Dendrimers-Based Drug Delivery System: A Novel Approach in Addressing Parkinson’s Disease
Abstract
1. Introduction
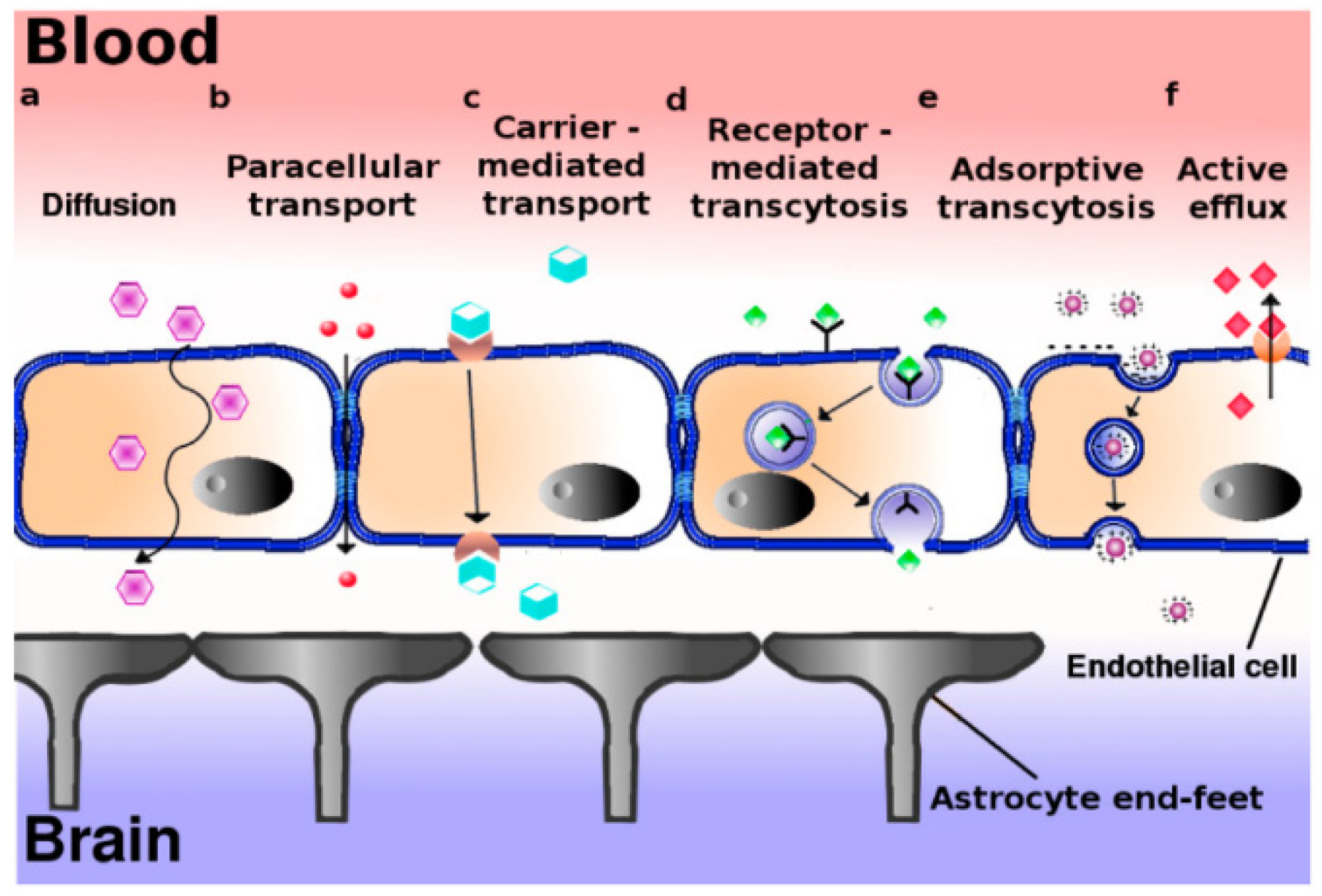
2. Blood–Brain Barrier Pathway: An Overview of the Problem
3. The Role of Dopamine and the Dopamine Receptors
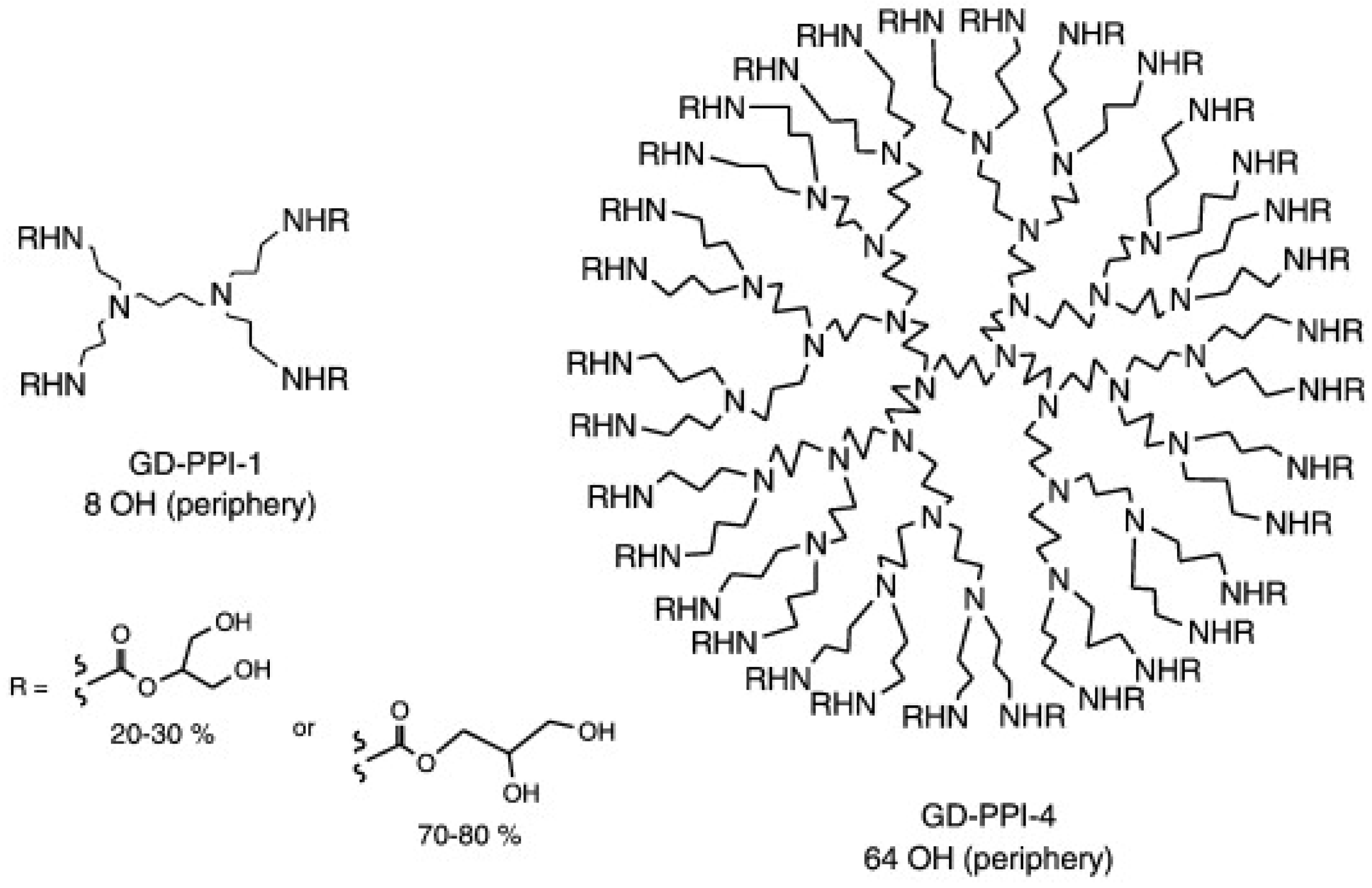
4. Dendrimers
5. Suitability of Dendrimers for Parkinson’s Disease
6. Anti-Parkinson’s Drugs and Dopamine
6.1. Rotigotine
6.2. Pramipexole
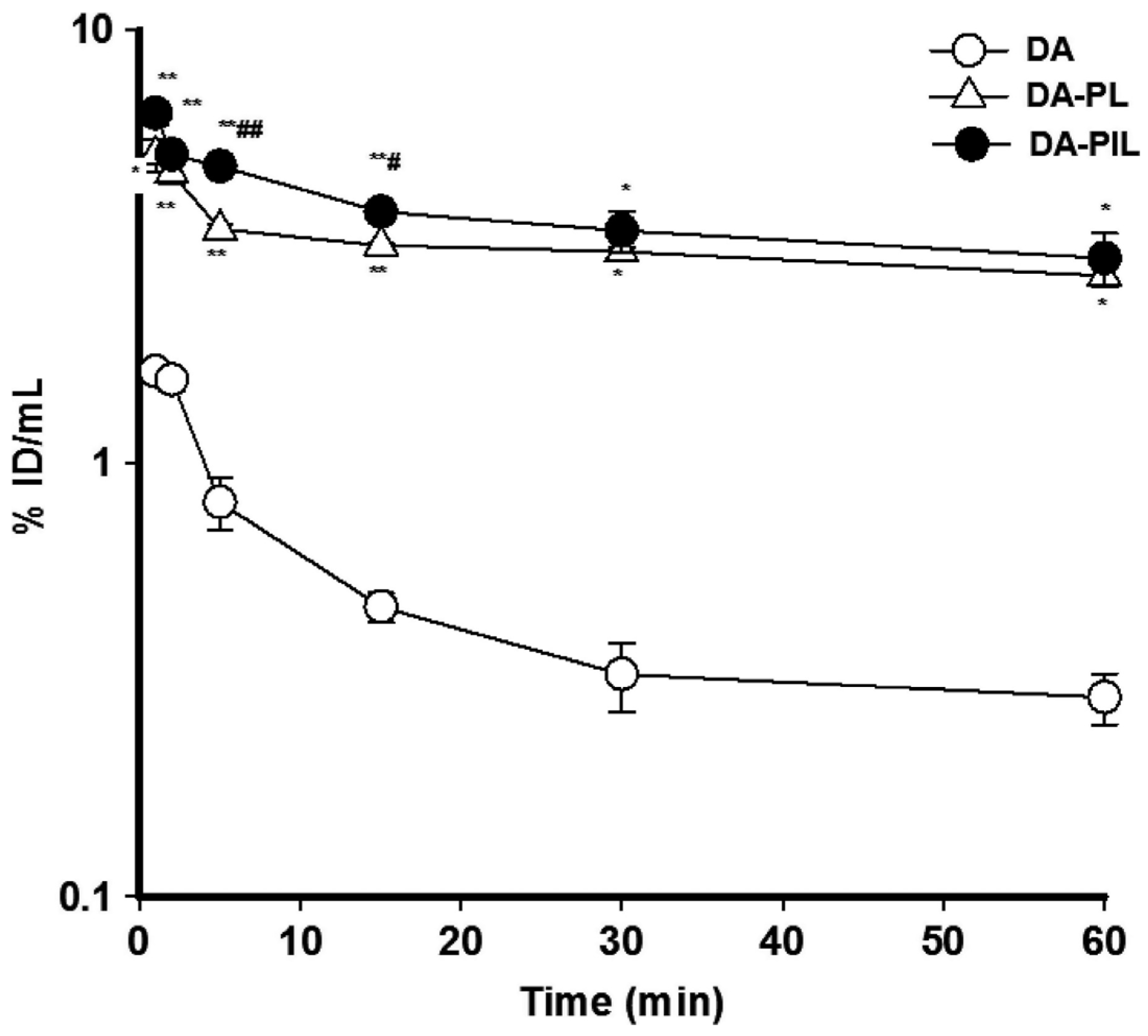
6.3. Dopamine
7. Discussion
7.1. Characterization Tests and Materials
7.2. Korsmeyer-Peppas Reaction Kinetics
7.3. Intranasal Route and Chitosan
8. Conclusions
9. Limitations and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ding, W.; Ding, L.J.; Li, F.; Han, Y.; Mu, L. Neurodegeneration and cognition in Parkinson’s disease: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2275–2281. [Google Scholar] [PubMed]
- Moreau, C.; Rolland, A.S.; Pioli, E.; Li, Q.; Odou, P.; Barthelemy, C.; Lannoy, D.; Demailly, A.; Carta, N.; Deramecourt, V.; et al. Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease. Neurobiol. Dis. 2020, 139, 104846. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, H. Diseases of the Nervous System; Academic Press: Cambridge, MA, USA, 2015; pp. 133–164. [Google Scholar]
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Neurodegeneration: What is it and where are we? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.; Vizcarra, J.; Marsili, L.; Lang, A.; Simon, D.; Merola, A.; Josephs, K.; Fasano, A.; Morgante, F.; Savica, R. