Selenium and Its Compounds in the Treatment of Anxiety and Related Disorders: A Scoping Review of Translational and Clinical Research
Abstract
1. Introduction
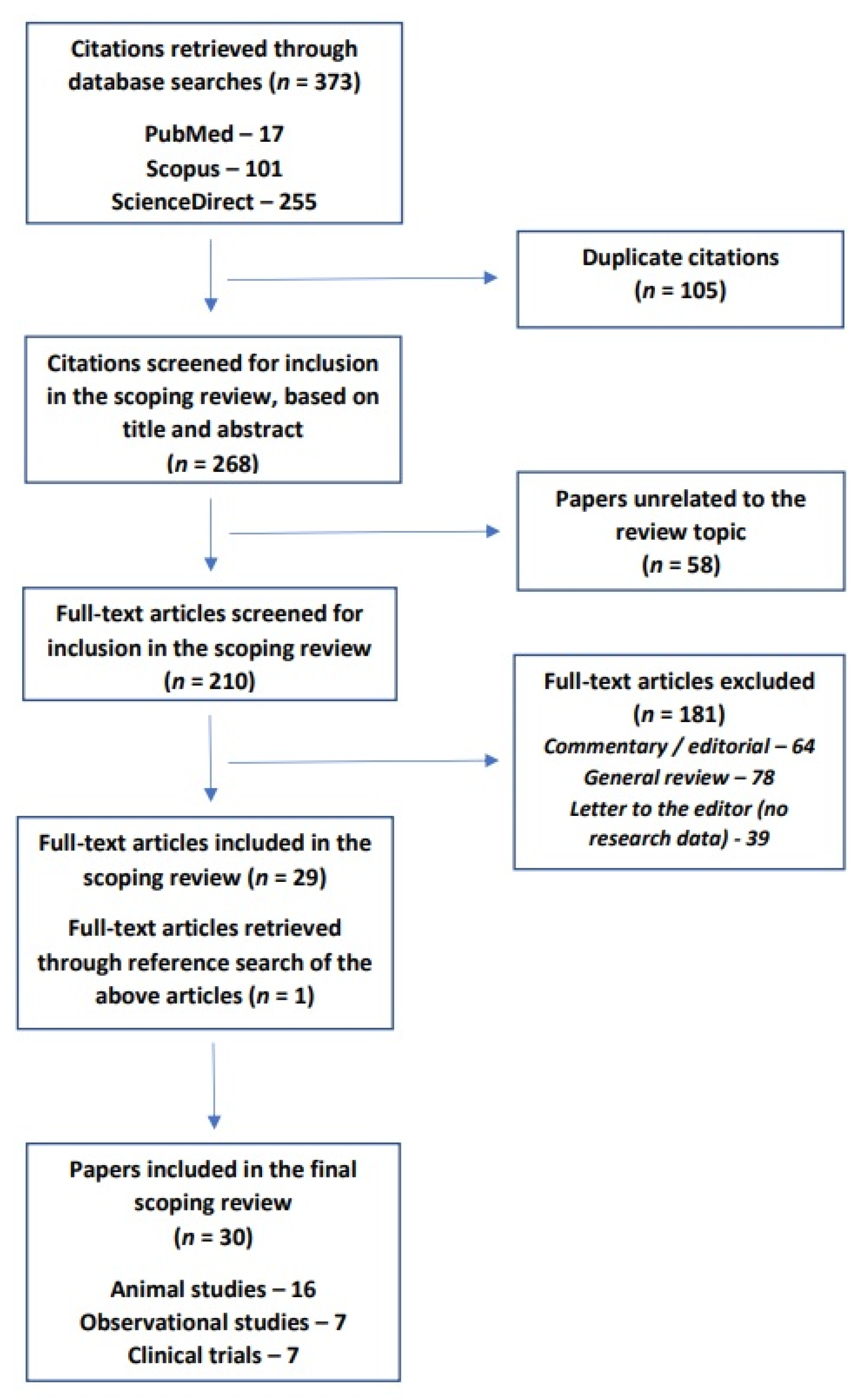
2. Materials and Methods
- in vitro or animal studies examining the relationship between selenium, selenoproteins, or selenium-containing compounds and experimentally-induced anxiety-like symptoms or behaviors;
- observational studies examining the links between levels of selenium or selenoproteins and symptoms of anxiety or syndromal anxiety disorders in human subjects; and
- interventional studies (clinical trials) examining the therapeutic effect of selenium or selenium-containing compounds in the management of anxiety and related disorders, including obsessive-compulsive disorder (OCD) and post-traumatic disorder (PTSD).
