Abstract
Selenium is one of the eight necessary trace elements humans require for active health balance. It contributes in several ways to the proper functioning of selenoprotein. Selenium has received enormous interest recently due to its therapeutic potential against a number of ailments. To date, numerous chemical compounds containing selenium have been investigated for the therapy of cancer and other disorders. Unifying the selenium atom into chemical components (typically organic) greatly increased their bioactivities. We foresee that the structure–property relationship of recently developed materials could significantly decrease the laborious work of background research to achieve target-oriented drug design in coming years. This review summarizes the research progress in the last 10 to 15 years and the application of selenium-containing compounds in the design and synthesis of those materials for potential antioxidant and anticancer agents.
1. Introduction
Selenium is a nonmetal/metalloid with characteristics halfway between those of sulfur and tellurium [1,2]. In the Earth’s crust, it hardly ever exists in either its elemental state or as a pure ore complex. Jöns Jacob Berzelius made the discovery of selenium in 1817 and remarked that it resembled the already-known element tellurium [1]. Although minute amounts of selenium are required for normal cellular function, elemental selenium and selenium salts are hazardous in even small levels and can lead to selenosis [3]. The antioxidants glutathione peroxidase and thioredoxin reductase, as well as deiodinase enzymes, contain selenium, which is listed as an element in many multivitamins and other nutritional supplements [4,5]. Thioredoxin reductase and glutathione peroxidase catalyze processes that are necessary for shielding biological components from oxidative and free radical damage. The amount of selenium needed by different plant species varies from high levels to zero level [6]. The current recommended daily selenium allowance or recommended dietary allowances (RDA) for adult men and women is 55 micrograms [7].
Selenium-based heterocycles have made a valuable contribution in material science due to their electron-donating and electron-accepting abilities and their ability to modify electronic, structural, and morphological properties [8,9,10,11]. Selenium-based heterocycles have been utilized in small molecules, oligomers, and polymers to improve the electronic properties in an incremental direction [12,13,14,15]. Multiple selenium-containing compounds [12,16] have also been developed to improve the electronic behavior with higher mobility value in field-effect transistor (FET) devices [17]. They have also been used as polymers in organic solar cells and organic electronic devices for better power conversion efficiency (PCE) and mobility value for implementation purposes [18].
Selenium-containing compounds have been of great significance in the domain of medicinal chemistry. Selenium and selenium-containing compounds continue to be topics of substantial interest in the fields of biochemistry, epidemiology, and pharmacology. Numerous diseases that are now known to be linked to selenium deficiency have historically affected specific groups [19,20]. When considering medicinal uses, it is vitally important to manage the right amount and the right molecular types of selenium-based pharmaceuticals. It is important to note that more than 40 years ago, the idea that selenium might have a preventive effect against human cancer was explored in mainstream scientific literature [21,22]. Cancer death rates were found to be much lower in US counties with moderate or high selenium levels, as opposed to counties with low selenium levels, which revealed an inverse relationship between selenium levels and cancer incidence [23]. Low selenium levels have been perceived in breast cancer patients and patients with pancreatic carcinomata. Selenium compounds have been shown to have antitumorigenic properties in several animal studies [24].
As a crucial trace element, selenium also performs a number of exclusive activities and has significant metabolic impacts on human health [25]. Numerous clinical studies have demonstrated that selenium deficit in the human body can lead to many significant life-threatening disorders, such as cancer, liver disease, organ failure, and cardiovascular disease [26,27]. Several studies have demonstrated that individuals with low or deficient selenium levels require an appropriate selenium supplement, which helps in boosting antioxidant activity and augmenting cellular defense capability [28]. As a result, the development of unique selenium compounds provides a desirable toolset for medical chemists in building effective pharmaceuticals. Selenium-containing compounds have been found to have antioxidants, anticancer agents, and antiparasitic, antibacterial, antiviral, antifungal, and neuroprotective properties [24,29,30]. We foresee that the structure–property relationship of recently developed materials could significantly decrease the laborious work of background research to achieve target-oriented drug design in coming years. This review summarizes the research progress that has taken place in last 10 to 15 years and the application of selenium-containing compounds from differential structural aspects, which will be helpful in increasing interest in the community in the design and synthesis of those materials for applications as potential antioxidant and anticancer agents.
2. Antioxidant Activity
Selenocysteine can substitute the effect of cysteine and shield healthy cells from the adverse effects of reactive oxygen species (ROS); in other ways, specific selenium compounds (Figure 1) are classified as antioxidant agents that preserve the redox environment in healthy cells [31]. In contrast, oxidation occurs at advanced levels in cancer cells and affects various aspects of their activity with ineffective antioxidant mechanisms [32]. Selenium can be administered in its elemental form or after combining it with other inorganic or organic compounds, where low doses promote cell development and high levels exhibit a cytotoxic impact [32].
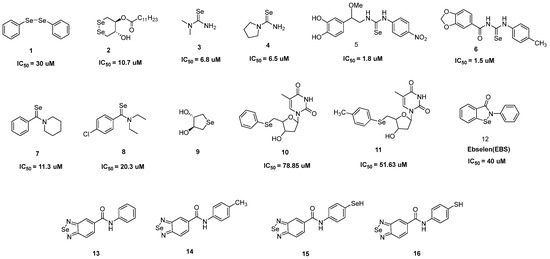
Figure 1.
Selenium-containing compounds with antioxidant activity [31,32,33,34,35,36,37,38,39,40,41,42,43].
The excellent antioxidant and glutathione-peroxidase-like properties of diphenyl diselenide 1 help to defend macrophages from atherogenic signaling. Pretreating J774A.1 macrophages with 1 substantially reduced the formation of ROS driven by oxidized low-density lipoprotein (oxLDL) [33]. Without affecting by inherent cytotoxicity, 1 was able to prevent breast cancer (MCF-7) cells from oxidative damage imposed by tamoxifen [34]. Cyclic diselenide, compound 2, outperformed in terms of anti-lipid peroxidation activity (IC50 = 10.7 μM) [35]. In actuality, the coexistence of dithiothreitol [Se⁻ Se−] caused by the diselenide (Se-Se) bond reduction resulted in highly active [Se−, Se−] that helped to convert H2O2 to H2O and reintroduce the original Se-Se form [36]. Moreover, Se-Se directly converted the lipid peroxide to the respective alcohol and inhibited the chain reaction of free radicals as an antioxidant in the process of lipid peroxidation of lecithin/cholesterol liposomes caused by 2,2′-azobis (2-aminopropane) dihydrochloride (AAPH).
