Abstract
Background: Despite proven efficacy of biologics in inflammatory bowel disease (IBD), many exhibit primary non-response or secondary loss of response and switch to subsequent biologic(s). Here, we identified early predictors of second- and/or third-line biologic persistence in IBD, in a real-world cohort of patients. Methods: A retrospective multicentre cohort study was conducted on patients receiving second- and/or third-line biologics for IBD from 2005–2021. Cox regression was applied to identify factors predictive of longer cumulative biologic persistence prior to treatment failure. Results: Of 179 patients who received ≥2 biologics, 159 (88.8%) received an anti-tumour necrosis factor (anti-TNF) first-line. There was a significantly increased likelihood of longer treatment persistence in recipients who received an anti-TNF first, versus those that received a non-anti-TNF agent first (p < 0.01). A diagnosis of CD (OR 7.1, 95% CI [2.3–21.7], p < 0.01), and endoscopic remission achieved on the first biologic (OR 10.4 [1.3–79.9], p = 0.03) were positive predictors of longer biologic persistence, whilst advancing age at IBD diagnosis (OR 0.97 [0.94–0.99], p = 0.04) and primary non-response to initial biologic (OR 0.3 [0.1–0.7], p < 0.01) were inversely associated with biologic persistence. Conclusions: These real-world data demonstrate multiple, simple to identify factors that offer the potential for early objectively assessed response to first-line biologic to predict future biologic persistence.
1. Introduction
Crohn’s disease (CD) and Ulcerative colitis (UC) are relapsing and remitting inflammatory diseases of the gastrointestinal tract. In those with more severe and aggressive phenotypes, adequate treatment mandates rapid stepwise progression to biological therapy to achieve and maintain clinical and endoscopic remission, utilising a “treat-to-target” approach which has been associated with improved long-term outcomes [1,2].
Biologic therapy has revolutionized medical treatment of inflammatory bowel disease (IBD), with improved disease control and mucosal healing rates. Nevertheless, there are a subgroup of patients who either do not respond to a biologic agent (primary non-response) or lose response over time (secondary loss of response, SLOR) [3]. Real-world data suggest that up to 50 percent of patients on a biologic will experience this in time, though it remains challenging to predict which patients this will affect [3]. The postulated mechanisms, best studied in the anti-tumour necrosis factor (anti-TNF) biologics, include: pharmacodynamic failure (adequate drug level with biologic resistance or loss of response through promotion of alternate inflammatory pathways); or pharmacokinetic failure (immune-mediated: low drug levels with rising anti-drug antibody titres; or non-immune mediated: no anti-drug antibodies present but other factors such as weight, high inflammatory burden or ‘antigen sink’ and/or genetic factors contribute) [4,5,6]. Strategies to overcome primary non-response and SLOR can include dose intensification or switching to another biologic, either within or out of biologic drug class.
Despite these strategies becoming standard practice, there is paucity of data surrounding the optimal choice and order of biologics, or modifiable factors to ensure longer term persistence with second and third-line biologics thereafter. From a patient’s perspective, it is the collective maintenance of remission and long-term disease stability, along with positive safety profile, regardless of whether a single or multiple sequential biologic therapies are employed that is integral to a consistent, robust quality of life. Furthermore, it has recently been suggested that with each subsequent biologic, the likelihood of durable response reduces [6,7]. Hence, this study aimed to (1) assess the relative persistence of sequential biologics in a real-world cohort, and (2) elucidate predictors of long-term treatment persistence with consecutive biologics in IBD.
2. Methods
A retrospective cohort study was conducted on patient data from 2005 to 2021 from two tertiary hospitals in Melbourne, Victoria, Australia. Data from all patients who received two or more biologic therapies was extracted from IBD databases at each site and patients’ individual electronic medical records. There were no defined exclusion criteria. Data extracted included date of birth, sex, weight (where available), diagnosis and phenotype (as per Montreal classification), age of diagnosis, smoking status, and surgical history. The start and stop dates for each biological drug therapy, reason for cessation, therapeutic drug monitoring and anti-drug antibody levels (where available) along with the closest corresponding c-reactive protein (CRP), albumin, faecal calprotectin, and endoscopic results guiding treatment decisions were additionally retrieved. Steroid duration and concomitant immunomodulator therapy were recorded to determine influence on outcomes.