- (a)
- (b)
- (c)
3. Results
3.1. Selenium, Selenoproteins, and Selenium-Containing Compounds in Animal Models of Anxiety
3.2. Observational Studies of Selenium or Related Biomarkers in Relation to Anxiety in Humans
3.3. Clinical Trials of Selenium or Selenium-Containing Supplements for Anxiety
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Crocq, M.A. A history of anxiety: From Hippocrates to DSM. Dialogues Clin. Neurosci. 2015, 17, 319–325. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Spottswood, M.; Davydow, D.S.; Huang, H. The prevalence of posttraumatic stress disorder in primary care: A systematic review. Harv. Rev. Psychiatry 2017, 25, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, I.; Marriott, S.; Pessina, E.; Fisher, C.; Govender, A.; Mohamed, H.; Chandler, A.; Tyagi, H.; Morris, L.; Pallanti, S. The global assessment of OCD. Compr. Psychiatry 2022, 118, 152342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Sharma, L.P.; Balachander, S.; Lin, B.; Manohar, H.; Khanna, P.; Lu, C.; Garg, K.; Thomas, T.L.; Au, A.C.L.; et al. Comorbidities in obsessive-compulsive disorder across the lifespan: A systematic review and meta-analysis. Front. Psychiatry 2021, 12, 703701. [Google Scholar] [CrossRef]
- Auxemery, Y. Post-traumatic psychiatric disorders: PTSD is not the only diagnosis. Presse Med. 2018, 47, 423–430. [Google Scholar] [CrossRef]
- Murphy, D.L.; Moya, P.R.; Fox, M.A.; Rubenstein, L.M.; Wendland, J.R.; Timpano, K.R. Anxiety and affective disorder comorbidity related to serotonin and other neurotransmitter systems: Obsessive-compulsive disorder as an example of overlapping clinical and genetic heterogeneity. Phil. Trans. R. Soc. B 2013, 368, 20120435. [Google Scholar] [CrossRef]
- Cerda, M.; Sagdeo, A.; Johnson, J.; Galea, S. Genetic and environmental influences on psychiatric comorbidity: A systematic review. J. Affect. Disord. 2010, 126, 14–38. [Google Scholar] [CrossRef]
- Serra-Blasco, M.; Radua, J.; Soriano-Mas, C.; Gomez-Benlloch, A.; Porta-Casteras, D.; Carulla-Roig, M.; Albajes-Eizagirre, A.; Arnone, D.; Klauser, P.; Canales-Rodriguez, E.J.; et al. Structural brain correlates in major depression, anxiety disorders and post-traumatic stress disorder: A voxel-based morphometry meta-analysis. Neurosci. Biobehav. Rev. 2021, 129, 269–281. [Google Scholar] [CrossRef]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef]
- Williams, L.M. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: A theoretical review of the evidence and future directions for clinical translation. Depress. Anxiety 2017, 34, 9–24. [Google Scholar] [CrossRef]
- Nikolaus, S.; Muller, H.W.; Hautzel, H. Different patterns of 5-HT receptor and transporter dysfunction in neuropsychiatric disorders—A comparative analysis of in vivo imaging findings. Rev. Neurosci. 2016, 27, 27–59. [Google Scholar] [CrossRef]
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate systems in DSM-5 anxiety disorders: Their role and a review of glutamate and GABA psychopharmacology. Front. Psychiatry 2020, 11, 548505. [Google Scholar] [CrossRef]
- Renna, M.E.; O’Toole, M.S.; Spaeth, P.E.; Lekander, M.; Mennin, D.S. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depress. Anxiety 2018, 35, 1081–1094. [Google Scholar] [CrossRef]
- Hovatta, I.; Juhila, J.; Donner, J. Oxidative stress in anxiety and comorbid conditions. Neurosci. Res. 2010, 68, 261–275. [Google Scholar] [CrossRef]
- Fedoce, A.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Andero, R.; Choi, D.C.; Ressler, K.J. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog. Mol. Biol. Transl. Sci. 2014, 122, 169–192. [Google Scholar] [CrossRef]
- Bandelow, B.; Allgulander, C.; Baldwin, D.S.; Costa, D.; Denys, D.; Dilbaz, N.; Domschke, K.; Eriksson, E.; Fineberg, N.A.; Hattenschwiler, J.; et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders—Version 3. Part I: Anxiety disorders. World J. Biol. Psychiatry 2022, 28, 1–39. [Google Scholar] [CrossRef]
- Bandelow, B.; Allgulander, C.; Baldwin, D.S.; Costa, D.; Denys, D.; Dilbaz, N.; Domschke, K.; Eriksson, E.; Fineberg, N.A.; Hattenschwiler, J.; et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders—Version 3. Part II: OCD and PTSD. World J. Biol. Psychiatry 2022, 28, 1–17. [Google Scholar] [CrossRef]
- He, H.; Xiang, Y.; Gao, F.; Bai, L.; Gao, F.; Fan, Y.; Lyu, J.; Ma, X. Comparative efficacy and acceptability of first-line drugs for the acute treatment of generalized anxiety disorder in adults: A network meta-analysis. J. Psychiatr. Res. 2019, 118, 21–30. [Google Scholar] [CrossRef]
- Williams, T.; Phillips, N.J.; Stein, D.J.; Ipser, J.C. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 2022, 3, CD002795. [Google Scholar] [CrossRef]
- Soomro, G.M.; Altman, D.; Rajagopal, S.; Oakley-Browne, M. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder. Cochrane Database Syst. Rev. 2008, 2008, CD001765. [Google Scholar] [CrossRef]
- Taylor, S.; Abramowitz, J.S.; McKay, D. Non-adherence and non-response in the treatment of anxiety disorders. J. Anxiety Disord. 2012, 26, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Castle, D.; Bosanac, P.; Rossell, S. Treating OCD: What to do when first-line therapies fail. Australas. Psychiatry 2015, 23, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Davidson, J.R. Post-traumatic stress disorder: An evaluation of existing pharmacotherapies and new strategies. Expert Opin. Pharmacother. 2007, 8, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Batelaan, N.M.; Bosman, R.C.; Muntingh, A.; Scholten, W.D.; Huijbregts, K.M.; van Balkom, A.J.L.M. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: Systematic review and meta-analysis of relapse prevention trials. BMJ 2017, 358, j3927. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; McGuire, J.; Landeros-Weisenberger, A.; Leckman, J.F.; Pittenger, C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol. Psychiatry 2010, 15, 850–855. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of anxiety disorders: Current and emerging treatment options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef]