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology 2019, 92, 329–337. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Michel, P.P.; Hirsch, E.C.; Hunot, S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron Cell Press J. Rev. 2016, 90, 675–691. [Google Scholar] [CrossRef]
- Cahill, C.; Aleyadeh, R.; Gao, J.; Wang, C.; Rogers, J. Alpha-synuclein in alcohol use disorder, connections with Parkinson’s disease and potential therapeutic role of 5′ Untranslated Region-Directed small molecules. Biomolecules 2020, 10, 1465. [Google Scholar] [CrossRef]
- Pearce, J.M.S. Aspects of the history of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1989, 52, 6–10. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for Drug Delivery to the Central Nervous System. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Y.; Jumah, F.; Coplan, L.; Krosser, A.; Sharif, K.; Tubbs, S. Blood-Brain Barrier: A review of its anatomy and physiology in health and disease. Clin. Anat. 2018, 31, 812–823. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.; Davis, T. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Holmes, J.; Bridges, A.; Gookin, J.L.; Coccaro, M.R.; Proctor, W.; Colegio, O.R.; Anderson, J.M. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 2008, 121, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling Drugs into the Brain: An Overview of Ligands Targeting Transcytosis for Drug Delivery across the Blood–Brain Barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef] [PubMed]
- Aradi, S.D.; Hauser, R.A. Medical Management and Prevention of Motor Complications in Parkinson’s Disease. Neurotherapeutics 2020, 17, 1339–1365. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Sawalha, M.; Khawaja, Y.; Naijar, A.; Karaman, R. Dopamine and Levodopa Prodrugs for the Treatment of Parkinson’s Disease. Molecules 2018, 23, 40. [Google Scholar] [CrossRef]
- Giladi, N.; Gurevich, T.; Djaldetti, R.; Adar, L.; Case, R.; Leibman-Barak, S.; Sasson, N.; Caraco, Y. ND0612 (levodopa/carbidopa for subcutaneous infusion) in patients with Parkinson’s disease and motor response fluctuations: A randomized, placebo-controlled phase 2 study. Parkinsonism Relat. Disord. 2021, 91, 139–145. [Google Scholar] [CrossRef]
- Carvey, P.M. Dopa-decarboxylase inhibitors. In Encyclopedia of Movement Disorders; Academic Press: Cambridge, MA, USA, 2010; pp. 313–316. [Google Scholar]
- Li, H.; Yang, P.; Knight, W.; Guo, Y.; Perlmutter, J.S.; Benzinger, T.; Morris, J.; Xu, J. The interactions of dopamine and oxidative damage in the striatum of patients with neurodegenerative diseases. J. Neurochem. 2019, 152, 235–251. [Google Scholar] [CrossRef]
- Kahana, M.; Weizman, A.; Weizman, A.; Gabay, M.; Loboda, Y.; Segal-Gavish, H.; Gavish, A.; Barhum, Y.; Offen, D.; Finberg, J.; et al. Liposome-based targeting of dopamine to the brain: A novel approach for the treatment of Parkinson’s disease. Mol. Psychiatry 2020, 26, 2626–2632. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control Release 2018, 287, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Monge-Fuentes, V.; Mayer, A.B.; Lima, M.R.; Geraldes, L.R.; Zanotto, L.N.; Moreira, K.G.; Martins, O.P.; Piva, H.L.; Felipe, M.S.S.; Amaral, A.C.; et al. Dopamine-loaded nanoparticle systems circumvent the blood–brain barrier restoring motor function in mouse model for Parkinson’s Disease. Sci. Rep. 2021, 11, 15185. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, R.; Seth, K.; Shukla, A.; Shukla, R.; Bhatnagar, P.; Chauhan, L.; Saxena, P.; Arun, J.; Chaudhari, B.; Patel, D.; et al. Trans-Blood Brain Barrier Delivery of Dopamine-Loaded Nanoparticles Reverses Functional Deficits in Parkinsonian Rats. Am. Chem. Soc. Nano 2015, 9, 4850–4871. [Google Scholar] [CrossRef] [PubMed]
- Rekha, M.R.; Sharma, P. Nanoparticle Mediated Oral Delivery of Peptides and Proteins: Challenges and Perspectives. In Peptide and Protein Delivery; Chapter 8; Elsevier Inc.: London, UK, 2011; pp. 165–194. [Google Scholar]
- Jin, K.; Lu, Z.; Chen, J.; Liu, Y.; Lan, H.; Dong, H.; Yang, F.; Zhao, Y.; Chen, X. Recent trends in nanocarrier-Based Targeted Chemotherapy: Selective Delivery of Anticancer Drugs for Effective Lung, Colon, Cervical, and Breast Cancer Treatment. J. Nanomater. 2020, 2020, 9184284. [Google Scholar] [CrossRef]
- Tam, V.; Sosa, C.; Liu, R.; Yao, N.; Priestley, R. Nanomedicine as non-invasive strategy for drug delivery across the blood brain barrier. Int. J. Pharm. 2016, 515, 331–342. [Google Scholar] [CrossRef]
- Asil, S.M.; Ahlawat, J.; Barrosoc, G.G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 15, 4109–4128. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ferdous, K.S.; Ahmed, M. Emerging promise of nanoparticle-based treatment for Parkinson’s disease. Biointerface Res. Appl. Chem. 2020, 2010, 7135–7151. [Google Scholar]