Persistence was defined as the duration of therapy from initiation to discontinuation of each biologic, whilst primary non-response was defined as failure to respond to treatment at conclusion of induction either biochemically or endoscopically. Pharmacodynamic failure was defined as primary non-response or loss of response with adequate drug levels. We additionally included immunogenic failure—high anti-drug antibody levels with low drug levels—in the definition to broaden applicability to a real world population. Biologic failure was defined by individual clinicians, using these data, in a real-world setting. Furthermore, patients tolerability or lifestyle factors contributed to cessation in some cases. There were no local or reimbursement directives that would potentially impact biologic choice, order and/or escalation strategies during the data collection period; these decisions were at the discretion of the treating clinician in discussion with the patient.
This study design was considered low risk by the relevant institutions’ ethics committees, having met criteria for Quality Assurance or Audit activity per the Australian National Health and Medical Research Council (NHMRC) guidelines.
Statistical Analysis
Data were assessed for normality with Shapiro–Wilk testing for relevant continuous variables and non-normality was assumed for all analyses. Continuous variables were expressed as medians and range, and compared with Kruskal–Wallis or Mann–Whitney U tests as appropriate. Proportions were expressed as percentages and compared with Fisher exact tests.
Given the disparate durations between first biologic commencement and end of study follow-up, Kaplan–Meier survival curve analyses were conducted to assess the proportion of patients maintaining treatment persistence over time, censored by failure of the first- and second-line biologic during the study period, and comparing between relevant patient, disease, and treatment-related factors at baseline. The total duration of follow-up since commencement of first biologic was the dependent variable in all analyses. Mantel-Cox log rank tests were applied to assess for statistical significance. Factors significantly associated with treatment persistence in these univariable analyses were then included in a Cox multiple regression analysis, again with total duration of follow-up as the dependent variable and censored by failure of the first- and second-line biologic therapies. A backwards conditional model was applied, with the (highest) Omnibus test of model coefficient value used to select the final model as presented. A p-value < 0.05 conferred significance for all tests. The software package used for data analysis was SPSS, IBM, Version 27.
3. Results
During the study period, 179 patients failed a biologic, and 162 patients whom received two or more biological therapies were included in final analysis. Those that were not included either started their therapy prior to engagement with our units, or moved shortly thereafter starting second biologic therapy. Baseline characteristics are presented in Table 1. 159 patients (88.8%) were commenced on an anti-TNF biologic as initial therapy, whilst conversely, the majority (n = 41; 77.4%) received an agent other than an anti-TNF biologic as their third-line therapy (Figure 1; p < 0.05).

Table 1.
Demographics and patient characteristics prior to biologic therapy (total n = 179).
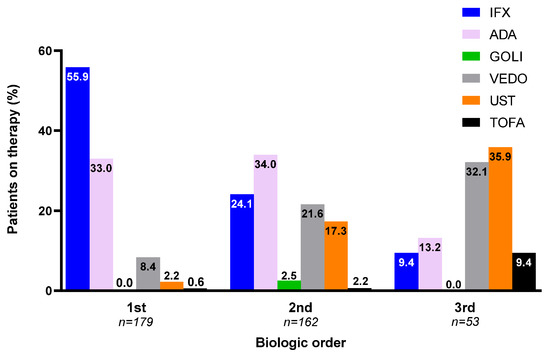
Figure 1.
Order of therapy by biologic agent (proportion (%) per total number of patients on first-, second-, and third-line biologic within study follow-up period) (IFX—infliximab; ADA—adalimumab; GOLI—golimumab; VEDO—vedolizumab; UST—ustekinumab; TOFA—tofacitinib).
Comparisons of characteristics per successive biologic period are presented in Table 2. In the subgroup where at least one cessation occurred during the study follow-up period, the median duration of biologic therapy did not differ according to chronologic order for either anti-TNF or non-anti-TNF biologics (Table 2). Additionally, in this subgroup, there was a significant difference in treatment persistence between anti-TNF and non-TNF biologics for the second biologic (median 36.7 [range 2.0, 193.1] versus 24.7 [5.5, 70.5] months, respectively, p = 0.004), though not for the third biologic (median 30.0 [8.0, 58.8] versus 16.1 [0.1, 71.9] months, respectively, p = 0.10).