Dimethylselenourea 3 and its pyrrolidine analog 4 with IC50 values of 6.8 and 6.5 μM, respectively, showed antioxidant behavior and rapidly scavenged O2− produced by polymorphonuclear leukocyte (PMNs). Selenoureas appeared to readily scavenge O2− and generate O2, with little to no NADPH oxidase activity inhibition. Selenourea analog 5 also showed excellent anti-free radical properties and acted as a biomimetic catalyst for glutathione peroxidase to scavenge H2O2 (IC50 value of 3.76 μM on HeLa cells) [37]. Similarly, selenourea-based compound 6 demonstrated strong radical scavenging activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylenebenzothiazoline)-6-sulfonic acid (ABTS) assays and had a preventive role on cells against oxidative stress [38]. Phenyl-ring-containing selenamides 7 and 8, with IC50 values of 11.3 and 20.3 μM, respectively, efficiently scavenged O2⁻ from PMNs [39].
With good water solubility, cycloselenide 9 catalyzed the dielectronic reduction of H2O2 to H2O and the oxidation of mercaptan (RSH) to disulfide (RSSR) through a catalytic cycle akin to the GPX [40]. The stimulation of the apoptotic signaling pathway in healthy cells was caused by a disparity in redox and immunological homeostasis initiated by the zidovudine derivatives 10 and 11 [41]. Ebselen (EBS) was demonstrated to be a glutathione peroxidase mimic and a peroxynitrite scavenger [42]. EBS pretreatments significantly reduced the amount of thiobarbituric acid-induced substance production on mouse skin. Leukocyte infiltration and activation were specifically prevented by EBS, which reduced the H2O2 level. Compounds 13, 14, and 15 were capable of neutralizing the DPPH activity by 20% at 0.25 mg/mL, whereas compound 16 scavenged the DPPH activity by >40% at 0.25 mg/mL. The result indicated that the selenadiazoles ability to scavenge free radicals was concentration-dependent [43].
3. Anticancer Activity
Cancer is one of the most serious threats to human health. It affects millions of individuals and imposes a tremendous burden on research, social life, and the economy [44]. There is a limit to current treatments’ efficacy for the prevention and treatment of cancer [45,46]. The creation of novel pharmaceuticals [47,48], radiopharmaceuticals [49,50,51], therapeutic targets, and modified molecules with improved drug transport and efficacy have received attention in recent years [52,53].
3.1. Inorganic Se Compound
Common inorganic selenium compounds are hydrogeno selenides (H2Se), hydrogeno metal selenides (HSeM), dimetal selenides (M2Se), hydrogeno diselenides (H2Se2), metal diselenides (M2Se2), metal selenocyanates (MSeCN), and metal selenosulfates (4) (SO3SeM2) (Table 1). Sodium selenite (Na2SeO3) is one of the imperative inorganic selenium salts. Na2SeO3 is a component of dietary supplements, such as multivitamins/minerals. The US Food and Drug Administration (FDA) approved a general form of Na2SeO3 as a supplement to animal diets. Cholangiocarcinoma (a type of malignancy) has been exploited to explore the effects of sodium selenite (Na2SeO3), primarily in preclinical models. Na2SeO3, when applied to cholangiocarcinoma cells at concentrations between 1 and 10 μM, significantly reduces invasion, migration, and the epithelial-to-mesenchymal transition by causing apoptosis and downregulating N-cadherin [54]. It has been demonstrated that at the molecular level, treatment (10 μM) with Na2SeO3 can restrict cell proliferation in gastric cancer. At 30 μM, it caused apoptosis and elevated Se-binding protein 1 (SBP1) expression levels, which are key mediators for anticancer effects of selenium compounds. Additionally, sodium selenite reduced the levels of β-catenin, glycogen synthase kinase 3β (GSK3β), and nuclear factor erythroid 2-related factor 2 (NRF2) [55].

Table 1.
Category of selenium-containing compounds with anticancer activity.
3.2. Peptides
The majority of inorganic Se compounds are poisonous and the minimum lethal dose is relatively low. Therefore, the ideal characteristics of these supplements include organic Se molecules with high biological activity, low toxicity, and few adverse effects. In addition, food proteolysis byproducts have been found to contain peptides with physiologically active properties, including the capacity to chelate minerals. Selenocystine (SeCys, 17), a diselenide product of selenocysteine (Figure 2) and nutritionally available selenoamino acid, displayed a significant cytotoxic effect against melanoma (A375 cells), nasopharyngeal carcinoma (CNE2 cells), acute myeloid leukemia (HL60 cells) cells and colorectal adenocarcinoma (SW620 and Colo201 cells), breast adenocarcinoma (MCF7 and MDA-MB-231 cells), and hepatocellular carcinoma (HepG2 cells) cancer cells with the IC50 values of 3.6, 5.6, 34.5, 7.3, 16.2, and 17.5 μM, respectively [56,57,58]. A monomethylated derivative of selenocysteine, Se-methylselenocysteine (MeSeCys, 18), might be converted into methylselenol by selenocysteine-conjugated β-lyase [59]. The intensity of the active metabolite methylselenol is the only factor affecting the potency of MeSeCys. In the micromolar range, MeSeCys showed cytotoxic effects against many human cancer cell lines, including melanoma (A375 cells), nasopharyngeal carcinoma (CNE2 cells), acute myeloid leukemia (HL60 cells), colorectal adenocarcinoma (SW620 cells), and breast adenocarcinoma (MCF7 and MDA-MB-231 cells) cancer cells (IC50 = 54, 138.7, 459.0, 632.8, 193, and 255.8 μM, respectively) [56]. Selenomethionine (SeMet, 19) demonstrated significant cytotoxicity against melanoma (UACC-375 cells), lung cancer (A-549 cells), breast cancer (MCF-7/S cells), colon cancer (HT-29 cells), prostate cancer (DU-145 cells), and other cancer cells (IC50 = 50, 65, 45, 130, and 40 μM, respectively) [60].