- De Marco, I. Supercritical Fluids and Nanoparticles in Cancer Therapy. Micromachines 2022, 13, 1449. [Google Scholar] [CrossRef]
- Kang, Y.; Jung, H.; Suk Oh, J.; Song, D. Use of PEGylated Immunoliposomes to Deliver Dopamine across the Blood-Brain Barrier in a Rat Model of Parkinson’s disease. CNS Neurosci. Ther. 2016, 22, 817–823. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, C.; Pang, Z. Dendrimer-Based Drug Delivery Systems for Brain Targeting. Biomolecules 2019, 9, 790. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Moscariello, P.; Ng, D.Y.W.; Jansen, M.; Weil, T.; Luhmann, H.J.; Hedrich, J. Brain Delivery of Multifunctional Dendrimer Protein Biconjugates. Adv. Sci. 2018, 5, 1700897. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Heylings, J.; Wan, K.; Mossa, G. Antimicrobial efficacy and mechanism of action of poly(amidoamine) (PAMAM) dendrimers against opportunistic pathogens. Int. J. Antimicrob. Agents 2019, 53, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Esumi, K.; Houdatsu, H.; Yoshimura, T. Antioxidant Action by Gold−PAMAM Dendrimer Nanocomposites. Langmuir 2004, 20, 2536–2538. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Merino, A.G.; Fraile-Martinez, O.; Recio-Ruiz, J.; Pekarek, L.; Guijarro, L.G.; Garcia-Honduvilla, N.; Alavrez-Mon, M.; Bujan, J.; Garcia-Gallego, S. Dendrimers and Dendritic Materials:From Laboratory to Medical Practice in Infectious Diseases. Pharmaceutics 2020, 12, 874. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Thakur, S.; Kesharwani, P.; Tekade, R.K.; Jain, N. Impact of pegylation on biopharmaceutical properties of dendrimers. Polymer 2015, 59, 67–92. [Google Scholar] [CrossRef]