- Malikowska-Racia, N.; Salat, K. Recent advances in the neurobiology of posttraumatic stress disorder: A review of possible mechanisms underlying an effective pharmacotherapy. Pharmacol. Res. 2019, 142, 30–49. [Google Scholar] [CrossRef]
- Grassi, G.; Pallanti, S. Current and up-and-coming pharmacotherapy for obsessive-compulsive disorder in adults. Expert Opin. Pharmacother. 2018, 19, 1541–1550. [Google Scholar] [CrossRef]
- Santos, P.; Herrmann, A.P.; Elisabetsky, E.; Piato, A. Anxiolytic properties of compounds that counteract oxidative stress, neuroinflammation, and glutamatergic dysfunction: A review. Braz. J. Psychiatry 2019, 41, 168–178. [Google Scholar] [CrossRef]
- Holben, D.H.; Smith, A.M. The diverse role of selenium within selenoproteins: A review. J. Am. Diet. Assoc. 1999, 99, 836–843. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. The role and mechanism of essential selenoproteins for homeostasis. Antioxidants 2022, 11, 973. [Google Scholar] [CrossRef]
- Schweizer, U.; Fabiano, M. Selenoproteins in brain development and function. Free Radic. Bio. Med. 2022, 190, 105–115. [Google Scholar] [CrossRef]
- Ferreira de Almeida, T.L.; Petarli, G.B.; Cattafesta, M.; Zandonade, E.; Bezerra, O.M.P.A.; Tristao, K.G.; Salaroli, L.B. Association of selenium intake and development of depression in Brazilian farmers. Front. Nutr. 2021, 8, 671377. [Google Scholar] [CrossRef]
- Jin, Y.; Coad, J.; Pond, R.; Kim, N.; Brough, L. Selenium intake and status of postpartum women and postnatal depression during the first year after childbirth in New Zealand—Mother and Infant Nutrition Investigation (MINI) study. J. Tr. Elem. Med. Biol. 2020, 61, 126503. [Google Scholar] [CrossRef]
- Saha, S.; Lim, C.C.W.; Cannon, D.L.; Burton, L.; Bremner, M.; Cosgrove, P.; Huo, Y.; McGrath, J.J. Co-morbidity between mood and anxiety disorders: A systematic review and meta-analysis. Depress. Anxiety 2021, 38, 286–306. [Google Scholar] [CrossRef]
- Janiri, D.; Moser, D.A.; Doucet, G.E.; Luber, M.J.; Rasgon, A.; Lee, W.H.; Murrough, J.W.; Sani, G.; Eickhoff, S.B.; Frangou, S. Shared neural phenotypes for mood and anxiety disorders: A meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry 2020, 77, 172–179. [Google Scholar] [CrossRef]
- Aucoin, M.; LaChance, L.; Naidoo, U.; Remy, D.; Shekdar, T.; Sayar, N.; Cardozo, V.; Rawana, T.; Chan, I.; Cooley, K. Diet and anxiety: A scoping review. Nutrients 2021, 13, 4418. [Google Scholar] [CrossRef]
- Torres, D.J.; Alfulaij, N.; Berry, M.J. Stress and the brain: An emerging role for selenium. Front. Neurosci. 2021, 15, 666601. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.M.; Hill, K.E.; Burk, R.F.; Weeber, E.J. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol. Neurodegener. 2006, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Ghisleni, G.; Kazlauckas, V.; Both, F.L.; Pagnussat, N.; Mioranzza, S.; Rocha, J.B.T.; Souza, D.O.; Porciuncula, L.O. Diphenyl diselenide exerts anxiolytic-like effect in Wistar rats: Putative roles of GABAA and 5HT receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Bruning, C.A.; Prigol, M.; Roehrs, J.A.; Nogueira, C.W.; Zeni, G. Involvement of the serotonergic system in the anxiolytic-like effect caused by m-trifluoromethyl-diphenyl diselenide in mice. Behav. Brain Res. 2009, 205, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Gai, B.M.; Bortolatto, C.F.; Heck, S.O.; Stein, A.L.; Duarte, M.M.M.F.; Zeni, G.; Nogueira, C.W. An organoselenium compound improves behavioral, endocrinal and neurochemical changes induced by corticosterone in mice. Psychopharmacology 2013, 231, 2119–2130. [Google Scholar] [CrossRef]
- Laureano-Melo, R.; Imperio, G.E.; da Silva-Almeida, C.; Kluck, G.E.G.; Cruz Seara, F.A.; da Rocha, F.F.; da Silveira, A.L.B.; Reis, L.C.; Ortiga-Carvalho, T.M.; da Silva Cortes, W. Sodium selenite supplementation during pregnancy and lactation promotes anxiolysis and improves mnemonic performance in wistar rats’ offspring. Pharmacol. Biochem. Behav. 2015, 138, 123–132. [Google Scholar] [CrossRef]
- Kedzierska, E.; Dudka, J.; Poleszak, E.; Kotlinska, J.H. Antidepressant and anxiolytic-like activity of sodium selenite after acute treatment in mice. Pharmacol. Rep. 2017, 69, 276–280. [Google Scholar] [CrossRef]
- Reis, A.S.; Pinz, M.; Duarte, L.F.B.; Roehrs, J.A.; Alves, D.; Luchese, C.; Wilhelm, E.A. 4-phenylselenyl-7-chloroquinoline, a novel multitarget compound with anxiolytic activity: Contribution of the glutamatergic system. J. Psychiatr. Res. 2017, 84, 191–199. [Google Scholar] [CrossRef]
- Sousa, F.S.S.; Birmann, P.T.; Balaguez, R.; Alves, D.; Bruning, C.A.; Savegnago, L. α-(phenylselanyl) acetophenone abolishes acute restraint stress induced-comorbid pain, depression and anxiety-related behaviors in mice. Neurochem. Int. 2018, 120, 112–120. [Google Scholar] [CrossRef]