3.3. Ebselen (EBS)
Numerous organoselenium compounds have been produced and studied for various pharmacological applications since 1984, when ebselen 12 (Figure 1) was evaluated as a mimic of the important antioxidant enzyme glutathione peroxidase (GPx) [3]. Since then, the wide range of uses for ebselen’s anti-inflammatory, antioxidant, and cytoprotective effects when administered orally or by subcutaneous injection has been investigated in various studies and clinical trials. Ebselen scavenges hydrogen peroxide and peroxynitrite in human cells by acting as a GSH peroxidase mimic and primarily as a peroxiredoxin mimic through thioredoxin (Trx) and thioredoxin reductase (TrxR) [61]. Ebselen inhibits TrxR in prokaryotic cells, which increases reactive oxygen species (ROS). Ebselen has been developed as a possible antibiotic in recent experiments using the distinction, particularly when combined with silver, that allows ebselen to kill multi-drug-resistant Gram-negative bacteria [61]. An analog of ebselen, called ethaselen 20 (Figure 2), can specifically inhibit TrxR activities, which reduces nuclear factor κB (NF-κB) activities and causes the overexpression of Bax protein and the downregulation of Bcl-2 protein [62]. It is often delivered orally or by intravenous injection. In K562/CDDP cells, the elevated Bax/Bcl-2 ratio subsequently causes a cytochrome c release from mitochondria to cytosol and Caspase-3 activation. Numerous cancer cell lines that have TrxR inactivation experience cell death through apoptosis commencing 24 h after treatment, particularly at ebselen concentrations between 20 and 40 μM [62]. In a phase II clinical trial for the treatment of non-small-cell lung tumors overexpressing TrxR, ethaselen was the second organoselenium to have achieved this milestone (Ethaselen for the Treatment of Thioredoxin Reductase High Expression Advanced Non-small Cell Lung Cancers. Available online: https://clinicaltrials.gov/ct2/show/NCT02166242 (accessed on 16 March 2022).
3.4. Seleninic Acid
Methylseleninic acid (CH3SeO2H) is an organoselenium compound with one carbon atom derived from seleninic acid, wherein the hydrogen atom next to selenium was swapped by a methyl functionality. It functions as a histone deacetylase inhibitor, an antineoplastic agent, and a human xenobiotic metabolite. Methylselenic acid showed inhibition potency for human pancreatic (PANC-1 cells), breast (MCF-7 cells), and prostate (PC-3 cells) cancer cells with IC50 values on MCF-7 cells of 2.6, 2.0, and 8.4 μM, respectively [63]. According to the mechanisms, CH3SeO2H suppressed several cancer cells by triggering numerous caspase pathways, inducing the unfolded protein response (UPR), cytochrome C release, and cleaving poly (ADP-ribose) polymerase (PARP) [64].
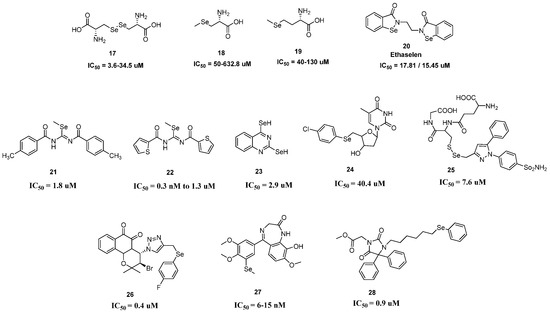
Figure 2.
Structural representation of peptide-based selenium-containing compounds (17–19) [56,57,58,59,60], ethaselen (20) [62], and selenides (21–28) [65,66,67,68,69,70,71,72] for anticancer activity and corresponding IC50 values.
3.5. Selenides
In comparison with their diselenide counterparts, organic selenides (R-Se-R), commonly known as selenoethers, are the selenium equivalents of ethers and sulfides (Figure 2). Antioxidant substances (redox-modulating, antioxidant, and chemopreventive chemicals) and antitumoral substances (antiproliferative, cytotoxic, and apoptotic) are the two main categories of selenides. Methylimidoseleno carbamates 21 [65], which are multi-kinase inhibitors, blocked PI3K/Akt/mTOR and ERK1/2, simultaneously causing autophagy and death in cancer cells. They demonstrated anticancer activity against the human prostate cancer cell line PC-3 with an IC50 value of 1.85 mM. The similar derivative 22 [66] showed antiproliferative action against breast cancer MCF-7 (IC50 = 3.4 nM), colon cancer HT-29 (IC50 = 0.3 nM), lymphocytic leukemia K-562 (IC50 = 6.1 μM), hepatocarcinoma Hep-G2 (IC50 = 0.6 μM), and prostate cancer PC-3 (IC50 = 1.3 μM) cells. The Se-containing quinazoline 23 had a potent cytotoxic impact on lymphocytic leukemia (CCRF-CEM cells) and breast cancer (MCF-7 cells) with IC50 values of 2.99 and 2.92 μM, respectively [67]. A series of Se-containing zidovudine derivatives, 10, 11, and 24, showed potential antitumor effects with IC50 values of 78.85, 51.63, and 40.4 μM, respectively, against human bladder cancer cell 5637 [68]. Targeting the COX-2 and PI3K/Akt signaling pathways, selecoxib-1-GSH, 25, demonstrated increased potency against melanoma cells with an IC50 value of 7.66 μM, compared with celecoxib (IC50 = 55.6 μM), and reduced xenograft tumor formation by almost 70% with minimal toxicity [69]. The IC50 values for compound 26 were 0.59 μM for human promyelocytic leukemia (HL-60 cells) and 0.37 μM for human colon cancer (HCT-116 cells), respectively [70]. With IC50 values between 6 and 15 nM, compound 27 with a benzodiazepine scaffold showed potent cytotoxicity against five cancer cell lines: A549, MDA-MB231, HepG2, HeLa, and HCT116 [71]. Mechanistic investigations revealed that 27 interfered with intracellular microtubule organization, stopped the cell cycle at the G2/M phase, and ultimately promoted cell death. With IC50 values of 0.67 and 0.90 μM, respectively, compound 28 with a phenylselenide tail effectively inhibited the proliferation of both sensitive (PAR) and resistant (MDR) mouse T-lymphoma cell lines [72].
