Applications and Utility of Three-Dimensional In Vitro Cell Culture for Therapeutics
Abstract
1. Introduction
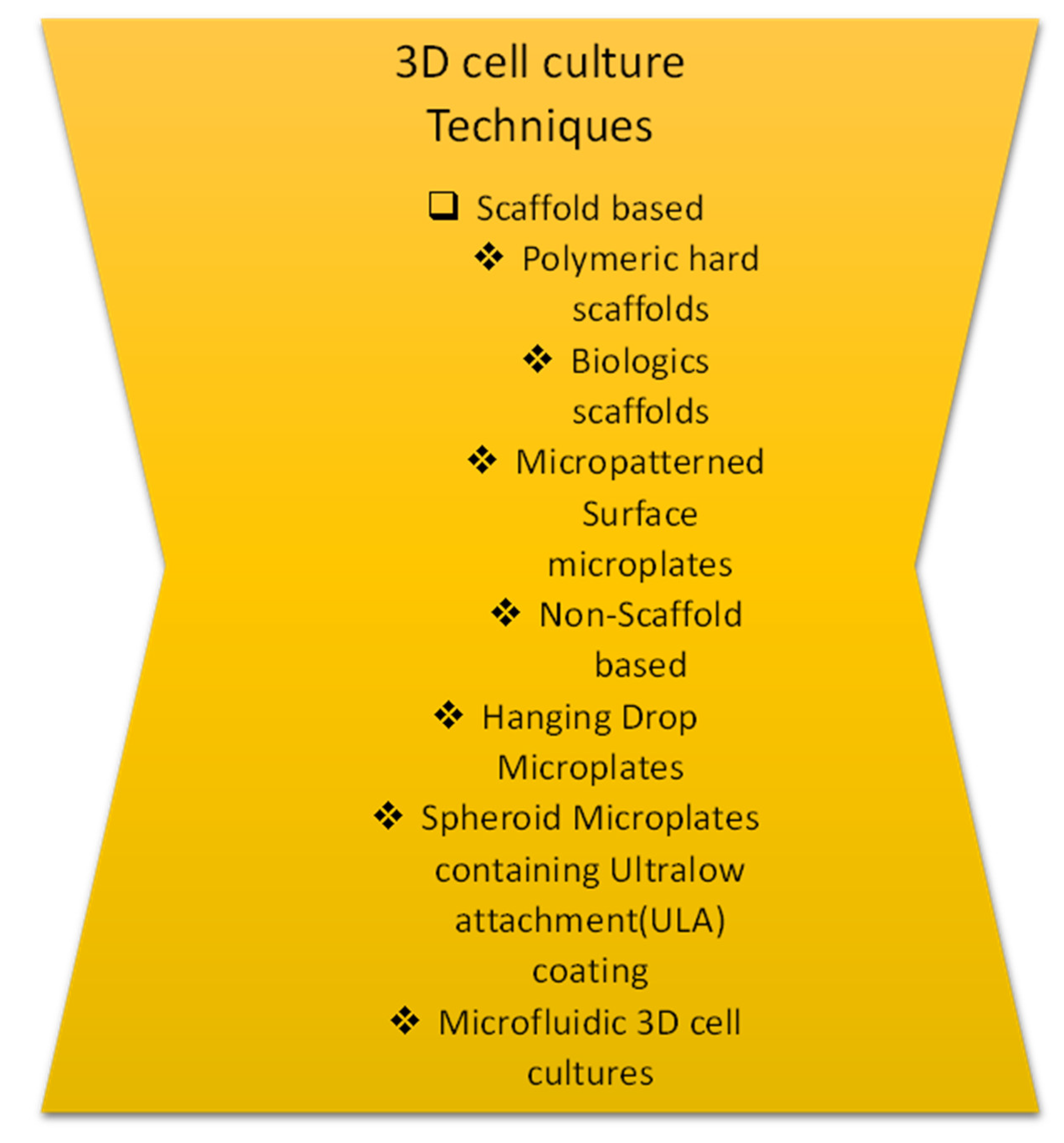
2. Models of 3D Culture
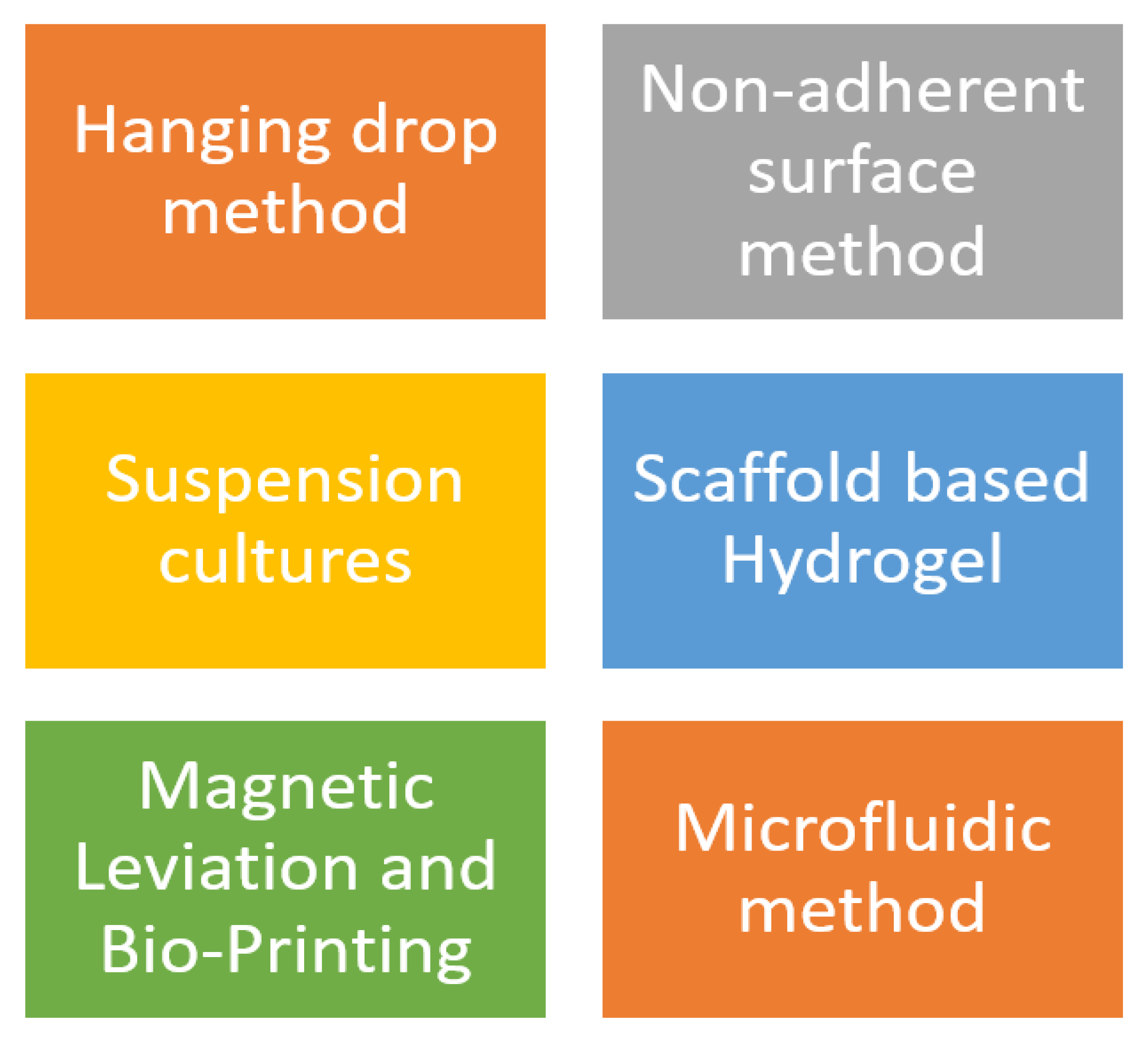
3. 3D Cell Culture Techniques
3.1. Hanging Drop Method
3.2. Formation of Spontaneous Spheroid: Non-Adherent Surface Approaches
- Microenvironment conditions such as hypoxia, and nutrient gradients can be stimulated.