- Bampi, S.R.; Casaril, A.M.; Sousa, F.S.S.; Pesarico, A.P.; Vieira, B.; Lenardao, E.J.; Savegnago, L. Repeated administration of a selenium-containing indolyl compound attenuates behavioural alterations by streptozotocin through modulation of oxidative stress in mice. Pharmacol. Biochem. Behav. 2019, 183, 46–55. [Google Scholar] [CrossRef]
- Casaril, A.M.; Domingues, M.; de Andrade Lourenco, D.; Birmann, P.T.; Padilha, N.; Vieira, B.; Begnini, K.; Seixas, F.K.; Collares, T.; Lenardao, E.J.; et al. Depression- and anxiogenic-like behaviors induced by lipopolysaccharide in mice are reversed by a selenium-containing indolyl compound: Behavioral, neurochemical and computational insights involving the serotonergic system. J. Psychiatr. Res. 2019, 115, 1–12. [Google Scholar] [CrossRef]
- Paltian, J.J.; dos Reis, A.S.; de Oliveira, R.L.; da Fonseca, C.A.R.; Domingues, W.B.; Dellagostin, E.N.; Campos, V.F.; Kruger, R.; Alves, D.; Luchese, C.; et al. The anxiolytic effect of a promising quinoline containing selenium with the contribution of the serotonergic and GABAergic pathways: Modulation of parameters associated with anxiety in mice. Behav. Brain Res. 2020, 393, 112797. [Google Scholar] [CrossRef]
- Birmann, P.T.; Domingues, M.; Casaril, A.M.; Smaniotto, T.A.; Hartwig, D.; Jacob, R.G.; Savegnago, L. A pyrazole-containing selenium compound modulates neuroendocrine, oxidative stress, and behavioral responses to acute restraint stress in mice. Behav. Brain. Res. 2021, 396, 112874. [Google Scholar] [CrossRef]
- Mansouri, M.; Sotoudeh, M.M.; Shamshirian, A.; Beheshti, F.; Hosseini, M.; Sadeghnia, H.R. Beneficial effects of selenium against the behavioral consequences of lipopolysaccharide administration in rats. Learn. Motiv. 2021, 74, 101713. [Google Scholar] [CrossRef]
- Pinz, M.P.; Vogt, A.G.; da Costa Rodrigues, K.; Dos Reis, A.S.; Duarte, L.; Fronza, M.G.; Domingues, W.B.; Blodorn, E.B.; Alves, D.; Campos, V.F.; et al. Effect of a purine derivative containing selenium to improve memory decline and anxiety through modulation of the cholinergic system and Na+/K+-ATPase in an Alzheimer’s disease model. Metab. Brain. Dis. 2021, 36, 871–888. [Google Scholar] [CrossRef]
- Samad, N.; Rao, T.; Rehman, M.H.; Bhatti, S.A.; Imran, I. Inhibitory effects of selenium on arsenic-induced anxiety-/depression-like behavior and memory impairment. Biol. Trace Elem. Res. 2022, 200, 689–698. [Google Scholar] [CrossRef]
- Situ, J.; Huang, X.; Zuo, M.; Huang, Y.; Ren, B.; Liu, Q. Comparative proteomic analysis reveals the effect of selenoprotein W deficiency on oligodendrogenesis in fear memory. Antioxidants 2022, 11, 999. [Google Scholar] [CrossRef]
- Zieker, J.; Zieker, D.; Jatzko, A.; Dietzsch, J.; Nieselt, K.; Schmitt, A.; Bertsch, T.; Fassbender, K.; Spanagel, R.; Northoff, H.; et al. Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Mol. Psychiatry 2007, 12, 116–118. [Google Scholar] [CrossRef]
- Ozdemir, E.; Cetinkaya, S.; Ersan, S.; Kucukosman, S.; Ersan, E.E. Serum selenium and plasma malondialdehyde levels and antioxidant enzyme activities in patients with obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 62–65. [Google Scholar] [CrossRef]
- Jamali, F.; Izadi, A.; Khalili, H.; Garmaroudi, G. Correlation between daily dietary micronutrients intake and mental health outcomes in Iranians living with HIV infection. J. Ass. Nurs. AIDS Care 2016, 27, 817–825. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L.; Keshteli, A.H.; Feizi, A.; Feinle-Bisset, C.; Adibi, P. Do patterns of nutrient intake predict self-reported anxiety, depression and psychological distress in adults? SEPAHAN study. Clin. Nutr. 2019, 38, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Wieder-Huszla, S.; Zabielska, P.; Kotwas, A.; Owsianowska, J.; Karakiewicz-Krawczyk, K.; Kowalczyk, R.; Jurczak, A. The severity of depressive and anxiety symptoms in postmenopausal women depending on their magnesium, zinc, selenium and copper levels. J. Elem. 2020, 25, 1305–1317. [Google Scholar] [CrossRef]
- Portnoy, J.; Wang, J.; Wang, F.; Um, P.; Irving, S.Y.; Hackl, L.; Liu, J. Lower serum selenium concentration associated with anxiety in children. J. Pediatr. Nurs. 2022, 63, e121–e126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jing, D.; Huang, X.; Xiao, Y.; Shu, Z.; Luo, D.; Duan, Y.; He, M.; Xiao, S.; Chen, X.; et al. Effects of co-exposure to multiple metals on children’s behavior problems in China. Sci. Tot. Environ. 2022, 826, 154062. [Google Scholar] [CrossRef] [PubMed]
- Gosney, M.A.; Hammond, M.F.; Shenkin, A.; Allsup, S. Effect of micronutrient supplementation on mood in nursing home residents. Gerontology 2008, 54, 292–299. [Google Scholar] [CrossRef]
- Rucklidge, J.; Johnstone, J.; Harrison, R.; Boggis, A. Micronutrients reduce stress and anxiety in adults with Attention-Deficit/Hyperactivity Disorder following a 7.1 earthquake. Psychiatry Res. 2011, 189, 281–287. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Rucklidge, J.J.; Romijn, A.R.; Dolph, M. A randomized trial of nutrient supplements to minimise psychological stress after a natural disaster. Psychiatry Res. 2015, 228, 373–379. [Google Scholar] [CrossRef]
- Voicehovskis, V.V.; Voicehovska, J.G.; Skesters, A.; Ancane, G.; Silova, A.; Ivascenko, T.; Micans, J.; Vaivads, N. Advances of selenium supplementation in posttraumatic stress disorder risk group patients. Biomed. Khim. 2014, 60, 125–132. [Google Scholar] [CrossRef][Green Version]