Table 2.
Comparison of characteristics per successive biologic period (1st, 2nd, 3rd).
Moreover, in this same subgroup where biologic cessation(s) occurred within the study follow-up period, there was a statistically significant trend of shorter duration with each successive biologic (Figure 2). Furthermore, there were reducing rates of both primary non-response and pharmacodynamic failure with each successive biologic therapy (chi-squared test, each p < 0.05) (Table 2).
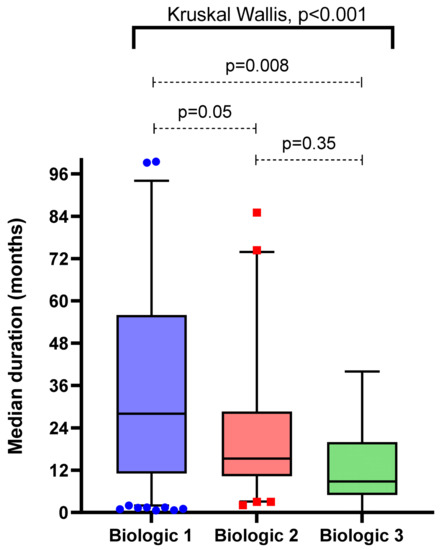
Figure 2.
Comparison of treatment persistence per order of biologic (subgroups including only when biologic ceased due to lack of efficacy or intolerance within study follow-up period).
3.1. Choice of Biologic Order on Overall Therapy Persistence
Kaplan–Meier survival curve analysis revealed no significant difference in treatment persistence between patients receiving anti-TNF therapy for their first- and second-line biologic compared with those receiving anti-TNF agent first, then a non-anti-TNF agent second (p = 0.99; Figure 3A). Despite being non-significant when comparing median durations (Table 2), there was a significantly increased likelihood of longer treatment persistence in patients receiving an anti-TNF agent first versus a non-anti-TNF therapy first (then any biologic(s) thereafter, respectively) when survival analysis and log rank test was performed (p < 0.01, Figure 3B).
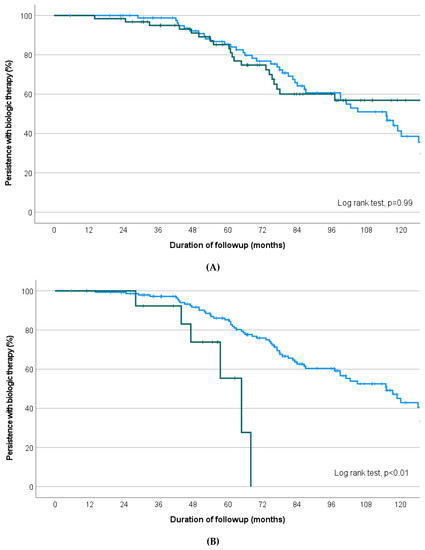
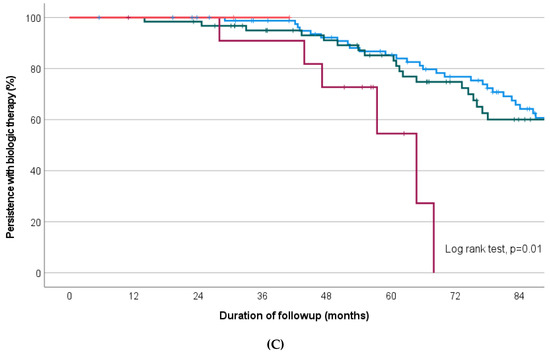
Figure 3.