- The cellular function can be differentiated.
- Two or more different types-cultures can be exhibited.
- Better proof diction in vivo responses upon drug treatment.
- 3D cultures are known to mimic tissue-like structures.
3.3. Suspension Culture
3.4. Spinner Flasks
3.5. Bioreactors
3.6. Scaffold–Based Models: Hydrogels
3.7. Magnetic Levitation
3.8. Bioprinting
3.8.1. The Porosity of the Hydrogel
3.8.2. Physical Properties of Hydrogel
3.8.3. Biochemical Properties of Hydrogel
3.9. Acellular Matrix-Cell Encapsulated Material
Cell Supporting Materials
3.10. Spheroid Models and Methods
Culturing of Spheroids
3.11. Organoid Methods and Models
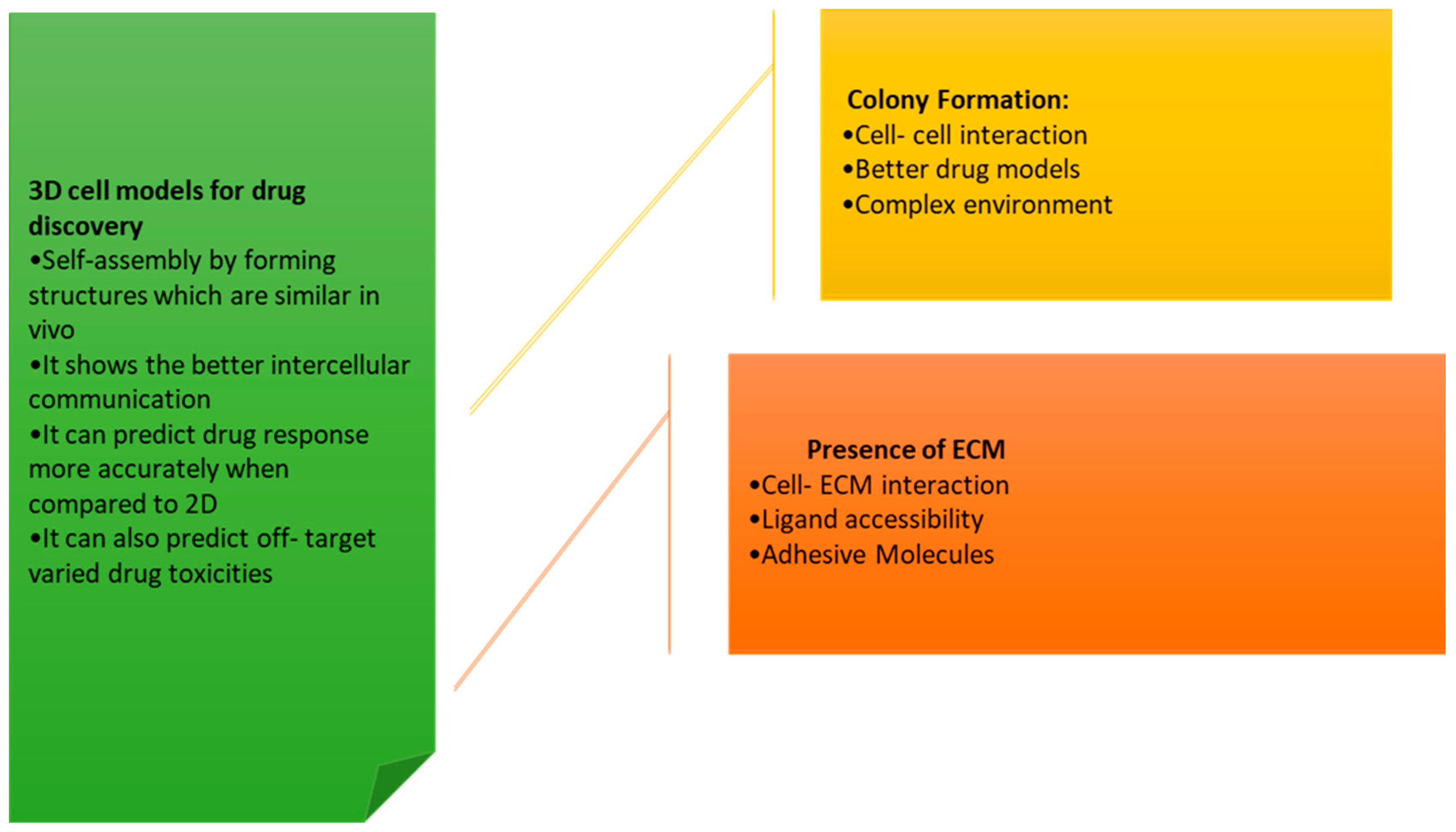
- Compared to traditional 2D cell cultures, 3D cell cultures more accurately replicate the in vivo environment of cells, making them more effective for studying cellular behavior and biological processes.
- The 3D cell cultures can be grown in a variety of different formats, including spheroids, organoids, and scaffold-based cultures.
- The 3D cell cultures have been used extensively over a wide range of biological processes, including cancer progression, tissue development, and drug metabolism.
- The 3D cell cultures have the potential to be used as an alternative to animal testing, as they can provide more accurate results and are more ethical.
- The use of 3D cell cultures has been increasing in recent times due to advances in technology, such as the development of automated systems for growing and analyzing 3D cultures.
3.12. How Do 3D Cell Cultures Simulate the Structure?
3.12.1. Molding
3.12.2. Microspheres
3.12.3. Channels
3.12.4. Composites
3.13. Applications of 3D Cell Culture
3.13.1. D Bioprinted Tissues/Organs for Transplantation
3.13.2. 3D Cultures in Cell Therapy and Tissue Engineering
3.13.3. Challenges and Future Perspectives
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef]
- Fröhlich, E. Issues with Cancer Spheroid Models in Therapeutic Drug Screening. Curr. Pharm. Des. 2020, 26, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers 2020, 12, 2727. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas. J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar] [CrossRef]
- Steinberg, M.S. Differential adhesion in morphogenesis: A modern view. Curr. Opin. Genet. Dev. 2007, 17, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.C. Spheroids and cell survival. Crit. Rev. Oncol. 2000, 36, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The Use of 3-D Cultures for High-Throughput Screening: The Multicellular Spheroid Model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef]
- Quereda, V.; Hou, S.; Madoux, F.; Scampavia, L.; Spicer, T.P.; Duckett, D. A Cytotoxic Three-Dimensional-Spheroid, High-Throughput Assay Using Patient-Derived Glioma Stem Cells. SLAS Discov. Adv. Life Sci. R D 2018, 23, 842–849. [Google Scholar] [CrossRef]