- Sayyah, M.; Andishmand, M.; Ganji, R. Effect of selenium as an adjunctive therapy in patients with treatment-resistant obsessive-compulsive disorder: A pilot randomized double blind placebo-controlled clinical trial. Arch. Psychiatr. Psychother. 2018, 4, 57–65. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Asemi, Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1594–1598. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Oliver, G.; Cribb, L.; Blair-West, S.; Castle, D.; Dean, O.M.; Camfield, D.A.; Brakoulias, V.; Bousman, C.; et al. Treatment of refractory obsessive-compulsive disorder with nutraceuticals (TRON): A 20-week, open label pilot study. CNS Spectr. 2022, 27, 588–597, Advance online publication. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Parra-Martinez, C.; Selma-Royo, M.; Callejon-Leblic, B.; Collado, M.C.; Abril, N.; Garcia-Barrera, T. Mice brain metabolomics after the exposure to a “chemical cocktail” and selenium supplementation through the gut-brain axis. J. Hazard. Mater. 2022, 438, 129443. [Google Scholar] [CrossRef]
- Werneke, U.; Turner, T.; Priebe, S. Complementary medicines in psychiatry: Review of effectiveness and safety. Br. J. Psychiatry 2006, 188, 109–121. [Google Scholar] [CrossRef]
- Mokhber, N.; Namjoo, M.; Tara, F.; Boskabadi, H.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Majdi, M.R.; Tavallaie, S.; Azimi-Nezhad, M.; et al. Effect of supplementation with selenium on postpartum depression: A randomized double-blind placebo-controlled trial. J. Matern. Fetal Neonatal Med. 2011, 24, 104–108. [Google Scholar] [CrossRef]
- Jamilian, M.; Mansury, S.; Bahmani, F.; Heidar, Z.; Amirani, E.; Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 2018, 11, 80. [Google Scholar] [CrossRef]
- Bot, M.; Brouwer, I.A.; Roca, M.; Kohls, E.; Penninx, B.; Watkins, E.; van Grootheest, G.; Cabout, M.; Hegerl, U.; Gili, M.; et al. MooDFOOD Prevention Trial Investigators. Effect of Multinutrient Supplementation and Food-Related Behavioral Activation Therapy on Prevention of Major Depressive Disorder Among Overweight or Obese Adults with Subsyndromal Depressive Symptoms: The MooDFOOD Randomized Clinical Trial. JAMA 2019, 321, 858–868. [Google Scholar] [CrossRef]
- Hurst, R.; Siyame, E.W.; Young, S.D.; Chilimba, A.D.; Joy, E.J.; Black, C.R.; Ander, E.L.; Watts, M.J.; Chilima, B.; Gondwe, J.; et al. Soil-type influences human selenium status and underlies widespread selenium deficiency risks in Malawi. Sci. Rep. 2013, 3, 1425. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Deter, H.C.; Orth-Gomér, K.; Rauch-Kröhnert, U.; Albus, C.; Ladwig, K.H.; Söllner, W.; de Zwaan, M.; Grün, A.S.; Ronel, J.; Hellmich, M.; et al. SPIRR-CAD-Study Group. Depression, anxiety, and vital exhaustion are associated with pro-coagulant markers in depressed patients with coronary artery disease—A cross sectional and prospective secondary analysis of the SPIRR-CAD trial. J. Psychosom. Res. 2021, 151, 110659. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Shao, D.; Li, J.; Yang, C.L.; Fan, M.H.; Cao, F.L. Positive Association Between Serum Levels of High-Sensitivity C-Reactive Protein and Depression/Anxiety in Female, but Not Male, Patients with Type 2 Diabetes Mellitus. Biol. Res. Nurs. 2020, 22, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Mechler, K.; Hoffmann, G.F.; Dittmann, R.W.; Ries, M. Defining the hidden evidence in autism research. Forty per cent of rigorously designed clinical trials remain unpublished—A cross-sectional analysis. Int. J. Methods Psychiatr. Res. 2017, 26, e1546. [Google Scholar] [CrossRef] [PubMed]
- Heres, S.; Davis, J.; Maino, K.; Jetzinger, E.; Kissling, W.; Leucht, S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am. J. Psychiatry 2006, 163, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Puar, P.; Zonouzi-Marand, M.; Chivers, D.P.; Niyogi, S.; Kwong, R. A comprehensive review on the neuropathophysiology of selenium. Sci. Total Environ. 2021, 767, 144329. [Google Scholar] [CrossRef] [PubMed]
- Derbeneva, S.A.; Bogdanov, A.R.; Pogozheva, A.V.; Gladyshev, O.A.; Vasilevskaia, L.S.; Zorin, S.N.; Mazo, V.K. Effect of diet enriched with selenium on the psycho-emotional and adaptive capacity of patients with cardiovascular diseases and obesity. Vorp. Pitan. 2012, 81, 35–41. (In Russian) [Google Scholar]
- Martens, I.B.; Cardoso, B.R.; Hare, D.J.; Niedzwiecki, M.M.; Lajolo, F.M.; Martens, A.; Cozzolino, S.M. Selenium status in preschool children receiving a Brazil nut-enriched diet. Nutrition 2015, 31, 1339–1343. [Google Scholar] [CrossRef]
| Study | Type of Selenium Compound Studied | Animal Species | Experimental Methods | Results—Behavioural | Results—Other |
|---|---|---|---|---|---|
| Peters et al., 2006 [43] | Sodium selenite, high-dose (1 mg/kg) and deficient (0 mg/kg) diets | Male and female mice with targeted disruption of the selenoprotein P gene (SEPP1) and control mice | Observation of anxiety during open-field test and elevated plus maze test; conditioned fear through the pairing of noise with an electric shock to the foot | No difference in anxiety-like behaviors or fear conditioning across SEPP1 genotypes | Reduced spatial learning, short-term plasticity, and long-term potentiation was seen with SEPP1 disruption and selenium- deficient diet |
| Ghisleni et al., 2008 [44] | Diphenyl diselenide (5, 25, and 50 µmol/kg) | Male Wistar rats (n = 40, divided into four groups) | Observation of anxiety during the open-field task and the elevated plus maze task | Reduced anxiety, as measured by reduced fecal boli in the open-field task and increased entries and time spent in the open arm of the elevated plus maze, with a 50 µmol/kg dose of diphenyl diselenide only | Anxiolytic effects of diphenyl diselenide abolished by administration of bicuculline (GABAA antagonist), ketanserin (5HT2A antagonist), or WAY100635 (5HT1A antagonist) |
