 1171–1184. [Google Scholar] [CrossRef]
- Kusmardiyani, S.; Suharli, Y.A.; Insanu, M.; Fidrianny, I. Phytochemistry and Pharmacological Activities of Annona Genus: A Review. Curr. Res. Biosci. Biotechnol. 2020, 2, 77–88. [Google Scholar] [CrossRef]
- Barbalho, S.; de Goulart, R.; Vasques Farinazzi-Machado, F.; da Soares de Souza, M.; Santos Bueno, P.; Guiguer, E.; Araujo, A.; Groppo, M. Annona Sp: Plants with Multiple Applications as Alternative Medicine—A Review. Curr. Bioact. Compd. 2012, 8, 277–286. [Google Scholar] [CrossRef]
- Sanchez-Gomez, K.F.; Cristóbal-Pérez, E.J.; Harvey, N.; Quesada, M. Isolation and Characterization of Microsatellite Loci in Astronium graveolens (Anacardiaceae) and Cross Amplification in Related Species. Mol. Biol. Rep. 2020, 47, 4003–4007. [Google Scholar] [CrossRef]
- Chagas Filho, S.F.; Figueiredo, C.M.; Da Costa Gomes, A.; Silva, L.P.; de Mello Peixoto, E.T.; Da Silva, R.G. Antitumor and Antioxidant Potential of the Astronium graveolens Extract. Planta Med. 2016, 82, 862. [Google Scholar] [CrossRef]
- Carter, C.T.; Northcutt, J.K. Raw or Roasted Brosimum alicastrum Seed Powder as a Nutritional Ingredient in Composite Sugar-snap Cookies. Cereal Chem. 2023, 100, 841–851. [Google Scholar] [CrossRef]
- Subiria-Cueto, R.; Larqué-Saavedra, A.; Reyes-Vega, M.L.; De La Rosa, L.A.; Santana-Contreras, L.E.; Gaytán-Martínez, M.; Vázquez-Flores, A.A.; Rodrigo-García, J.; Corral-Avitia, A.Y.; Núñez-Gastélum, J.A.; et al. Brosimum alicastrum Sw. (Ramón): An Alternative to Improve the Nutritional Properties and Functional Potential of the Wheat Flour Tortilla. Foods 2019, 8, 613. [Google Scholar] [CrossRef]
- Dussol, L.; Elliott, M.; Michelet, D.; Nondédéo, P. Ancient Maya Sylviculture of Breadnut (Brosimum alicastrum Sw.) and Sapodilla (Manilkara zapota (L.) P. Royen) at Naachtun (Guatemala): A Reconstruction Based on Charcoal Analysis. Quat. Int. 2017, 457, 29–42. [Google Scholar] [CrossRef]
- Ozer, H.K. Phenolic Compositions and Antioxidant Activities of Maya Nut (Brosimum alicastrum): Comparison with Commercial Nuts. Int. J. Food Prop. 2017, 20, 2772–2781. [Google Scholar] [CrossRef]
- Losoya-Sifuentes, C.; Pinto-Jimenez, K.; Cruz, M.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Loredo-Treviño, A.; López-Badillo, C.M.; Belmares, R. Determination of Nutritional and Antioxidant Properties of Maya Nut Flour (Brosimum alicastrum) for Development of Functional Foods. Foods 2023, 12, 1398. [Google Scholar] [CrossRef]
- Gullian Klanian, M.; Terrats Preciat, M. Optimization of the Ultrasound-Assisted Extraction of Phenolic Compounds from Brosimum alicastrum Leaves and the Evaluation of Their Radical-Scavenging Activity. Molecules 2017, 22, 1286. [Google Scholar] [CrossRef]
- Pereira, V.V.; Borel, C.R.; Silva, R.R. Phytochemical Screening, Total Phenolic Content and Antioxidant Activity of Byrsonima Species. Nat. Prod. Res. 2015, 29, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Poot, M.A.; Valencia-Chan, L.S.; Moo-Puc, R.E.; Richomme-Peniguel, P.; Rupasinghe, H.V.; Peña-Rodríguez, L.M. Exploring the Potential Health Benefits of Plants and Fruits Traditionally Consumed in the Yucatan Peninsula. J. Diabetes Treat. 2022, 7, 10111. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial Use of Pepper (Capsicum annum L.) Derived Products: Technological Benefits and Biological Advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Raigón, M.D.; Moreno-Peris, E.; Fita, A.; Rodríguez-Burruezo, A. Response to Organic Cultivation of Heirloom Capsicum Peppers: Variation in the Level of Bioactive Compounds and Effect of Ripening. PLoS ONE 2018, 13, e0207888. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; la Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sanchez, E.; Muñoz-Marquez, E.; Sida-Arreola, J.; Flores-Cordova, M. Bioactive Compounds and Antioxidant Activity in Different Grafted Varieties of Bell Pepper. Antioxidants 2015, 4, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.U.E.; Taher, M.; Sanad, M.I.; Tadros, L.K. Chemical Properties, Phenolic Profiles and Antioxidant Activities of Pepper Fruits. J. Agric. Chem. Biotechnol. 2019, 10, 133–140. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Martínez-Guirado, C.; del Mar Rebolloso-Fuentes, M.; Carrique-Pérez, A. Nutrient Composition and Antioxidant Activity of 10 Pepper (Capsicum annuun) Varieties. Eur. Food Res. Technol. 2006, 224, 1–9. [Google Scholar] [CrossRef]
- Blanco-Ríos, A.K.; Medina-Juárez, L.Á.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant Activity of the Phenolic and Oily Fractions of Different Sweet Bell Peppers. J. Mex. Chem. Soc. 2017, 57, 137–143. [Google Scholar] [CrossRef]
- Sora, G.T.S.; Haminiuk, C.W.I.; da Silva, M.V.; Zielinski, A.A.F.; Gonçalves, G.A.; Bracht, A.; Peralta, R.M. A Comparative Study of the Capsaicinoid and Phenolic Contents and in Vitro Antioxidant Activities of the Peppers of the Genus Capsicum: An Application of Chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. [Google Scholar] [CrossRef]
- Qiao, G.H.; Wexxin, D.; Zhigang, X.; Sami, R.; Khojah, E.; Amanullah, S. Antioxidant and Anti-Inflammatory Capacities of Pepper Tissues. Ital. J. Food Sci. 2020, 32, 265–274. [Google Scholar] [CrossRef]
- Ilic, Z.S.; Mirecki, N.; Fallik, E. Cultivars Differences in Keeping Quality and Bioactive Constituents. J. Adv. Biotechnol. 2014, 4, 313–318. [Google Scholar] [CrossRef]
- Yang, E.; Song, K. The Ameliorative Effects of Capsidiol Isolated from Elicited Capsicum annuum on Mouse Splenocyte Immune Responses and Neuroinflammation. Phyther. Res. 2021, 35, 1597–1608. [Google Scholar] [CrossRef]
- Ebel, R.; de Jesús Méndez Aguilar, M.; Castillo Cocom, J.A.; Kissmann, S. Genetic Diversity in Nutritious Leafy Green Vegetable—Chaya (Cnidoscolus aconitifolius). In Genetic Diversity in Horticultural Plants; Nandwani, D., Ed.; Springer: Cham, Switzerland, 2019; Volume 22, pp. 161–189. [Google Scholar]
- Chukwu, E.C.; Osuocha, K.U.; Uhegbu, F.O. Nutrient Composition and Selected Biochemical Effects of Cnidoscolus aconitifolius Leaf Extracts in Male Albino Rats. J. Forensic Res. 2018, 9, 409. [Google Scholar] [CrossRef]
- Ross-Ibarra, J.; Molina-Cruz, A. The Ethnobotany of Chaya (Cnidoscolus aconitifolius (Mill.) I.M. Johnst. Subsp. Aconitifolius (Mill.) I.M. Johnst.): A Nutritious Maya Vegetable. Econ. Bot. 2002, 56, 350–365. [Google Scholar] [CrossRef]