- Fhayli, K.; Gatard, S.; Mohamadou, A.; Dupont, L.; Bouquillon, S. Poly(propylene imine) (PPIs) dendrimers modified with glyceryl moieties: Powerful catalysts for catecholase. Inorg. Chem. Commun. 2013, 27, 101–104. [Google Scholar] [CrossRef]
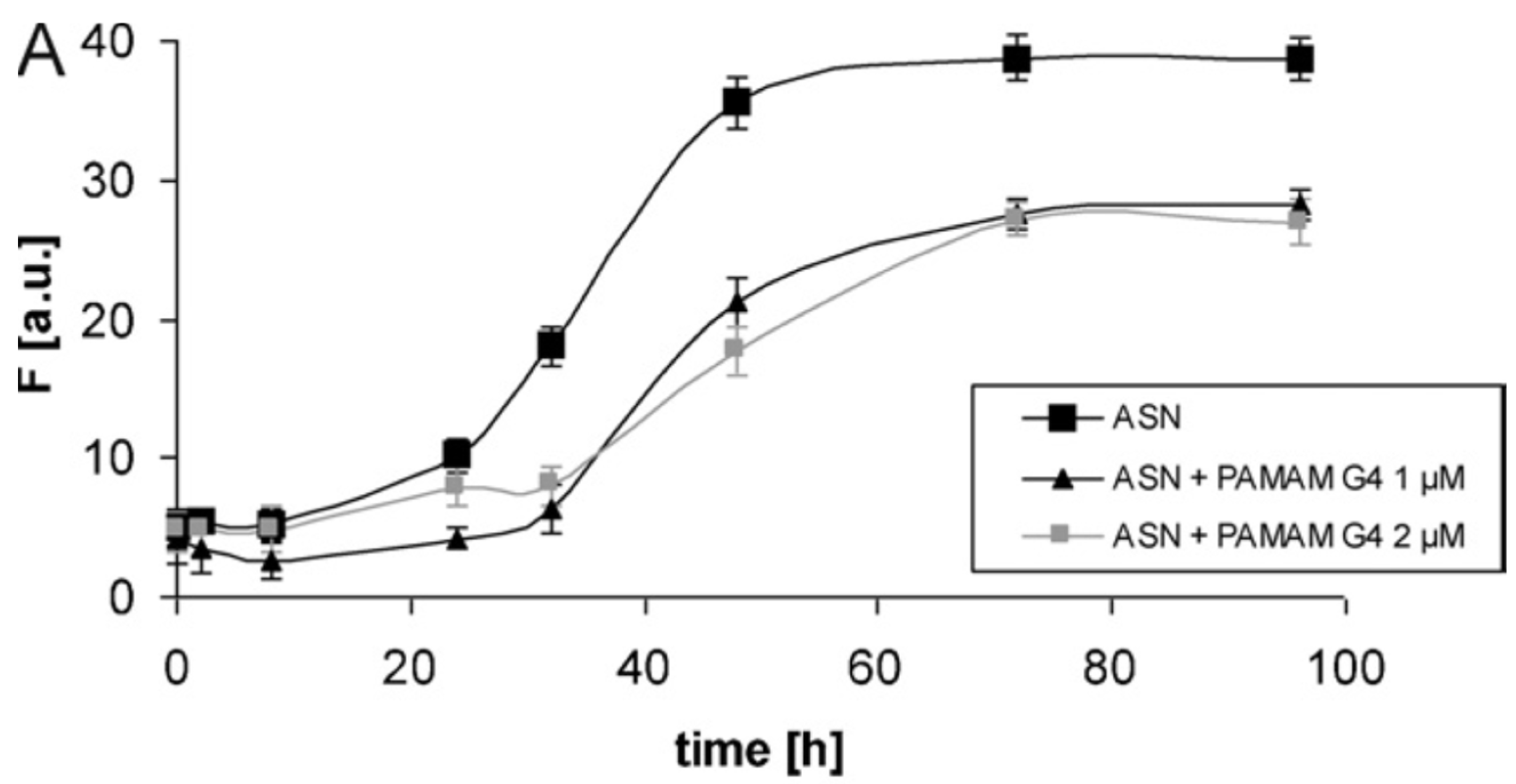
- Milowska, K.; Malachowska, M.; Gabryelak, T. PAMAM G4 dendrimers affect the aggregation of α-synuclein. Int. J. Biol. Macromol. 2011, 48, 742–746. [Google Scholar] [CrossRef]
- Milowska, K.; Szwed, A.; Mutrynowska, M.; Gomez-Ramirez, R.; Javier de la Mata, F.; Gabryelak, T.; Bryszewska, M. Carbosilane dendrimers inhibit α-synuclein fibrillation and prevent cells from rotenone-induced damage. Int. J. Pharm. 2015, 484, 268–275. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Stroylova, Y.; Shifrina, Z.; Muronetz, V. Disruption of Amyloid Prion Protein Aggregates by Cationic Pyridylphenylene Dendrimers. Macromol. Biosci. 2016, 16, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Zakaria, N.F.; Tilang, P.A.; Tzeyung, A.; Pandey, M.; Chatterjee, B.; Alhakamy, N.; Bhattamishra, S.; Kesharwani, P.; Gorain, B.; et al. Formulation development and evaluation of rotigotine mucoadhesive nanoemulsion for intranasal delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101301. [Google Scholar] [CrossRef]
- Thornton, P. Neupro Patch. 2019. Available online: https://www.drugs.com/neupro.html (accessed on 15 September 2020).