Kaplan–Meier survival curves depicting proportion of treatment persistence on biologic therapy during follow-up period comparing (A) Anti-TNF therapy as first biologic, then anti-TNF therapy second (blue line) versus anti-TNF therapy first then non-anti-TNF therapy second (green line), (B) Anti-TNF therapy first (blue) versus non-anti-TNF therapy first (green) (regardless of second/third biologic thereafter), (C) All possible permutations for biologic 1 and 2 sequentially (Blue: anti-TNF therapy first and second-line, Green: anti-TNF first with non-anti-TNF agent second-line (black), Maroon: Non-anti-TNF first with anti-TNF second-line, Red: Non-anti-TNF agent first and second (limited numbers only).
All possible permutations for first and second-line biologic are represented in Figure 3C. Within the group receiving first- and second-line anti-TNF agents, there was no difference in the median duration of treatment persistence between infliximab as first choice (then adalimumab or golimumab, n = 59) or adalimumab as first choice (then infliximab or golimumab, n = 37; estimated median 82.6 versus 84.6 months, p = 0.46) (Figure 4).
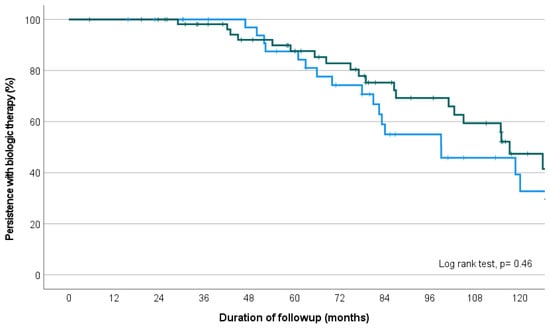
Figure 4.
Kaplan–Meier survival curve depicting proportion of treatment persistence on infliximab then adalimumab/golimumab (green line), versus adalimumab then infliximab/golimumab (blue line), over the study follow-up period.
3.2. Factors Associated with Treatment Persistence
Kaplan–Meier univariate analysis revealed the following factors were significantly associated with treatment persistence: elevated CRP (≥3 mg/L) at baseline (p < 0.01), reduced serum albumin (≤35 g/L) at baseline (p = 0.07), active smoking at commencement of first biologic (p = 0.004) and early introduction of first biologic therapy post-diagnosis (≤12 months; p = 0.02). When accounting for other cofactors however, these were not significant in the multivariate Cox regression model. Additionally, sex (p = 0.27), prior surgical resection (p = 0.57), structuring or penetrating disease behaviour (CD, p = 0.27), extensive colitis (p = 0.86), concomitant immunomodulator at baseline (p = 0.84), and moderate-severe endoscopic activity at baseline (p = 0.64) were not found to predict treatment persistence in second- or third-line biologic therapy.
The final multivariate Cox regression model revealed the following factors independently associated with treatment persistence on consecutive biologics: diagnosis of CD (OR 7.1 (95% CI 2.3–21.7), p < 0.01) and endoscopic remission achieved on the first biologic (OR 10.4 (95% CI 1.3–79.9), p = 0.03). Conversely, advancing age at IBD diagnosis (per 1 year increase; OR 0.97 (95% CI 0.94–0.99), p = 0.04) and primary non-response to initial biologic (OR 0.3 (95% CI 0.1–0.7), p <0.01) were each associated with shorter persistence overall (Table 3).

Table 3.
Cox regression analysis (final model) depicting factors associated with increased likelihood of treatment persistence over time during the study follow-up period.
4. Discussion
Biologic therapies have revolutionized the medical treatment of IBD, but a substantial proportion of patients either lack primary response or lose response with time [3,8,9]. While the efficacy of individual biologic agents has been well demonstrated in the pivotal registration trials and open label extension studies, there is paucity of data available to predict and guide clinicians to which patients are likely to respond to and the durability of second- and third-line biologic therapy. This is despite increasing prevalence of this common scenario.
In this cohort, we have shown sustained duration of therapy with second- and third-line biologic therapies ranging from 24–36 months, and 16–30 months, respectively. Patients who had primary non-response to first-line biologic showed shorter persistence with each subsequent biologic. Treatment persistence was longest if the first biologic was an anti-TNF, but there were no significant differences in those that subsequently had an anti-TNF versus another biologic class. These findings are in line with the results of a prior network meta-analysis which supported the efficacy of infliximab and adalimumab therapy in combination with an immunomodulator to have the highest efficacy as first line treatment in Crohn’s disease [10]. Additionally, there is the possibility that anti-TNF therapy use was longer as initial treatment as other biologic therapies were not available in Australia until 2016. Multivariate Cox-regression analysis revealed CD and endoscopic remission on first-line biologic were independent factors increasing likelihood of persistence of two or more biologics over time. Conversely, advancing age at IBD diagnosis and primary non-response to initial biologic were each associated with shorter persistence of biologic therapy [11].