3.6. Diselenides
Organic diselenides (R–Se–Se–R) have two covalently linked selenium atoms in the same molecular construct. Numerous diselenides were produced using various synthetic techniques and demonstrated potential antitumor efficacy, Figure 3. Through ERK1/2-mediated apoptosis, diphenyl diselenide 1 demonstrated blatant cytotoxicity on neuroblastoma cell SHSY5Y (IC50 = 30 μM) [73]. Subsequently, it was shown that substituted diaryl diselenide, 3-(trifluoromethyl) diphenyl diselenides 29 and 4-methoxydiphenyl diselenides 30 activated caspase-dependent and independent pathways to cause apoptosis in HT-29 cells [74]. One of the earliest examples of a diselenide that showed synergistic potency when paired with well-known antitumor medications was diphenyl diselenide 31, which had a significant effect on MCF-7 cells (IC50 = 15 μM) [75]. Diselenide 32, which has a pyridazine scaffold, significantly inhibited the growth of human breast cancer cells MCF-7 (IC50 = 10.3 μM) [76]. Aggregation induced emission (AIE) properties were visible in the fluorescent 9,10-distyryllanthracene (DSA) derivative 33 that contains a diselenide group [77]. SeDSA-SePTX-Co-NPs (Co-NPs), which provided improved advantages in cell imaging and antitumor activity with strong selectivity between tumor and normal cells, were produced by the combination of 33 and a paclitaxel analog. With IC50 values of 3.9 (Caco2 cells), 8.3 (BGC-823 cells), 6.8 (MCF-7 cells), and 6.6 (PC-3 cells), μM compound 34 showed powerful anticancer properties [78]. Diselenide 35, which has a lengthy selenide chain, demonstrated effective early melanocytic lesion prevention and showed reduced HDAC activity and controlled Akt activity [79].
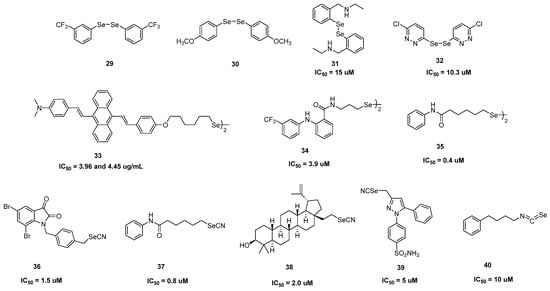
Figure 3.
Structural representation of diselenides (29–35) [73,74,75,76,77,78,79] and selenocyanates (36–40) [79,80,81,82,83] for anticancer activity.
3.7. Selenocyanates
Selenocyanate (SeCN) (Figure 3) has been demonstrated to be a crucial pharmacophore for drugs that enhance anticancer activity through synergistic effects brought on by the combination of both functions (Se and CN). SeCN compounds like p-xylene selenocyanate and benzyl selenocyanate have been investigated for their anticancer properties for a very long time and numerous new active selenocyanate compounds with strong cytotoxic properties have been found in recent years. The tubulin polymerization and Akt phosphorylation were inhibited by compound 36, a benzyl selenocyanate derivative that exhibited cytotoxicity against the MCF-7 breast cancer cell line with an IC50 value of 1.5 μM [80]. Compound 37 was an SeCN-containing derivative of vorinostat (an FDA-approved drug) that demonstrated cytotoxicity against melanoma cell line WM115 with an IC50 value of 0.8 μM [79]. It was also utilized to stop the onset of early melanocytopathy. Without compromising the development of human fibroblasts, selenium-containing triterpenoids 38 demonstrated low micromolar cytotoxic action with an IC50 value of 2 μM against T-lymphoblastic leukemia [81]. Selenocyanate analog selnocoxib-1 39 inhibited the development of the prostate cancer cells PAIII and PC-3M with low IC50 values (5 μM for each) by downregulating the levels of p-AKT, COX-2, and HIF-1a [82]. Phenylalkyl isoselenocyanates (ISCS), which were discovered to be effective antitumor drugs, are isoselenium analogs of phenylalkyl isothiocyanates (ITCs). Phenylbutyl isoselenocyanate 40 exhibited strong anti-proliferative activity against the cell lines of fibrosarcoma HT-1080 (IC50 = 11 μM), melanoma UACC 903 (IC50 = 10 μM), fibrosarcoma HT-1080 (IC50 = 11 μM), glioblastoma T98G (IC50 = 27 μM), prostate cancer PC-3 (IC50 = 19 μM), fibrosarcoma HT-1080 (IC50 = 11 μM), and colon cancer Caco-2 (IC50 = 12 μM) [83].
3.8. Selenoureas
Seleniourea, a functional group containing a Se instead of an O in the urea group, has been reported to have antitumor potential (Figure 4). As a mimic of glutathione peroxidase, compound 41 with an N-phenylselenourea group is an efficient reactive oxygen species scavenger [84]. On HeLa cells, it had a potent antiproliferative impact (IC50 = 1.9–11.8 μM). Low IC50 values (0.7–6.6 μM) for compound 42 with two selenourea groups were perceived in a number of cancer cell lines, including HCT116, A549, 1205Lu, DU145, and PANC-1 [85]. With IC50 values ranging from 0.7 to 2.5 μM, 42 effectively lowered the viability of three colon cancer cell lines (HCT116, HT29, and RKO). Selenourea 43 exhibited potent antiproliferative, glutathione peroxidase-like, and antiradical properties when used against HeLa cancer cells [37].
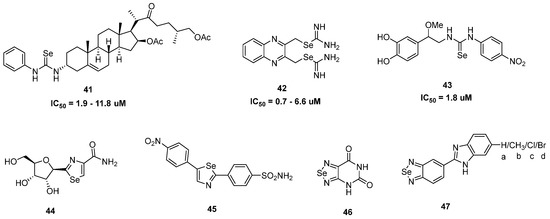
Figure 4.
Structural representation of selenoureas (41–43) [84,85] and selenazoles (44–47) [86,87,88,89] for anticancer activity.