| Bruning et al., 2009 [45] | m-trifluoromethyl-diphenyl diselenide (0.1, 10, and 100 mg/kg) | Female Swiss mice (n = 40, divided into four groups) | Observation of anxiety during the elevated plus maze task and light/dark box | Reduced anxiety, as measured by time spent in the illuminated side of a light/dark box and increased entries and time spent in the open arm of an elevated plus maze, with 100 mg/kg m-trifluoromethyl diphenyl diselenide | Significant inhibition of cortical MAO-A with 100 mg/kg m-trifluoromethyl diphenyl diselenide; anxiolytic effect abolished by administration of WAY100635 (5HT1A antagonist), ritanserin (5HT2A antagonist), or ondansetron (5HT3 antagonist) |
| Gai et al., 2013 [46] | 3-(4-fluorophenyselenyl)-2,5-diphenylselenophene F-DPS) (0.1 mg/kg/day for 1 week) | Male Swiss mice (n = 48, divided into four groups) | Induction of anxiety by administration of corticosterone 20 µg/mL of water for 4 weeks | Reduced anxiety, as measured by transition to the dark zone and time spent in the light zone of the light/dark box, with F-DPS | Normalization of ACTH and corticosterone levels, inhibition of cortical MAO-A, and increased 5-HT and glutamate synaptosomal uptake with F-DPS |
| Laureano-Melo et al., 2015 [47] | Sodium selenite, 1 mg/kg, administered to mother rats during pregnancy | Twenty-three Wistar rat offspring, assessed both in childhood and adulthood | Observation of anxiety during the open field test, light–dark test and elevated plus maze test | Reduced anxiety-like behaviors, as measured by increased transitions and time spent in the illuminated side in the light–dark test and an increased time spent in the open arms of the elevated plus maze test during childhood offspring of mothers treated with sodium selenite; the elevated plus maze finding remained significant even when the offspring reached adulthood | Increased serum T3 and T4, reduced hippocampal AChE activity, and reduced hippocampal TPH2 mRNA expression in offspring of mothers treated with sodium selenite |
| Kedzierska et al., 2017 [48] | Sodium selenite, 0.5, 1, and 2 mg/kg | Albino Swiss mice (n = 40, divided into four groups) | Observation of anxiety during the adapted elevated plus maze test | Reduced anxiety, as measured by increased time spent in the open arm of the elevated plus maze with all three doses of sodium selenite | This study did not examine any biomarkers related to anxiety |
| Reis et al., 2017 [49] | 4-phenylselenyl-7-chloroquinoline (4-PSQ), 5–50 mg/kg | Male Swiss mice (n = 32, divided into four groups) | Observation of anxiety during the elevated plus maze test, light–dark test, and open field test; induction of anxiety by administration of kainate (15 mg/kg) | Reduced anxiety, as measured by increased time spent in the open arm of the elevated plus maze (25 mg/kg), and reduced transitions (50 mg/kg) and time spent on illuminated size during the light–dark test (25 and 50 mg/kg), with 4-PSQ | A decrease in cortical glutamate uptake, but not glutamate release or Na+, K+-ATPase activity, with 4-PSQ; blockade of kainate-induced anxiety by 4-PSQ (50 mg/kg) |
| Sousa et al., 2018 [50] | α-(phenylselanyl) acetophenone (PSAP), 10 mg/kg | Male Swiss mice (n = 21, divided into three groups) | Induction of anxiety by acute restraint stress for 4 h | Reduced anxiety, as measured by a reduced number of marbles buried in the marble burying test and increased entries and time spent in the open arm of the elevated plus maze, with PSAP | A reduction in stress-induced elevations in lipid peroxidation, reactive species, nitrites/nitrates, and corticosterone with PSAP |
| Bampi et al., 2019 [51] | 1-methyl-3-(phenylselanyl)-1H-indole (MFSeI), 10 mg/kg | Male Swiss mice (n = 24, divided into four groups) | Induction of anxiety by intra-cerebral administration of streptozotocin (0.2 mg/4 µL) | Reduced anxiety, as measured by reduced grooming in the open-field test and increased entries and time spent in the open arm of the elevated plus maze, with MFSeI | A reduction in streptozotocin-induced increases in lipid peroxidation, reactive species, nitrites and AChE activity with MFSeI |
| Casaril et al., 2019 [52] | 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole (CMI), 1 mg/kg | Male Swiss mice (n = 32, divided into four groups) | Induction of anxiety by administration of single-dose LPS (0.83 mg/kg) | Reduced anxiety, as measured by a reduced number of marbles buried in the marble burying test and increased entries and time spent in the open arm of the elevated plus maze, with CMI | A reduction in LPS-induced elevations in IDO, IL-1β, TNF-α with CMI |
| Paltian et al., 2020 [53] | 7-chloro-4-(phenylselanyl) quinoline (4-PSQ), 50 mg/kg | Male Swiss mice (n = 119) | Observation of anxiety during the open-field test and elevated plus maze task | Reduced anxiety as measured by increased time spent in the open arm of the elevated plus maze with CMI, but no change in behavior during the open-field test | Anxiolytic effects of 4-PSQ abolished by administration of PTZ (GABAA antagonist), pindolol (5-HT1A antagonist), or ketanserin (5-HT2A antagonist); 4-PSQ reduced PTZ-induced increase in corticosterone and PTZ-induced decrease in cortical BDNF, CREB, and NF-κB expressions |