- Babalola, O.J.; Opeyemi, O.A. Effect of Processing Methods on Nutritional Composition, Phytochemicals, and Anti-Nutrient Properties of Chaya Leaf (Cnidoscolus aconitifolius). Afr. J. Food Sci. 2015, 9, 560–565. [Google Scholar]
- Manzanilla Valdez, M.L.; Acevedo Fernández, J.J.; Segura Campos, M.R. Antidiabetic and Hypotensive Effect of Cnidoscolus aconitifolius (Mill) I.M Johnst Leaves Extracts. J. Food Meas. Charact. 2021, 15, 5245–5255. [Google Scholar] [CrossRef]
- Quintal-Martínez, J.P.; Quintal-Ortiz, I.G.; Alonzo-Salomón, L.G.; Muñoz-Rodríguez, D.; Segura-Campos, M.R. Antithrombotic Study and Identification of Metabolites in Leaf Extracts of Chaya [Cnidoscolus aconitifolius (Mill.) I.M. Johnst.]. J. Med. Food 2021, 24, 1304–1312. [Google Scholar] [CrossRef]
- Manzanilla Valdez, M.L.; Segura Campos, M.R. Renal and Hepatic Disease: Cnidoscolus aconitifolius as Diet Therapy Proposal for Prevention and Treatment. J. Am. Coll. Nutr. 2021, 40, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Us-Medina, U.; Millán-Linares, M.D.C.; Arana-Argaes, V.E.; Segura-Campos, M.R. In Vitro Antioxidant and Anti-Inflammatory Activity of Chaya Extracts (Cnidoscolus aconitifolius (Mill.) I.M. Johnst)]. Nutr. Hosp. 2020, 37, 46–55. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, R.V.; Gutiérrez-Rebolledo, G.A.; Méndez-Bolaina, E.; Sánchez-Medina, A.; Maldonado-Saavedra, O.; Domínguez-Ortiz, M.Á.; Vázquez-Hernández, M.; Muñoz-Muñiz, O.D.; Cruz-Sánchez, J.S. Cnidoscolus chayamansa Mc Vaugh, an Important Antioxidant, Anti-Inflammatory and Cardioprotective Plant Used in Mexico. J. Ethnopharmacol. 2014, 151, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela Soto, R.; Morales Rubio, M.E.; Verde Star, M.J.; Oranday Cárdenas, A.; Preciado-Rangel, P.; Antonio González, J.; Esparza-Rivera, J.R. Cnidoscolus chayamansa Hidropónica Orgánica y Su Capacidad Hipoglucemiante, Calidad Nutraceutica y Toxicidad. Rev. Mex. Ciencias Agrícolas 2017, 6, 815–825. [Google Scholar] [CrossRef][Green Version]
- Loarca-Piña, G.; Mendoza, S.; Ramos-Gómez, M.; Reynoso, R. Antioxidant, Antimutagenic, and Antidiabetic Activities of Edible Leaves from Cnidoscolus chayamansa Mc. Vaugh. J. Food Sci. 2010, 75, H68–H72. [Google Scholar] [CrossRef]
- Prajanban, B.; Fangkrathok, N. In Vitro Study of Cnidoscolus aconitifolius Leaf Extracts on Foam Cells and Their Antioxidant. Pharmacogn. Res. 2022, 14, 121–126. [Google Scholar] [CrossRef]
- Yam-Chin, C.; Montañez-Escalante, P.; Ruenes-Morales, R. Crecimiento de Plantas Jóvenes de Cordia dodecandra (Boraginaceae) En Tres Etapas Sucesionales de Vegetación En Calotmul, Yucatán. Rev. Mex. Biodivers. 2014, 85, 589–597. [Google Scholar] [CrossRef]
- Ruiz-Valencia, J.A.; Vázquez-Sánchez, M.; Burgos-Hernández, M.; Gutiérrez, J.; Terrazas, T. Physicochemical and Antioxidant Changes of Black Sapote (Diospyros Digyna, Ebenaceae) during on-Tree Fruit Development. Acta Bot. Mex. 2022, 129, 1–17. [Google Scholar]
- Yahia, E.M.; Gutierrez-Orozco, F. Black Sapote (Diospyros Digyna Jacq.); Woodhead Publishing Limited: Sawston, UK, 2011. [Google Scholar]