3.9. Selenazoles
Selenazoles (Figure 4) are selenium-containing compounds in the series of heterocyclic 5-membered ring azoles with nitrogen heteroatoms. Significant efforts have been made to develop selenazoles as effective cancer chemotherapeutics. In this category, compound 44 was the onset compound having substantial antitumor activity against mouse melanoma [86]. Next-generation selenazole 45 displayed effective antitumor activity for breast (MDA-MB-231) and human prostate (PC3) cancer cell lines [87]. Among the other selenazoles, compound 46 also showed potent antitumor activity against melanoma (A375 cells IC50 14.3 μM), breast cancer (MCF-7 cells IC50 46.0 μM), and liver cancer (HepG2 cells IC50 19.6 μM) [88]. On the other hand, compound 47 showed a practical antiproliferative effect with benzimidazole functionality [89]. Compounds 47a, 47b, 47c, and 47d displayed antiproliferative effects for MCF-7 and MDA-MB-231 cells with lower ranges (1–26 μM) IC50 values. Selenium-based small molecules have been explored not only as potential antioxidant and anticancer agents but also as antimicrobial, antialzheimer, antidepressant, antidiabetic, antifibrolytic, antiparasitic, antiviral, antifungal, and neuroprotective agents. Recent interest focused on selenium-based nanomedicines in confronting various diseases, especially as antitumor and antidiabetic agents. However, most of the work involving selenium compounds is still in the preclinical stage. We can expect that new selenium-based pharmaceutical compounds with therapeutic promise against human diseases will continue to be developed in coming years, because our knowledge of the biology, biochemistry, and pharmacology of selenium is quickly expanding.
4. Conclusions
Selenium-containing compounds with a variety of functional groups have been found to have antioxidant and anticancer activities. In the search for effectiveness in the prevention and treatment of cancer and other related disorders, selenocyanates, selenoureas, selenoesters, selenium-containing heterocycles, selenium nanoparticles, selenides, and diselenides have been taken into consideration. In addition to the antioxidant and anticancer activities of selenium-containing compounds, they have also been explored as antifibrolytic, antiparasitic, antibacterial, antiviral, antifungal, neuroprotective, and even anti-Alzheimer agents. Although selenium in its elemental form is usually seen as a harmful element with few or no benefits, significant progress has been made when it is incorporated into bioactive compounds. However, due to the high toxicity of selenium, limited clinical exploration of selenium-based drug components has been observed. Efforts must be focused on finding the proper balance of the dose a person could receive as a drug molecule that should not exceed the safe amount of elemental selenium. The conjugation of selenium in organic or inorganic compounds under a structural modification approach or in combination with other reactive safe chemical functionalities or hybrid platforms could lead to a solution towards more clinically approved selenium-based drug molecules. As our fundamental knowledge of selenium biology, biochemistry, and pharmacology grows, it will be possible to predict future innovative approaches for the logical development of new therapeutics that are selenium-based or that target particular parts of selenium metabolism.
Author Contributions
S.D. developed the outline for the systematic review writing. S.D. and A.B. carried out the thorough literature search and categorized selenium-containing drugs. S.D., A.A., N.R.K. and A.B. undertook the drafting and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Agrawal, A.R.; Kumar, N.R.; Debnath, S.; Das, S.; Kumar, C.; Zade, S.S. Radical-cascade avenue for 3,4-fused-ring-substituted thiophenes. Org. Lett. 2018, 20, 4728–4731. [Google Scholar] [CrossRef] [PubMed]
- Lenardão, E.J.; Santi, C.; Sancineto, L. Bioactive organoselenium compounds and therapeutic perspectives. In New Frontiers in Organoselenium Compounds; Springer: Berlin/Heidelberg, Germany, 2018; pp. 99–143. [Google Scholar]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.-W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [PubMed]
- Krinsky, N.I.; Beecher, G.R.; Burk, R.F.; Chan, A.C.; Erdman, J.J.; Jacob, R.A.; Jialal, I.; Kolonel, L.N.; Marshall, J.R.; Taylor Mayne, P.R.; et al. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Inst. Med. 2000, 19, 95–185. [Google Scholar]
- Bedi, A.; Debnath, S.; Chandak, H.S.; Zade, S.S. Phenyl-capped cyclopenta [c] chalcogenophenes: Synthesis, crystal structures, electrochemistry and theoretical insights. RSC Adv. 2014, 4, 35653–35658. [Google Scholar] [CrossRef]
- Bedi, A.; Debnath, S.; Zade, S.S. Diselenolodiselenole: A selenium containing fused heterocycle for conjugated systems. Chem. Commun. 2014, 50, 13454–13456. [Google Scholar] [CrossRef]
- Patra, A.; Agrawal, V.; Bhargav, R.; Bhardwaj, D.; Chand, S.; Sheynin, Y.; Bendikov, M. Metal free conducting PEDOS, PEDOT, and their analogues via an unusual bromine-catalyzed polymerization. Macromolecules 2015, 48, 8760–8764. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Cai, G.; Zhang, Y.; Lu, X.; Lin, Y. Selenium heterocyclic electron acceptor with small urbach energy for as-cast high-performance organic solar cells. J. Am. Chem. Soc. 2020, 142, 18741–18745. [Google Scholar] [CrossRef]
- Debnath, S.; Chithiravel, S.; Sharma, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Selenium-Containing Fused Bicyclic Heterocycle Diselenolodiselenole: Field Effect Transistor Study and Structure–Property Relationship. ACS Appl. Mater. Interfaces 2016, 8, 18222–18230. [Google Scholar] [CrossRef]