| Birmann et al., 2021 [54] | 3,5-dimethyl-1-phenyl-4-(phenylselanyl)-1H-pyrazole (SePy) (1 and 10 mg/kg) | Male Swiss mice (n = 21, divided into three groups) | Induction of anxiety by acute restraint stress for 2 h | Reduced anxiety, as measured by reduced rearing and grooming in the open-field test, reduced the number of marbles buried in the marble burying test, and increased entries and time spent in the open arm of the elevated plus maze, with both doses of SePy | A reduction in stress-induced elevations in corticosterone, reactive species, lipid peroxidation, and an increase in stress-induced reductions of CAT and SOD with 10 mg/kg SePy |
| Mansouri et al., 2021 [55] | Selenium (specific compound not mentioned in the paper) (100–200 µg/kg) | Male Wistar rats (n = 32, divided into four groups) | Induction of anxiety by administration of single-dose LPS (1 mg/kg) | Reduced anxiety, as measured by increased time spent in the open arm of the elevated plus maze test with 200 µg/kg selenium | Increased cortical CAT and SOD in rats treated with Se; greater changes were seen with 200 µg/kg Se |
| Pinz et al., 2021 [56] | 6-((4-fluorophenyl)selanyl)-9H-purine (FSP), 1 mg/kg | Male Swiss mice (n = 28, divided into four groups) | Induction of anxiety by intracerebral administration of streptozotocin (2 µL of 2.5 mg/mL solution) | Reduced anxiety, as measured by reversal of streptozotocin- induced reduction in dives and open-arm entries during the elevated plus maze test, with FSP | A reduction in streptozotocin- induced an increase in cortical and hippocampal AChE activity and AChE mRNA expression with FSP |
| Samad et al., 2022 [57] | Sodium selenite (0.175 mg/mL/kg) | Male Wistar rats (n = 36, divided into three groups) | Induction of anxiety by exposure to arsenic (2.5 mg/mL/kg for 4 weeks) | Reduced anxiety-like behaviors, as measured by increased time spent in the open arm of the elevated plus maze and light–dark activity tests | Increased levels of GPx, CAT, and SOD in rats treated with Se |
| Situ et al., 2022 [58] | - | Male and female mice with targeted disruption of the selenoprotein W gene (SEPW1) and control mice | Observation of anxiety during the open-field test and elevated plus maze test; conditioned fear through the pairing of noise with an electric shock to the foot | Reduced anxiety-like behavior on the open-field and elevated plus maze tests and impaired fear conditioning in female mice with disruption of SEPW1 | Abnormal hippocampal Nissl bodies neuronal damage and reduced amygdala dendrite spine density in female mice with disruption of SEPW1; no effect of SEPW1 on levels of lipid peroxidation |
| Study | Country of Origin | Study Population and Sample Size | Measure of Anxiety | Parameter Measured | Results |
|---|---|---|---|---|---|
| Zieker et al., 2007 [59] | Germany | Patients with post-traumatic stress disorder (PTSD) following a disaster (n = 8) Healthy controls (n = 8) | Clinical diagnosis of PTSD according to the Diagnostic and Statistical Manual, 4th edition (DSM-IV) criteria | Expression of selenium-related genes—thioredoxin reductase (TXR1) and superoxide dismutase (SOD1)—in DNA microarray chips | Significant down-regulation of TXR1 and SOD1 in patients with PTSD |
| Ozdemir et al., 2009 [60] | Turkey | Patients with obsessive-compulsive disorder (OCD) (n = 28) Age- and sex-matched healthy controls (n = 28) | Clinical diagnosis of OCD according to the Diagnostic and Statistical Manual, 4th edition (DSM-IV) criteria | Serum selenium, measured by atomic absorption spectrometry plasma malondialdehyde (MDA), erythrocyte hemolysate glutathione peroxidase (GSH-Px) activity, erythrocyte superoxide dismutase (SOD), and catalase (CAT) activity | Significantly lower selenium GSH-Px and CAT, and higher MDA and SOD, in patients with OCD. No correlation between serum selenium and OCD symptom severity |
| Jamali et al., 2016 [61] | Iran | Patients living with HIV/AIDS (n = 100) | Depression, Anxiety, and Stress Scale, 42-item version (DASS-42) | Dietary selenium intake (µg/day) was measured using a 168-item food frequency questionnaire | A significant negative correlation was observed between dietary selenium intake and anxiety score |
| Salehi-Abargouei et al., 2019 [62] | Iran | Adults from the general population (n = 3846) | Hospital Anxiety and Depression Scale (HADS) | A selenium-rich diet, measured using a 106-item food frequency questionnaire assessing the daily intake of 57 nutrients | No significant association between a selenium-rich diet and anxiety scores |
| Wieder-Huszla et al., 2020 [63] | Poland | Healthy postmenopausal women (n = 102) | State-Trait Anxiety Inventory (STAI) | Serum selenium, measured by absorption spectrometry | No significant association between serum selenium and anxiety score |
| Portnoy et al., 2022 [64] | China | Children from the general population (n = 831) | Screen for Child Anxiety Related Emotional Disorders (SCARED) | Serum selenium, measured by atomic absorption spectrophotometry | Lower serum selenium was significantly associated with generalized, social, panic, school-related, and overall anxiety, but not separation anxiety |
| Zhang et al., 2022 [65] | China | Rural children aged 7–11 from the general population (n = 831) | Conners’ Parent Rating Scale (CPRS) | Urinary selenium, measured by inductively coupled plasma-mass spectrometry | No significant association between urinary selenium and anxiety score |
| Study | Trial Population and Sample Size | Trial Design | Intervention | Duration | Outcome Measure(s) | Result(s) | Safety and Tolerability Outcomes |