- Halfter, K.; Hoffmann, O.; Ditsch, N.; Ahne, M.; Arnold, F.; Paepke, S.; Grab, D.; Bauerfeind, I.; Mayer, B. Testing chemotherapy efficacy in HER2 negative breast cancer using patient-derived spheroids. J. Transl. Med. 2016, 14, 123. [Google Scholar] [CrossRef]
- Tomás-Bort, E.; Kieler, M.; Sharma, S.; Candido, J.B.; Loessner, D. 3D approaches to model the tumor microenvironment of pancreatic cancer. Theranostics 2020, 10, 5074–5089. [Google Scholar] [CrossRef]
- Raghavan, S.; Mehta, P.; Ward, M.R.; Bregenzer, M.E.; Fleck, E.M.A.; Tan, L.; McLean, K.; Buckanovich, R.J.; Mehta, G. Personalized Medicine–Based Approach to Model Patterns of Chemoresistance and Tumor Recurrence Using Ovarian Cancer Stem Cell Spheroids. Clin. Cancer Res. 2017, 23, 6934–6945. [Google Scholar] [CrossRef]
- Zhang, L.; Su, P.; Xu, C.; Yang, J.; Yu, W.; Huang, D. Chondrogenic differentiation of human mesenchymal stem cells: A comparison between micro mass and pellet culture systems. Biotechnol. Lett. 2010, 32, 1339–1346. [Google Scholar] [CrossRef]
- Timmins, N.E.; Nielsen, L.K. Generation of Multicellular Tumor Spheroids by the Hanging-Drop Method. In Tissue Engineering; Methods in Molecular MedicineTM; Humana Press: Totowa, NJ, USA, 2007; Volume 140, pp. 141–151. [Google Scholar] [CrossRef]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In Vitro Expansion of Human Gastric Epithelial Stem Cells and Their Responses to Bacterial Infection. Gastroenterology 2015, 148, 126–136.e6. [Google Scholar] [CrossRef]
- Garcez, P.P.; Loiola, E.C.; Madeiro Da Costa, R.; Higa, L.M.; Trindade, P.; DelVecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef]
- Li, X.; Nadauld, L.; Ootani, A.; Corney, D.C.; Pai, R.K.; Gevaert, O.; Cantrell, M.A.; Rack, P.G.; Neal, J.T.; Chan, C.W.-M.; et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 2014, 20, 769–777. [Google Scholar] [CrossRef]
- Cruz, N.M.; Song, X.; Czerniecki, S.M.; Gulieva, R.E.; Churchill, A.J.; Kim, Y.K.; Winston, K.; Tran, L.M.; Diaz, M.A.; Fu, H.; et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat. Mater. 2017, 16, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Pringle, S.; Maimets, M.; van der Zwaag, M.; Stokman, M.A.; van Gosliga, D.; Zwart, E.; Witjes, M.J.H.; de Haan, G.; van Os, R.; Coppes, R.P. Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cells 2016, 34, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Kasagi, Y.; Chandramouleeswaran, P.M.; Whelan, K.A.; Tanaka, K.; Giroux, V.; Sharma, M.; Wang, J.; Benitez, A.J.; DeMarshall, M.; Tobias, J.W.; et al. The Esophageal Organoid System Reveals Functional Interplay Between Notch and Cytokines in Reactive Epithelial Changes. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; Van Houdt, W.; Van Gorp, J.; Taylor-Weiner, A.; Kester, L. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef]