- Debnath, S.; Bedi, A.; Zade, S.S. Thienopentathiepine: A sulfur containing fused heterocycle for conjugated systems and their electrochemical polymerization. Polym. Chem. 2015, 6, 7658–7665. [Google Scholar] [CrossRef]
- Mecik, P.; Pigulski, B.; Szafert, S. Serendipitous Formation of Various Selenium Heterocycles Hidden in the Classical Synthesis of Selenophene. Org. Lett. 2021, 23, 1066–1070. [Google Scholar] [CrossRef]
- Xia, J.; Li, T.; Lu, C.; Xu, H. Selenium-containing polymers: Perspectives toward diverse applications in both adaptive and biomedical materials. Macromolecules 2018, 51, 7435–7455. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Chen, H.; Zheng, L.; Zhu, C.; Zhang, L.; Tan, S.; Wang, H.; Guo, Y.; Tang, Q. High-performance, air-stable field-effect transistors based on heteroatom-substituted naphthalenediimide-benzothiadiazole copolymers exhibiting ultrahigh electron mobility up to 8.5 cm V− 1 s− 1. Adv. Mater. 2017, 29, 1602410. [Google Scholar] [CrossRef]
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Synthesis, optoelectronic, and transistor properties of BODIPY-and cyclopenta [c] thiophene-containing π-conjugated copolymers. J. Phys. Chem. C 2015, 119, 15859–15867. [Google Scholar] [CrossRef]
- Fan, B.; Lin, F.; Wu, X.; Zhu, Z.; Jen, A.K.-Y. Selenium-containing organic photovoltaic materials. Acc. Chem. Res. 2021, 54, 3906–3916. [Google Scholar] [CrossRef]
- Walter, R.; Schwartz, I.; Roy, J. Can selenoamino acids act as reversible biological antioxidants. Ann. N. Y. Acad. Sci. 1972, 192, 175–180. [Google Scholar] [CrossRef]
- Olcott, H.; Brown, W.D.; Van der Veen, J. Selenomethionine as an antioxidant. Nature 1961, 191, 1201–1202. [Google Scholar] [CrossRef]
- Schrauzer, G.; Rhead, W. Interpretation of the methylene blue reduction test of human plasma and the possible cancer protecting effect of selenium. Experientia 1971, 27, 1069–1071. [Google Scholar] [CrossRef]
- Shamberger, R.; Frost, D. Possible protective effect of selenium against human cancer. Can. Med. Assoc. J. 1969, 100, 682. [Google Scholar]
- Clark, L.C.; Cantor, K.P.; Allaway, W. Selenium in forage crops and cancer mortality in US counties. Arch. Environ. Health Int. J. 1991, 46, 37–42. [Google Scholar] [CrossRef]
- May, S.W. Selenium-based drug design: Rationale and therapeutic potential. Expert Opin. Investig. Drugs 1999, 8, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.; Hoffmann, P. The human selenoproteome: Recent insights into functions and regulation. Cell. Mol. Life Sci. 2009, 66, 2457–2478. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- May, S.W. Selenium-based pharmacological agents: An update. Expert Opin. Investig. Drugs 2002, 11, 1261–1269. [Google Scholar] [CrossRef]
- May, S.W.; Pollock, S.H. Selenium-based antihypertensives. Drugs 1998, 56, 959–964. [Google Scholar] [CrossRef]
- Collery, P. Strategies for the development of selenium-based anticancer drugs. J. Trace Elem. Med. Biol. 2018, 50, 498–507. [Google Scholar] [CrossRef]
- Wallenberg, M.; Misra, S.; Björnstedt, M. Selenium cytotoxicity in cancer. Basic Clin. Pharmacol. Toxicol. 2014, 114, 377–386. [Google Scholar] [CrossRef]
- Straliotto, M.R.; Hort, M.A.; Fiuza, B.; Rocha, J.B.T.; Farina, M.; Chiabrando, G.; de Bem, A.F. Diphenyl diselenide modulates oxLDL-induced cytotoxicity in macrophage by improving the redox signaling. Biochimie 2013, 95, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; De Oliveira, I.; Grivicich, I.; Guecheva, T.; Saffi, J.; Henriques, J.; Rosa, R. Diphenyl diselenide protects cultured MCF-7 cells against tamoxifen-induced oxidative DNA damage. Biomed. Pharmacother. 2013, 67, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Sato, Y.; Nakajima, I.; Saito, M.; Sasaki, M.; Kanamori, A.; Iwaoka, M. Glutathione peroxidase-like functions of 1,2-diselenane-4, 5-diol and its amphiphilic derivatives: Switchable catalytic cycles depending on peroxide substrates. Bioorg. Med. Chem. 2021, 29, 115866. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Ueno, H.; Asano, Y.; Chakrabarty, G.; Shimodaira, S.; Mugesh, G.; Iwaoka, M. Protein Folding in the Presence of Water-Soluble Cyclic Diselenides with Novel Oxidoreductase and Isomerase Activities. ChemBioChem 2018, 19, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Begines, P.; Oliete, A.; Lopez, O.; Maya, I.; Plata, G.B.; Padron, J.M.; Fernandez-Bolanos, J.G. Chalcogen-containing phenolics as antiproliferative agents. Future Med. Chem. 2018, 10, 319–334. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Ramos-Inza, S.; Aydillo, C.; Talavera, I.; Encío, I.; Plano, D.; Sanmartín, C. Novel N,N′-disubstituted acylselenoureas as potential antioxidant and cytotoxic agents. Antioxidants 2020, 9, 55. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Koketsu, M.; Kato, M.; Kurabayashi, M.; Nishina, A.; Kimura, H. Superoxide radical-scavenging effects from polymorphonuclear leukocytes and toxicity in human cell lines of newly synthesized organic selenium compounds. FEBS J. 2007, 274, 6046–6054. [Google Scholar] [CrossRef]
- Arai, K.; Tashiro, A.; Osaka, Y.; Iwaoka, M. Glutathione peroxidase-like activity of amino-substituted water-soluble cyclic selenides: A shift of the major catalytic cycle in methanol. Molecules 2017, 22, 354. [Google Scholar] [CrossRef]
- Ecker, A.; Ledur, P.C.; da Silva, R.S.; Leal, D.B.R.; Rodrigues, O.E.; Ardisson-Araújo, D.; Waczuk, E.P.; da Rocha, J.B.T.; Barbosa, N.V. Chalcogenozidovudine derivatives with antitumor activity: Comparative toxicities in cultured human mononuclear cells. Toxicol. Sci. 2017, 160, 30–46. [Google Scholar] [CrossRef]