|---|---|---|---|---|---|---|---|
| Gosney et al., 2008 [66] | Nursing home residents aged >60 years, with intact cognition and no major depression or critical medical illness (n = 73) | Randomized controlled trial (n = 36 in active group, n = 37 in placebo group) | A multivitamin and mineral supplement containing selenium (60 µg/tablet), given as four tablets/day (i.e., 240 µg/day selenium) vs. placebo | 8 weeks | Hospital Anxiety and Depression Scale (HADS) | No significant difference between supplement and placebo on HADS anxiety scores | A significantly higher drop-out rate in the supplement group (10/36) than in the placebo group (3/37) |
| Rucklidge et al., 2011 [67] | Adults with attention-deficit hyperactivity disorder (ADHD) exposed to an earthquake (n = 33) | Retrospective analysis of data from two open trials and one randomized controlled trial (n = 16 on supplement and n = 17 not on treatment or placebo) | Micronutrient supplement containing 36 vitamins and minerals, including selenium (26 µg/capsule), given as 15 capsules/day (i.e., 390 µg/day selenium) | 8 weeks | Depression, Anxiety, and Stress Scale, 42-item version (DASS-42) | Significantly lower anxiety scores in the supplement group at 2 weeks post-earthquake (estimated effect size 0.69) | Not reported |
| Voicehovskis et al., 2014 [68] | Military personnel considered at risk for post-traumatic stress disorder (PTSD) (n = 97) | Controlled clinical trial; randomization not mentioned (n = 64 for selenium and n = 33 for placebo) | Selenium (200 µg/day) vs. placebo | 6 months | Changes in PTSD Symptom Checklist—Military Version (PCL-M) Measurement of malondialdehyde (MDA) in a subset (n = 62) of the sample | A reduction of 46.03% in those screening positive for PTSD on the PCL-M in the selenium group; a significant reduction in MDA with selenium compared to the placebo | Not reported |
| Kaplan et al., 2015 [69] | Adults aged 23–66 exposed to a flood (n = 56) | Randomized clinical trial (n = 17 in vitamin D group, n = 21 in few-nutrients group, n = 18 in broad-spectrum mineral/vitamin group) | Broad-spectrum mineral/vitamin (BSMV) supplement including selenium (45.2 µg/capsule), given as four capsules/day (i.e., ≈ 180 µg/day selenium) vs. vitamin D (1000 IU/day) and B-complex supplement (1 capsule/day) | 6 weeks | Depression, Anxiety, and Stress Scale, 42-item version (DASS-42) | A significant reduction in anxiety in the BSMV and B-complex groups compared to vitamin D (estimated effect size 0.89); no significant difference between the BSMW and B-complex groups | Minor adverse events (headache, nausea, rash) were comparable across groups; no significant difference in drop-out rates between groups |
| Sayyah et al., 2018 [70] | Patients with treatment-resistant obsessive-compulsive disorder (n = 32) | Randomized controlled trial (n = 16 in each group) | Selenium (200 µg/day) vs. placebo | 6 weeks | The mean reduction in the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score response, defined as ≥25% reduction in Y-BOCS score | The mean reduction in Y-BOCS was significantly greater in selenium; response rates were 43.7% in the selenium group and 7.1% in the placebo group | Adverse events (sedation, constipation, nausea, tremor, sexual dysfunction) comparable between selenium and placebo; no significant difference in drop-out rates between groups |
| Raygan et al., 2019 [71] | Patients with diabetes mellitus (type II) and coronary heart disease (n = 54) | Randomized controlled trial (n = 27 in each group) | Combined selenium (200 µg/day) and probiotic (8 × 109 CFU/day) vs. placebo | 12 weeks | Beck Anxiety Inventory (BAI) | A significant reduction in BAI in the supplement group compared to the placebo group (mean difference −1.46) | No adverse effects were reported by participants; no significant difference in drop-out rates between groups |
| Sarris et al., 2021 [72] | Patients with treatment-resistant obsessive-compulsive disorder (n = 28) | Open-label trial | A supplement containing selenium, zinc, magnesium, pyridoxal 5′-phosphate, N-acetyl cysteine, and L-theanine | 20 weeks | A mean reduction in the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score response, defined as ≥35% reduction in Y-BOCS score | A significant mean reduction (7.13 points) in Y-BOCS; 23% of patients were classified as responders | The treatment was reported as well-tolerated overall; no treatment drop-outs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkumar, R.P. Selenium and Its Compounds in the Treatment of Anxiety and Related Disorders: A Scoping Review of Translational and Clinical Research. Future Pharmacol. 2022, 2, 608-624. https://doi.org/10.3390/futurepharmacol2040037
Rajkumar RP. Selenium and Its Compounds in the Treatment of Anxiety and Related Disorders: A Scoping Review of Translational and Clinical Research. Future Pharmacology. 2022; 2(4):608-624. https://doi.org/10.3390/futurepharmacol2040037
Chicago/Turabian StyleRajkumar, Ravi Philip. 2022. "Selenium and Its Compounds in the Treatment of Anxiety and Related Disorders: A Scoping Review of Translational and Clinical Research" Future Pharmacology 2, no. 4: 608-624. https://doi.org/10.3390/futurepharmacol2040037
APA StyleRajkumar, R. P. (2022). Selenium and Its Compounds in the Treatment of Anxiety and Related Disorders: A Scoping Review of Translational and Clinical Research. Future Pharmacology, 2(4), 608-624. https://doi.org/10.3390/futurepharmacol2040037