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G.-L. Generation of human brain region–specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 2018, 13, 565–580. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Huh, D. A Human Breathing Lung-on-a-Chip. Ann. Am. Thorac. Soc. 2015, 12, S42–S44. [Google Scholar] [CrossRef]
- Shrestha, J.; Bazaz, S.R.; Es, H.A.; Azari, D.Y.; Thierry, B.; Warkiani, M.E.; Ghadiri, M. Lung-on-a-chip: The future of respiratory disease models and pharmacological studies. Crit. Rev. Biotechnol. 2020, 40, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Chou, L.-F.; Chien, C.-C.M.; Chang, H.-Y. Dynamic analysis of hepatoma spheroid formation: Roles of E-cadherin and β1-integrin. Cell Tissue Res. 2006, 324, 411–422. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Barra, G.; Ciaramella, V.; Di Liello, R.; Vicidomini, G.; Zappavigna, S.; Luce, A.; Abate, M.; Fiorelli, A.; Caraglia, M.; et al. Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J. Exp. Clin. Cancer Res. 2019, 38, 253. [Google Scholar] [CrossRef]
- Jeppesen, M.; Hagel, G.; Glenthoj, A.; Vainer, B.; Ibsen, P.; Harling, H.; Thastrup, O.; Jørgensen, L.N.; Thastrup, J. Short-term spheroid culture of primary colorectal cancer cells as an in vitro model for personalizing cancer medicine. PLoS ONE 2017, 12, e0183074. [Google Scholar] [CrossRef]
- Linxweiler, J.; Hammer, M.; Muhs, S.; Kohn, M.; Pryalukhin, A.; Veith, C.; Bohle, R.M.; Stöckle, M.; Junker, K.; Saar, M. Patient-derived, three-dimensional spheroid cultures provide a versatile translational model for the study of organ-confined prostate cancer. J. Cancer Res. Clin. Oncol. 2018, 145, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Ishiguro, T.; Okumura, M.; Iwanaga, T.; Kadosawa, T.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp. Hematol. 2004, 32, 502–509. [Google Scholar] [CrossRef] [PubMed]
- McCauley, H.A.; Wells, J.M. Pluripotent stem cell-derived organoids: Using principles of developmental biology to grow human tissues in a dish. Development 2017, 144, 958–962. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Ootani, A.; Li, X.; Sangiorgi, E.; Ho, Q.T.; Ueno, H.; Toda, S.; Sugihara, H.; Fujimoto, K.; Weissman, I.L.; Capecchi, M.R.; et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009, 15, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter-and intra-tumoral heterogeneity. Cell 2020, 180, 188–204.e22. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, B.D.; Dong, Z.; Nör, J.E. RAIN-Droplet: A novel 3D in vitro angiogenesis model. Lab. Investig. 2012, 92, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, K.; Tsurkan, M.V.; Freudenberg, U.; Werner, C. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci. Rep. 2014, 4, 4414. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Yi, S.H.; Jeong, S.H.; Ku, B.; Kim, J.; Lee, M.-Y. Plastic pillar inserts for three-dimensional (3D) cell cultures in 96-well plates. Sens. Actuators B Chem. 2013, 177, 78–85. [Google Scholar] [CrossRef]