- Nakamura, Y.; Feng, Q.; Kumagai, T.; Torikai, K.; Ohigashi, H.; Osawa, T.; Noguchi, N.; Niki, E.; Uchida, K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant: Implication for inflammation-associated carcinogenesis. J. Biol. Chem. 2002, 277, 2687–2694. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Plano, D.; Encío, I.; Aydillo, C.; Sharma, A.K.; Sanmartín, C. Novel selenadiazole derivatives as selective antitumor and radical scavenging agents. Eur. J. Med. Chem. 2018, 157, 14–27. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C. The global burden of cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Aquilano, K. Pushing the limits of cancer therapy: The nutrient game. Front. Oncol. 2018, 8, 148. [Google Scholar] [CrossRef]
- Society, A. Cancer Treatment & Survivorship Facts & Figure 2019, Figure 2020 and Figure 2021; American Cancer Society Atlanta: Atlanta, GA, USA, 2019. [Google Scholar]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef]
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-Targeting Imaging and Theranostic Agents—Current Status and Future Perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef]
- Vasdev, N.; Alavi, A. Novel PET Radiotracers with Potential Clinical Applications, An Issue of PET Clinics; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; Volume 12. [Google Scholar]
- Debnath, S.; Stevens, C.; Brandenburg, O.; Sovich, J.; Gonzalez, P.; Qin, Q.; Haldeman, S.; Tcheuyap, V.T.; Christie, A.; Thapa, P. Development of a Novel HIF2a PET Tracer: From Proof of Concept to a Clinical Trial. Cancer Res. 2022, 82 (Suppl. S12), 2478. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, N.; Wu, C.-Y.; Li, S.; Chen, Y.-A.; Debnath, S.; Hofstad, M.; Ma, S.; Raj, G.V.; He, D. Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 13085. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Debnath, S.; Hao, G.; Guan, B.; Thapa, P.; Hao, J.; Hammers, H.; Sun, X. Theranostic small-molecule prodrug conjugates for targeted delivery and controlled release of toll-like receptor 7 agonists. Int. J. Mol. Sci. 2022, 23, 7160. [Google Scholar] [CrossRef]
- Dai, X.; Thongchot, S.; Dokduang, H.; Loilome, W.; Khuntikeo, N.; Titapun, A.; Ungarreevittaya, P.; Yongvanit, P.; Techasen, A.; Namwat, N. Potential of selenium compounds as new anticancer agents for cholangiocarcinoma. Anticancer Res. 2016, 36, 5981–5988. [Google Scholar] [CrossRef]
- Gong, J.; Li, L. Sodium selenite inhibits proliferation of gastric cancer cells by inducing SBP1 expression. Tohoku J. Exp. Med. 2016, 239, 279–285. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.-S. Selenocystine induces reactive oxygen species–mediated apoptosis in human cancer cells. Biomed. Pharmacother. 2009, 63, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Poerschke, R.L.; Moos, P.J. Thioredoxin reductase 1 knockdown enhances selenazolidine cytotoxicity in human lung cancer cells via mitochondrial dysfunction. Biochem. Pharmacol. 2011, 81, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wong, Y. Selenocystine induces apoptosis of A375 human melanoma cells by activating ROS-mediated mitochondrial pathway and p53 phosphorylation. Cell. Mol. Life Sci. 2008, 65, 2763–2775. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Kurasaki, K.; Suzuki, N. Selenocysteine β-lyase and methylselenol demethylase in the metabolism of Se-methylated selenocompounds into selenide. Biochim. Biophys. Acta (BBA) Gen. Subj. 2007, 1770, 1053–1061. [Google Scholar] [CrossRef]
- Redman, C.; Scott, J.A.; Baines, A.T.; Basye, J.L.; Clark, L.C.; Calley, C.; Roe, D.; Payne, C.M.; Nelson, M.A. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998, 125, 103–110. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Lu, J.; Holmgren, A. Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free. Radic. Biol. Med. 2018, 127, 238–247. [Google Scholar] [CrossRef]
- Ye, S.-F.; Yang, Y.; Wu, L.; Ma, W.-W.; Zeng, H.-H. Ethaselen: A novel organoselenium anticancer agent targeting thioredoxin reductase 1 reverses cisplatin resistance in drug-resistant K562 cells by inducing apoptosis. J. Zhejiang Univ.-Sci. B 2017, 18, 373–382. [Google Scholar] [CrossRef]
- de Miranda, J.X.; de Oliveira Andrade, F.; de Conti, A.; Dagli, M.L.Z.; Moreno, F.S.; Ong, T.P. Effects of selenium compounds on proliferation and epigenetic marks of breast cancer cells. J. Trace Elem. Med. Biol. 2014, 28, 486–491. [Google Scholar] [CrossRef]
- Singh, U.; Null, K.; Sinha, R. In vitro growth inhibition of mouse mammary epithelial tumor cells by methylseleninic acid: Involvement of protein kinases. Mol. Nutr. Food Res. 2008, 52, 1281–1288. [Google Scholar] [CrossRef]
- Plano, D.; Sanmartín, C.; Moreno, E.; Prior, C.; Calvo, A.; Palop, J.A. Novel potent organoselenium compounds as cytotoxic agents in prostate cancer cells. Bioorg. Med. Chem. Lett. 2007, 17, 6853–6859. [Google Scholar] [CrossRef]
- Ibáñez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel symmetrical alkylthio-and alkylseleno-imidocarbamates. Eur. J. Med. Chem. 2011, 46, 265–274. [Google Scholar] [CrossRef]
- Moreno, E.; Plano, D.; Lamberto, I.; Font, M.; Encío, I.; Palop, J.A.; Sanmartín, C. Sulfur and selenium derivatives of quinazoline and pyrido [2, 3-d] pyrimidine: Synthesis and study of their potential cytotoxic activity in vitro. Eur. J. Med. Chem. 2012, 47, 283–298. [Google Scholar] [CrossRef]
- de Souza, D.; Mariano, D.O.; Nedel, F.; Schultze, E.; Campos, V.F.; Seixas, F.; da Silva, R.S.; Munchen, T.S.; Ilha, V.; Dornelles, L. New organochalcogen multitarget drug: Synthesis and antioxidant and antitumoral activities of chalcogenozidovudine derivatives. J. Med. Chem. 2015, 58, 3329–3339. [Google Scholar] [CrossRef]