- Jiménez-González, O.; González-Pérez, J.; Mejía-Garibay, B.; López-Malo, A.; Guerrero-Beltrán, J.Á. Caramel colour pigments from black sapote (Diospyros digyna): Obtention and food application. Sustainable Food Technol. 2023, 1, 555–566. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Hostettmann, K. Geschichte Einer Pflanze Am Beispiel von Echinacea. Complement. Med. Res. 2003, 10, 9–12. [Google Scholar] [CrossRef]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef]
- Geng, X.; Tian, X.; Tu, P.; Pu, X. Neuroprotective Effects of Echinacoside in the Mouse MPTP Model of Parkinson’s Disease. Eur. J. Pharmacol. 2007, 564, 66–74. [Google Scholar] [CrossRef]
- Brown, P.N.; Chan, M.; Betz, J.M. Optimization and Single-Laboratory Validation Study of a High-Performance Liquid Chromatography (HPLC) Method for the Determination of Phenolic Echinacea Constituents. Anal. Bioanal. Chem. 2010, 397, 1883–1892. [Google Scholar] [CrossRef]
- Hou, R.; Xu, T.; Li, Q.; Yang, F.; Wang, C.; Huang, T.; Hao, Z. Polysaccharide from Echinacea purpurea Reduce the Oxidant Stress in Vitro and in Vivo. Int. J. Biol. Macromol. 2020, 149, 41–50. [Google Scholar] [CrossRef]
- The Impact on Trade and Development of the War in Ukraine. Available online: https://unctad.org/system/files/official-document/osginf2022d1_en.pdf (accessed on 23 March 2023).
- Andrade-Cetto, A.; Heinrich, M. Mexican Plants with Hypoglycaemic Effect Used in the Treatment of Diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, R.M.; Pérez-González, C.; Zavala-Sánchez, M.A.; Pérez-Gutiérrez, S. Actividad Hipoglucemiante de Bouvardia Terniflora, Brickellia Veronicaefolia y Parmentiera Edulis. Salud Publica Mex. 1998, 40, 354–358. [Google Scholar] [CrossRef]
- Santiago Ruiz, C.; Nuricumbo Lievano, V.N.; Chapa Barrios, M.G.; Vela Gutiérrez, G.; Velázquez, A. Antimicrobial Activity, Phenolic and Antioxidant Content of Extracts from Cuajilote (Parmentiera Aculeata Kunth) Fruits at Different Degrees of Ripening. J. Mex. Chem. Soc. 2021, 65, 161–169. [Google Scholar] [CrossRef]
- Sethuraman, C.; Mohd, S.F.; Govindaraju, S.; Tiau, W.J.; Farouk, N.D.M.; Hassan, H.H.C. Severe Hypokalemia ECG Changes Mimicking Those of Acute Coronary Syndrome (ACS) in Patient with Underlying Ischaemic Heart Disease: A Case Review. Open J. Emerg. Med. 2020, 8, 53–58. [Google Scholar] [CrossRef]
- Nur, M.A.; Khan, M.; Biswas, S.; Hossain, K.M.D.; Amin, M.Z. Nutritional and Biological Analysis of the Peel and Pulp of Pouteria Campechiana Fruit Cultivated in Bangladesh. J. Agric. Food Res. 2022, 8, 100296. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S.; Meeso, N. Phytochemicals, Vitamin C and Sugar Content of Thai Wild Fruits. Food Chem. 2011, 126, 972–981. [Google Scholar] [CrossRef]
- Adiyaman, P.; Kanchana, S.; Usharani, T.; Ilaiyaraja, N.; Kalaiselvan, A.; Anila Kumar, K.R. Identification and Quantification of Polyphenolic Compounds in Underutilized Fruits (Star Fruit and Egg Fruit) Using HPLC. Indian J. Tradit. Knowl. 2016, 15, 487–493. [Google Scholar]
- Kong, K.W.; Khoo, H.E.; Prasad, N.K.; Chew, L.Y.; Amin, I. Total Phenolics and Antioxidant Activities of Pouteria CampechianaFruit Parts. Sains Malays. 2013, 42, 123–127. [Google Scholar]
- Hidayah, N.; Fitriansyah, S.N.; Aulifa, D.L.; Dewi, S.; Barkah, W. Determination of Total Phenolic, Flavonoid Content and Antioxidant Activity of Campolay (Pouteria campechiana (Kunth) Baehni) Extract. In Proceedings of the 2nd Bakti Tunas Husada-Health Science International Conference (BTH-HSIC 2019), Tasikmalaya, Indonesia, 5–6 October 2019; Atlantis Press: Paris, France, 2020. [Google Scholar]