- Di, Z.; Klop, M.J.D.; Rogkoti, V.-M.; Le Dévédec, S.; Van De Water, B.; Verbeek, F.J.; Price, L.S.; Meerman, J.H.N. Ultra High Content Image Analysis and Phenotype Profiling of 3D Cultured Micro-Tissues. PLoS ONE 2014, 9, e109688. [Google Scholar] [CrossRef]
- Poincloux, R.; Collin, O.; Lizárraga, F.; Romao, M.; Debray, M.; Piel, M.; Chavrier, P. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc. Natl. Acad. Sci. USA 2011, 108, 1943–1948. [Google Scholar] [CrossRef]
- Gunness, P.; Mueller, D.; Shevchenko, V.; Heinzle, E.; Ingelman-Sundberg, M.; Noor, F. 3D Organotypic Cultures of Human HepaRG Cells: A Tool for In Vitro Toxicity Studies. Toxicol. Sci. 2013, 133, 67–78. [Google Scholar] [CrossRef]
- Mueller, D.; Krämer, L.; Hoffmann, E.; Klein, S.; Noor, F. 3D organotypic HepaRG cultures as in vitro model for acute and repeated dose toxicity studies. Toxicol. In Vitro 2014, 28, 104–112. [Google Scholar] [CrossRef]
- Thoma, C.R.; Stroebel, S.; Rösch, N.; Calpe, B.; Krek, W.; Kelm, J.M. A High-Throughput–Compatible 3D Microtissue Co-Culture System for Phenotypic RNAi Screening Applications. J. Biomol. Screen. 2013, 18, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, C.; Kapadia, A.; Zhou, Q.; Harper, M.K.; Schaack, J.; Labarbera, D.V. 3D Models of Epithelial-Mesenchymal Transition in Breast Cancer Metastasis: High-Throughput Screening Assay Development, Validation, and Pilot Screen. J. Biomol. Screen. 2011, 16, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawabata, K.; Nagamoto, Y.; Kishimoto, K.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Kanda, K.; Hayakawa, T.; Furue, M.K.; et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 2013, 34, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Doyonnas, R.; Havenstrite, K.; Koleckar, K.; Blau, H.M. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr. Biol. 2009, 1, 59–69. [Google Scholar] [CrossRef]
- Håkanson, M.; Köbel, S.; Lutolf, M.P.; Textor, M.; Cukierman, E.; Charnley, M. Controlled Breast Cancer Microarrays for the Deconvolution of Cellular Multilayering and Density Effects upon Drug Responses. PLoS ONE 2012, 7, e40141. [Google Scholar] [CrossRef]
- Vonk, L.; Roël, G.; Hernigou, J.; Kaps, C.; Hernigou, P. Role of Matrix-Associated Autologous Chondrocyte Implantation with Spheroids in the Treatment of Large Chondral Defects in the Knee: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7149. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough In Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Booij, T.H.; Price, L.S.; Danen, E.H.J. 3D Cell-Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS Discov. Adv. Life Sci. R D 2019, 24, 615–627. [Google Scholar] [CrossRef]
- Picot, J.; Guerin, C.L.; Le Van Kim, C.; Boulanger, C.M. Flow cytometry: Retrospective, fundamentals and recent instrumentation. Cytotechnology 2012, 64, 109–130. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, N.; Yang, X.; Peng, B.; Jiang, H. New advances in microfluidic flow cytometry. Electrophoresis 2019, 40, 1212–1229. [Google Scholar] [CrossRef]