- Gowda, R.; Madhunapantula, S.V.; Desai, D.; Amin, S.; Robertson, G.P. Simultaneous Targeting of COX-2 and AKT Using Selenocoxib-1-GSH to Inhibit MelanomaTargeting Melanoma Using Selenocoxib-1-GSH. Mol. Cancer Ther. 2013, 12, 3–15. [Google Scholar] [CrossRef]
- Jardim, G.A.; da Cruz, E.H.; Valença, W.O.; Lima, D.J.; Cavalcanti, B.C.; Pessoa, C.; Rafique, J.; Braga, A.L.; Jacob, C.; da Silva Júnior, E.N. Synthesis of selenium-quinone hybrid compounds with potential antitumor activity via Rh-catalyzed CH bond activation and click reactions. Molecules 2017, 23, 83. [Google Scholar] [CrossRef]
- Pang, Y.; Lin, H.; Ou, C.; Cao, Y.; An, B.; Yan, J.; Li, X. Design, synthesis, and biological evaluation of novel benzodiazepine derivatives as anticancer agents through inhibition of tubulin polymerization in vitro and in vivo. Eur. J. Med. Chem. 2019, 182, 111670. [Google Scholar] [CrossRef]
- Ali, W.; Spengler, G.; Kincses, A.; Nove, M.; Battistelli, C.; Latacz, G.; Starek, M.; Dąbrowska, M.; Honkisz-Orzechowska, E.; Romanelli, A. Discovery of phenylselenoether-hydantoin hybrids as ABCB1 efflux pump modulating agents with cytotoxic and antiproliferative actions in resistant T-lymphoma. Eur. J. Med. Chem. 2020, 200, 112435. [Google Scholar] [CrossRef]
- Posser, T.; de Paula, M.T.; Franco, J.L.; Leal, R.B.; da Rocha, J.B.T. Diphenyl diselenide induces apoptotic cell death and modulates ERK1/2 phosphorylation in human neuroblastoma SH-SY5Y cells. Arch. Toxicol. 2011, 85, 645–651. [Google Scholar] [CrossRef]
- Nedel, F.; Campos, V.F.; Alves, D.; McBride, A.J.; Dellagostin, O.A.; Collares, T.; Savegnago, L.; Seixas, F.K. Substituted diaryl diselenides: Cytotoxic and apoptotic effect in human colon adenocarcinoma cells. Life Sci. 2012, 91, 345–352. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium compounds as novel adjuvants of chemotherapy drugs—A promising approach to fight cancer drug resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.; Park, M.-S. Synthesis of new diorganodiselenides from organic halides: Their antiproliferative effects against human breast cancer MCF-7 cells. Arch. Pharmacal Res. 2015, 38, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhang, S.; Qian, J.; Zhang, J.; Wang, X.; Xie, Z.; Xu, B.; Han, Y.; Tian, W. Redox-responsive Fluorescent Nanoparticles Based on Diselenide-containing AIEgens for Cell Imaging and Selective Cancer Therapy. Chem. Asian J. 2019, 14, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhong, M.; Li, S.; Li, X.; Zhang, Y.; Zhang, Y.; He, X. Synthesis and potential anticancer activity of some novel selenocyanates and diselenides. Chem. Biodivers. 2020, 17, e1900603. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Madhunapantula, S.V.; Desai, D.; Amin, S.; Robertson, G.P. Selenium-containing histone deacetylase inhibitors for melanoma management. Cancer Biol. Ther. 2012, 13, 756–765. [Google Scholar] [CrossRef]
- Krishnegowda, G.; Gowda, A.P.; Tagaram, H.R.S.; Staveley-O’Carroll, K.F.; Irby, R.B.; Sharma, A.K.; Amin, S. Synthesis and biological evaluation of a novel class of isatin analogs as dual inhibitors of tubulin polymerization and Akt pathway. Bioorg. Med. Chem. 2011, 19, 6006–6014. [Google Scholar] [CrossRef]
- Sidoryk, K.; Rárová, L.; Oklešťková, J.; Pakulski, Z.; Strnad, M.; Cmoch, P.; Luboradzki, R. Synthesis of 28a-homoselenolupanes and 28a-homoselenolupane saponins. Org. Biomol. Chem. 2016, 14, 10238–10248. [Google Scholar] [CrossRef]
- Desai, D.; Sinha, I.; Null, K.; Wolter, W.; Suckow, M.A.; King, T.; Amin, S.; Sinha, R. Synthesis and antitumor properties of selenocoxib-1 against rat prostate adenocarcinoma cells. Int. J. Cancer 2010, 127, 230–238. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, A.; Desai, D.; Madhunapantula, S.V.; Huh, S.J.; Robertson, G.P.; Amin, S. Synthesis and anticancer activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. J. Med. Chem. 2008, 51, 7820–7826. [Google Scholar] [CrossRef]
- Romero-Hernandez, L.L.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega-Báez, J.L.; Abasolo, I.; Schwartz, S., Jr.; Lopez, O.; Fernandez-Bolanos, J.G. Diosgenin-based thio (seleno) ureas and triazolyl glycoconjugates as hybrid drugs. Antioxidant and antiproliferative profile. Eur. J. Med. Chem. 2015, 99, 67–81. [Google Scholar] [CrossRef]
- Alcolea, V.; Plano, D.; Karelia, D.N.; Palop, J.A.; Amin, S.; Sanmartin, C.; Sharma, A.K. Novel seleno-and thio-urea derivatives with potent in vitro activities against several cancer cell lines. Eur. J. Med. Chem. 2016, 113, 134–144. [Google Scholar] [CrossRef]
- Boritzki, T.J.; Berry, D.A.; Besserer, J.A.; Cook, P.D.; Fry, D.W.; Leopold, W.R.; Jackson, R.C. Biochemical and antitumor activity of tiazofurin and its selenium analog (2-β-d-ribofuranosyl-4-selenazolecarboxamide). Biochem. Pharmacol. 1985, 34, 1109–1114. [Google Scholar] [CrossRef]
- Angeli, A.; Trallori, E.; Ferraroni, M.; Mannelli, L.D.C.; Ghelardini, C.; Supuran, C.T. Discovery of new 2, 5-disubstituted 1, 3-selenazoles as selective human carbonic anhydrase IX inhibitors with potent anti-tumor activity. Eur. J. Med. Chem. 2018, 157, 1214–1222. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, W.; Wong, Y.-S.; Yang, F. Mitochondria-mediated apoptosis in human breast carcinoma MCF-7 cells induced by a novel selenadiazole derivative. Biomed. Pharmacother. 2008, 62, 77–84. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, Y.; Deng, S.; Chen, T. Microwave-assisted syntheses of benzimidazole-containing selenadiazole derivatives that induce cell-cycle arrest and apoptosis in human breast cancer cells by activation of the ROS/AKT pathway. ChemMedChem 2016, 11, 2339–2346. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).