- Aseervatham, G.S.B.; Manthra, V.; Ireen, C.; Thilagameena, S.; Akshaya, S.; Clara Mary, A.; Giriprashanthini, S.; Sivasudha, T. Free Radical Scavenging Potential and Antihaemolytic Activity of Methanolic Extract of Pouteria Campechiana (Kunth) Baehni. and Tricosanthes Tricuspidata Linn. Biocatal. Agric. Biotechnol. 2019, 18, 101031. [Google Scholar] [CrossRef]
- Rao, G.N. Physico-Chemical, Mineral, Amino Acid Composition, in Vitro Antioxidant Activity and Sorption Isotherm of Pithecellobium dulce L. Seed Protein Flour. J. Food Pharm. Sci. 2013, 1, 74–80. [Google Scholar]
- Rao, B.G.; Samyuktha, P.; Ramadevi, D.; Battu, H. Review of Literature: Phyto Pharmacological Studies on Pithecellobium Dulce. Glob. Trends Pharm. Sci. 2018, 9, 4797–4808. [Google Scholar]
- Nagmoti, D.M.; Khatri, D.K.; Juvekar, P.R.; Juvekar, A.R. Antioxidant Activity Free Radical-Scavenging Potential of Pithecellobium dulce Benth Seed Extracts. Free. Radic. Antioxid. 2012, 2, 37–43. [Google Scholar] [CrossRef]
- Katekhaye, S.D.; Kale, M.S. Antioxidant and Free Radical Scavenging Activity of Pithecellobium dulce (Roxb.) Benth Wood Bark and Leaves. Free. Radic. Antioxid. 2012, 2, 47–57. [Google Scholar] [CrossRef]
- Kumari, S. Evaluation of phytochemical analysis and antioxidant and antifungal activity of Pithecellobium dulce leaves’ extract. Asian J. Pharm. Clin. Res. 2016, 10, 370–375. [Google Scholar] [CrossRef]
- Pío-León, J.F.; Díaz-Camacho, S.; Montes-Avila, J.; López-Angulo, G.; Delgado-Vargas, F. Nutritional and Nutraceutical Characteristics of White and Red Pithecellobium dulce (Roxb.) Benth Fruits. Fruits 2013, 68, 397–408. [Google Scholar] [CrossRef]
- López-Angulo, G.; Montes-Avila, J.; Sánchez-Ximello, L.; Díaz-Camacho, S.P.; Miranda-Soto, V.; López-Valenzuela, J.A.; Delgado-Vargas, F. Anthocyanins of Pithecellobium dulce (Roxb.) Benth. Fruit Associated with High Antioxidant and α-Glucosidase Inhibitory Activities. Plant Foods Hum. Nutr. 2018, 73, 308–313. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uuh-Narvaez, J.J.; Segura-Campos, M.R.; Sytar, O. Antioxidant Potential and Known Secondary Metabolites of Rare or Underutilized Plants of Yucatan Region. Future Pharmacol. 2023, 3, 664-685. https://doi.org/10.3390/futurepharmacol3040042
Uuh-Narvaez JJ, Segura-Campos MR, Sytar O. Antioxidant Potential and Known Secondary Metabolites of Rare or Underutilized Plants of Yucatan Region. Future Pharmacology. 2023; 3(4):664-685. https://doi.org/10.3390/futurepharmacol3040042
Chicago/Turabian StyleUuh-Narvaez, Jonatan Jafet, Maira Rubi Segura-Campos, and Oksana Sytar. 2023. "Antioxidant Potential and Known Secondary Metabolites of Rare or Underutilized Plants of Yucatan Region" Future Pharmacology 3, no. 4: 664-685. https://doi.org/10.3390/futurepharmacol3040042
APA StyleUuh-Narvaez, J. J., Segura-Campos, M. R., & Sytar, O. (2023). Antioxidant Potential and Known Secondary Metabolites of Rare or Underutilized Plants of Yucatan Region. Future Pharmacology, 3(4), 664-685. https://doi.org/10.3390/futurepharmacol3040042