Primary non-response to index biologic therapy is associated with subsequent diminished response to a second-line biologic. A large systematic review of 8 randomised controlled trials, inclusive of non-anti-TNF second-line biologic data (ustekinumab), found a 24 percent reduction in response to second-line biologic therapies when compared to those with SLOR [7]. A further meta-analysis and systematic review also supports these findings [11]. Our results confirm these data, showing less robust treatment persistence in this cohort. We postulate that patients with primary non-response are intrinsically more treatment refractory for multiple reasons, including that biologic pharmacokinetics are altered in severely inflamed and nutritionally deplete states [12,13,14]. This is exemplified in patients with acute severe colitis where in the setting of a significant inflammatory burden and low serum albumin, higher and/or accelerated doses of infliximab may be more effective than standard induction in selected patients [15,16,17,18]. Further, there is evidence that patients who have primary non-response to anti-TNF therapy may have molecular polymorphisms contributing to primary non-response, and even a paradoxical increased inflammatory state after induction [19,20,21,22]. Hence, patients with primary non-response to an initial biologic would likely benefit from increased prescribing vigilance and optimization of the chosen second-line biologic, such as high induction doses, combination with an immunomodulator or proactive drug level monitoring. Prospective data are clearly needed to confirm this hypothesis.
Moreover, there was a trend towards lower rates of primary non-response with second- and third-line biologic therapies in this cohort (Table 2). This is possibly explained by an overall reduced inflammatory burden at the initiation of second- or third- line biologic therapies, or indeed, increased prescribing vigilance with time.
In addition to primary non-response, shorter persistence of therapy was also associated with age such that each year of age had a three percent reduction in persistence; paralleling previous data [23,24]. Possible explanations include immunosenescence and altered immunogenicity driving biologic refractory disease. Furthermore, altered immunity has been linked with higher rates of anti-TNF antibody formation in elderly populations, potentially forcing alternate biologic therapies in quicker succession [25,26]. Elderly populations also appear to have reduced flare frequency requiring hospitalisation, or indeed, altered and atypical symptomatology possibly driving under recognition of flares [27,28,29,30,31]. Other colonic pathology such as those driven by non-steroidal anti-inflammatory drugs, diverticular disease, ischemic colitis, malignancy or other age-related colonic disease confound this further and perhaps reflect an increased willingness to cease a biologic agent. Indeed, relative scarcity of safety data on second- and third-line biologics in this age group may also invoke reluctance to trial alternate agents, despite a growing body of evidence regarding safety [32].
CD was shown to be independently associated with persistence of subsequent treatment. This may reflect the comparative higher inflammatory burden seen in moderate to severe UC, perhaps driving antibody formation and SLOR, as shown in previous studies [33,34]. Perturbed pharmacokinetics of biologics in excessively inflamed and nutritional states may also contribute [12,13,14,35,36]. Additionally, CD phenotype can be indolent, with flares often underreported or falsely attributed to alternate causes (e.g., irritable bowel syndrome). This is confounded by relative difficulty in quantifying inflammatory activity in small bowel disease given the lower sensitivity of faecal calprotectin, and difficulties in readily access to magnetic resonance imaging or intestinal ultrasound [37,38].
Our finding that previous attainment of endoscopic remission on first-line therapy is associated with increased persistence for second- and third-line therapies, demonstrates the importance of early, objective assessment of disease activity. Not only for early biologic optimisation, but also to potentially predict term disease stability. Indeed, this finding reinforces guidelines suggesting alternate biologics, even within the same class, upon SLOR [4,39]. Furthermore, if a biologic therapy was ceased due to an infusion reaction driven by antibody formation, it is easy to surmise that this intolerance will not be present upon switch of biologic. Thereafter, if endoscopic remission had been achieved, recapture and treatment persistence of second- and third-line agents should be expected.