- Lei, K.F.; Wu, M.H.; Hsu, C.W.; Chen, Y.D. Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens. Bioelectron. 2014, 51, 16–21. [Google Scholar] [CrossRef] [PubMed]
- De Bournonville, S.; Lambrechts, T.; Vanhulst, J.; Luyten, F.P.; Papantoniou, I.; Geris, L. Towards Self-Regulated Bioprocessing: A Compact Benchtop Bioreactor System for Monitored and Controlled 3D Cell and Tissue Culture. Biotechnol. J. 2019, 14, e1800545. [Google Scholar] [CrossRef] [PubMed]

| Property | 2D | 2.5D | 3D |
|---|---|---|---|
| Culture Matrix | Flat, inert | A curved surface, bioactive in nature | Stereoscopic, bioactive |
| Cell Polarity | No | yes | yes |
| Biological factor diffusion | Fast, Passive | Fast, Passive | Slow, active |
| Microenvironment | Static with the partial connection between cells, Imperfect physiological functions | Dynamic, interconnected cells observed in the 2D microenvironment | Dynamic, Reflects the interaction between cells, cells + ECM, cells, and tissues |
| Characteristics | Spheroids | Organoids |
|---|---|---|
| Source | Primary cell lines in tumors, multicellular mixtures | Embryonic and adult stem cells, or induced pluripotent cells, tumor cells, and tissues |
| Organization(3D) | Cell-cell in self-assembly, aggregation, and adhesion. In vivo models as self-organization | To respond to physical and chemical cues in forming complex structures, organoids undergo self-organization and self-assembly |
| Organs Physiology | This shows various layers of proliferation in heterogeneous cells- necrotic tissues resembling 3D cellular organization | Diversified cell lineages that reflect the structure and function of the organ |
| culture conditions(3D) | Extracellular matrix presence or absence of growth factors. | Input as extracellular matrix along with a cocktail of growth factors |
| Model | Natural | Synthetic |
|---|---|---|
| Biocompatibility | High | Medium-High |
| Bioactivity | Inherently bioactive | Inert state |
| Cell Modification of ECM | Cannot be adjusted | Can be adjusted |
| Endogenous factors | Present naturally | None |
| Tunability | Low | High |
| Reproducibility | Low | High |
| Microenvironment | Complex | Simple |
| Batch-batch variations | High | Low |
| Parameters | Extrusion Bioprinting | Stereolithography | Laser-Assisted Bioprinting | Inkjet Bioprinting |
|---|---|---|---|---|
| Resolution (um) | Moderate | High (100) | High (50) | High (50–300) |
| Speed | Low | High | Medium | High |
| Cell viability | 40–80% | >85% | >85% | >85% |
| Cell density | High (spheroids) | High | 106–107 cells/mL | 106–107 cells/mL |
| Ink viscosity (mPa/s) | Up to 6 × 107 | No limitation | 1–300 | 3.5–12 |