The data presented here is retrospective in nature and cannot account for all possible confounders that may predict persistence. Further, outside of standard therapeutic drug monitoring doses of biologic induction were not accounted for. Antibodies additionally were not measured at both sites in those that had adequate drug levels. Furthermore, there was no standardised definition between units defining when biologics should be dose increased, or changed, as a surrogate for biologic failure during data collection. Nevertheless, this data does shed light on factors associated with persistence, or lack thereof, of second- and third-line biologic agents. The unmodifiable nature of these variables emphasises the importance of ongoing research to identify those that can be readily altered. Additionally, in time, identifying those at risk of primary non-response through testing of genetic polymorphisms and disease susceptibilities may be a possibility, and indeed personalised biologic prescribing strategies may one day guide clinical practice. Until then, it is reassuring that recapture is possible, and that second- and third-line biologic agents are not futile.
Here, we have shown that a diagnosis of CD opposed to UC, and the attainment of endoscopic remission during first-line biologic therapy are independently associated with increased likelihood of persistence of two or more biologics over time. Advancing age at IBD diagnosis and primary non-response to initial biologic are each associated with shorter persistence of biologic therapy overall. These factors should be considered when prescribing subsequent biologic agents in refractory disease.
Author Contributions
T.P.H.: writing—original draft (lead); conceptualization (supportive); data curation (supportive), methodology (supportive); formal analysis (supportive); writing—review and editing (equal); project administration (lead). R.C.: data curation (lead, site 1). D.T.: data curation (lead, site 2). N.S.D.: supervision; writing—review and editing (equal). C.B.: writing—review and editing (equal). J.S.: writing—review and editing (equal), A.V.: writing—review and editing (equal). M.K.: writing—review and editing (equal). M.D.G.: writing—review and editing (equal). D.R.v.L.: formal analysis (lead); methodology (equal); writing—review and editing (equal); supervision; conceptualization (supportive). O.N.: methodology (equal); supervision; conceptualization (lead). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective nature and overarching ethics approval for retrospective studies in our institute (LR25/2017).
Informed Consent Statement
Patient consent was waived due to the retrospective, deidentified nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
TP Hanrahan, R Chan, D Tassone, N Ding, C Basnayake, A Vasudevan, M Kamm, M De Gregorio and O Niewiadomski have no conflict of interest to declare. J Schulberg has received a grant from Pfizer for meeting attendance. DR van Langenberg has received speaker support from Janssen, Takeda and Sandoz, received research funding from Janssen, Pfizer and Takeda, received conference sponsorship from Pfizer, and has served on advisory boards and consulted for Abbvie, Janssen, Takeda, Pfizer.
References
- Ungaro, R.; Colombel, J.-F.; Lissoos, T.; Peyrin-Biroulet, L. A Treat-to-Target Update in Ulcerative Colitis: A Systematic Review. Am. J. Gastroenterol. 2019, 114, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; D’haens, G.; Lee, W.-J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2019, 14, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.-F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroen. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Mitrev, N.; Casteele, N.V.; Seow, C.H.; Andrews, J.M.; Connor, S.J.; Moore, G.T.; Barclay, M.; Begun, J.; Bryant, R.; Chan, W.; et al. Review article: Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 46, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Pugliese, D.; Lopetuso, L.R.; Scaldaferri, F.; Neri, M.; Guidi, L.; Gasbarrini, A.; Armuzzi, A. Novel trends with biologics in inflammatory bowel disease: Sequential and combined approaches. Ther. Adv. Gastroenter. 2021, 14, 17562848211006668. [Google Scholar] [CrossRef]