| Advantages | Simple, Capable of printing, across biomaterials | Nozzle-free technique, Printing time, Independent model, High accuracy | Deposition in the solid or liquid phase, High spatial resolution, No issues with print head clotting | Ability to print low-viscosity biomaterials, low volumes of solutions, and cells required |
| Disadvantages | Applicable for viscous liquids | Uv light is toxic to cells, unable to print multiple cells | Thermal damage due to laser irritation | Poor functionality for vertical structures. |
| Cell Cultures | Advantages | Disadvantages | References |
|---|---|---|---|
| (1) Hydrogel matrix | Cell-cell communications (cell-ECM) | Upon 3D formation, the disposal of cells and changing growth media confers very low throughput and thus, makes it difficult for recovery. | In vitro angiogenesis and drug testing [44] Drug response study [45,46,47] Cancer research [48] |
| Growth factors were incorporated quite handy | |||
| Microenvironment (in-vivo) | |||
| Uniformly spread spheroid | |||
| (2) Method of Hanging Drop | The homogenous spheroids can be quite easily formed. | This demands frequent growth in media change. However, analysis is required which demands labor and time. Cells were subjected to mechanical shocks very often. | Hepatotoxicity testing with HepaRG cells ([49,50]; Target identification and validation using RNAi [51] |
| (3) Method of Liquid Overlay | Quite easy to use and handy for long cultures. | With extensive labor and time, centrifugation yields very low throughput, along heterogeneous cells were produced massively. | Evaluation of the therapeutic response of anticancer drugs [52] Identification of anticancer drugs [53] hepatoxicity testing with iPSC- derived hepatocytes [54] |
| (4) Method of Microwell Platform | HCL compatibility with different spheroid sizes. | Cross-contamination takes place with microwells. Therefore, testing compounds turn out quite difficult. | Study of self-renewal and differentiation process of stem cells [55] Study of cancer and drug development [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajjarapu, S.M.; Tiwari, A.; Kumar, S. Applications and Utility of Three-Dimensional In Vitro Cell Culture for Therapeutics. Future Pharmacol. 2023, 3, 213-228. https://doi.org/10.3390/futurepharmacol3010015
Ajjarapu SM, Tiwari A, Kumar S. Applications and Utility of Three-Dimensional In Vitro Cell Culture for Therapeutics. Future Pharmacology. 2023; 3(1):213-228. https://doi.org/10.3390/futurepharmacol3010015
Chicago/Turabian StyleAjjarapu, Suchitra Maheswari, Apoorv Tiwari, and Sundip Kumar. 2023. "Applications and Utility of Three-Dimensional In Vitro Cell Culture for Therapeutics" Future Pharmacology 3, no. 1: 213-228. https://doi.org/10.3390/futurepharmacol3010015
APA StyleAjjarapu, S. M., Tiwari, A., & Kumar, S. (2023). Applications and Utility of Three-Dimensional In Vitro Cell Culture for Therapeutics. Future Pharmacology, 3(1), 213-228. https://doi.org/10.3390/futurepharmacol3010015