- Ding, N.S.; Hart, A.; Cruz, P.D. Systematic review: Predicting and optimising response to anti-TNF therapy in Crohn’s disease—Algorithm for practical management. Aliment. Pharmacol. Ther. 2016, 43, 30–51. [Google Scholar] [CrossRef]
- Singh, S.; George, J.; Boland, B.S.; Casteele, N.V.; Sandborn, W.J. Primary Non-Response to Tumor Necrosis Factor Antagonists is Associated with Inferior Response to Second-line Biologics in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2018, 12, 635–643. [Google Scholar] [CrossRef]
- Yanai, H.; Hanauer, S.B. Assessing Response and Loss of Response to Biological Therapies in IBD. Am. J. Gastroenterol. 2011, 106, 685–698. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Chowers, Y. Review article: Loss of response to anti-TNF treatments in Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 33, 987–995. [Google Scholar] [CrossRef]
- Singh, S.; Fumery, M.; Sandborn, W.J.; Murad, M.H. Systematic review and network meta-analysis: First- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment. Pharmacol. Ther. 2018, 48, 394–409. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Marín, A.C.; McNicholl, A.G.; Chaparro, M. Systematic review with meta-analysis: The efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment. Pharmacol. Ther. 2015, 41, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Fasanmade, A.A.; Adedokun, O.J.; Olson, A.; Strauss, R.; Davis, H.M. Serum albumin concentration: A predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int. J. Clin. Pharmacol. Ther. 2010, 48, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Fasanmade, A.A.; Adedokun, O.J.; Ford, J.; Hernandez, D.; Johanns, J.; Hu, C.; Davis, H.M.; Zhou, H. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur. J. Clin. Pharmacol. 2009, 65, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Brandse, J.F.; van den Brink, G.R.; Wildenberg, M.E.; van der Kleij, D.; Rispens, T.; Jansen, J.M.; Mathôt, R.A.; Ponsioen, C.Y.; Löwenberg, M.; D’Haens, G.R.A.M. Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology 2015, 149, 350–355.e2. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.C.; Seah, D.; Gorelik, A.; An, Y.; Chen, C.; Macrae, F.A.; Sparrow, M.P.; Connell, W.R.; Moore, G.T.; Radford-Smith, G.; et al. Predicting response after infliximab salvage in acute severe ulcerative colitis. J. Gastroenterol. Hepatol. 2018, 33, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.C.; Seah, D.; Faleck, D.M.; Shah, S.C.; Chao, C.-Y.; An, Y.-K.; Radford-Smith, G.; Bessissow, T.; Dubinsky, M.C.; Ford, A.C. Systematic Review and Meta-analysis: Optimal Salvage Therapy in Acute Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 1169–1186. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Heetun, Z.S.; Redmond, C.E.; Nanda, K.S.; Keegan, D.; Byrne, K.; Mulcahy, H.E.; Cullen, G.; Doherty, G.A. An Accelerated Infliximab Induction Regimen Reduces the Need for Early Colectomy in Patients With Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 330–335.e1. [Google Scholar] [CrossRef]
- An, Y.; Chen, C.; White, L.; Howlett, M.; Lord, A.; Radford-Smith, G. Accelerated dosing of infliximab induction and endoscopic mucosal healing in patients with acute severe ulcerative colitis. J. Gastroenterol. Hepatol. 2017, 32, 121–154. [Google Scholar] [CrossRef]
- Louis, E.; Ghoul, Z.E.; Vermeire, S.; Dall’Ozzo, S.; Rutgeerts, P.; Paintaud, G.; Belaiche, J.; de Vos, M.; van Gossum, A.; Colombel, J.-F.; et al. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn’s disease. Aliment. Pharmacol. Ther. 2004, 19, 511–519. [Google Scholar] [CrossRef]
- Louis, E.J.; Watier, H.E.; Schreiber, S.; Hampe, J.; Taillard, F.; Olson, A.; Thorne, N.; Zhang, H.; Colombel, J.-F. Polymorphism in IgG Fc receptor gene FCGR3A and response to infliximab in Crohn’s disease: a subanalysis of the ACCENT I study. Pharmacogenet. Genom. 2006, 16, 911–914. [Google Scholar] [CrossRef]
- Louis, E.; Ribbens, C.; Godon, A.; Franchimont, D.; Groote, D.D.; Hardy, N.; Boniver, J.; Belaiche, J.; Malaise, M. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin. Exp. Immunol. 2000, 120, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Cleynen, I.; Vermeire, S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat. Rev. Gastroenterol. 2012, 9, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Zator, Z.A.; Silva P de Nguyen, D.D.; Korzenik, J.; Yajnik, V.; Ananthakrishnan, A.N. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 19, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lobatón, T.; Ferrante, M.; Rutgeerts, P.; Ballet, V.; Assche, G.V.; Vermeire, S. Efficacy and safety of anti-TNF therapy in elderly patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 42, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Roblin, X. Letter: Immunogenicity of anti-TNF in elderly IBD patients. Aliment. Pharmacol. Ther. 2019, 50, 336. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Grimm, I.S.; Friedman, L.S. Inflammatory bowel disease in the elderly. Gastroenterol. Clin. N. Am. 1990, 19, 361–389. [Google Scholar] [CrossRef]
- Riegler, G.; Tartaglione, M.T.; Carratú, R.; D’incá, R.; Valpiani, D.; Russo, M.I.; Papi, C.; Fiorentini, M.T.; Ingrosso, M.; Andreoli, A.; et al. Age-Related Clinical Severity at Diagnosis in 1705 Patients with Ulcerative Colitis. Dig. Dis. Sci. 2000, 45, 462–465. [Google Scholar] [CrossRef]
- Del Val, J.H. Old-age inflammatory bowel disease onset: A different problem? World J. Gastroenterol. 2011, 17, 2734–2739. [Google Scholar] [CrossRef]
- Heresbach, D.; Alexandre, J.-L.; Bretagne, J.-F.; Cruchant, E.; Dabadie, A.; Dartois-Hoguin, M.; Girardot, P.-M.; Jouanolle, H.; Kerneis, J.; Le Verger, J.-C.; et al. Crohn’s disease in the over-60 age group. Eur. J. Gastroenterol. Hepat. 2004, 16, 657–664. [Google Scholar] [CrossRef]
- Freeman, H.J. Age-Dependent Phenotypic Clinical Expression of Crohn’s Disease. J. Clin. Gastroenterol. 2005, 39, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Stitt, L.W.; Zou, G.; Khanna, R.; Dulai, P.S.; Sandborn, W.J.; Feagan, B.G.; Jairath, V. Early combined immunosuppression may be effective and safe in older patients with Crohn’s disease: Post hoc analysis of REACT. Aliment. Pharmacol. Ther. 2019, 49, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.I.; Lichtenstein, G.R. Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease. Curr. Treat. Options Gastroenterol. 2014, 12, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, I.; Blümel, S.; Schreiner, J.; Boyman, O.; Bögeholz, J.; Cheetham, M.; Rogler, G.; Biedermann, L.; Scharl, M. Clinical Relevance of Anti-TNF Antibody Trough Levels and Anti-Drug Antibodies in Treating Inflammatory Bowel Disease Patients. Inflamm. Intest. Dis. 2021, 6, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Xu, Z.; Marano, C.W.; Johanns, J.; Zhou, H.; Davis, H.M.; Cornillie, F.; et al. Association between Serum Concentration of Infliximab and Efficacy in Adult Patients with Ulcerative Colitis. Gastroenterology 2014, 147, 1296–1307.e5. [Google Scholar] [CrossRef]
- Paul, S.; Moreau, A.C.; Tedesco, E.D.; Rinaudo, M.; Phelip, J.-M.; Genin, C.; Peyrin-Biroulet, L.; Roblin, X. Pharmacokinetics of Adalimumab in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2014, 20, 1288–1295. [Google Scholar] [CrossRef]
- D’Arcangelo, G.; Imondi, C.; Terrin, G.; Catassi, G.; Aloi, M. Is Fecal Calprotectin a Useful Marker for Small Bowel Crohn’s Disease? J. Pediatr. Gastroenterol. Nutr. 2021, 73, 242–246. [Google Scholar] [CrossRef]
- Ye, L.; Cheng, W.; Chen, B.; Lan, X.; Wang, S.; Wu, X.; Huang, W.; Wang, F.-Y. Levels of Faecal Calprotectin and Magnetic Resonance Enterocolonography Correlate with Severity of Small Bowel Crohn’s Disease: A Retrospective Cohort Study. Sci. Rep. 2017, 7, 1970. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).