- Okura, T.; Ito, R.; Ishiguro, N.; Tamai, I. Blood-brain barrier transport of pramipexole, a dopamine D2 agonist. Life Sci. 2007, 80, 1564–1571. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Milowksa, K.; Grochowina, J.; Katir, N.; Kadib, A.; Majoral, J.P.; Bryszewska, M.; Gabryelak, T. Viologen-Phosphorus Dendrimers Inhibit α-Synuclein Fibrillation. Mol. Pharm. 2013, 10, 1131–1137. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.M.; Majoral, J. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef]
- Aliev, G.; Ashraf, G.; Tarasov, V.; Chubarev, V.; Keszek, J.; Gasiorowski, K.; Makhmutova, A.; Baeesa, S.; Avila-Rodriguez, M.; Ustyugov, A.; et al. Alzheimer’s Disease–Future Therapy Based on Dendrimers. Curr. Neuropharmacol. 2019, 17, 288–294. [Google Scholar] [CrossRef]
- Mignani, S.; Majoral, J.P. Can dendrimer based nanoparticles fight neurodegenerative diseases? Current situation versus other established approaches. Prog. Polym. Sci. 2017, 64, 23–51. [Google Scholar] [CrossRef]
- Kleindienst, A.; Dunbar, J.G.; Glisson, R.; Okuno, K.; Marmarou, A. Effect of dimethyl sulfoxide on blood-brain barrier integrity following middle cerebral artery occlusion in the rat. Neurosci. Lett. 2006, 89, 74–79. [Google Scholar] [CrossRef]
- Sridhar, V.; Gaud, R.; Bajaj, A.; Wairkar, S. Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson’s disease. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Skalko-Basnet, N.; Pio di Cagno, M. Interpreting non-linear drug diffusion data: Utilizing Korsemeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 1–14. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Helder, M.; Naderinezhad, S. A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 169–177. [Google Scholar] [CrossRef] [PubMed]
| Research Articles | Materials and Methods | Results |
|---|---|---|
| PAMAM G4 dendrimers affect the aggregation of α-synuclein |
|
|
| Carbosilane dendrimers inhibit α-synuclein fibrillation and prevent cells from rotenone-induced damage |
|
|
| Disruption of Amyloid Prion Protein Aggregates by Cationic Pyridylphenylene Dendrimers |
|
|
| Trans-Blood–Brain Barrier Delivery of Dopamine-Loaded Nanoparticles Reverses Functional Deficits in Parkinsonian Rats |
|
|
| Formulation development and evaluation of rotigotine mucoadhesive nanoemulsion for intranasal delivery |
|
|
| Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson’s activity |
|
|
| Use of PEGylated Immunoliposomes to Deliver Dopamine across the Blood–Brain Barrier in a Rat Model of Parkinson’s disease |
|
|
| Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson’s disease |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordonio, M.B.; Zaki, R.M.; Elkordy, A.A. Dendrimers-Based Drug Delivery System: A Novel Approach in Addressing Parkinson’s Disease. Future Pharmacol. 2022, 2, 415-430. https://doi.org/10.3390/futurepharmacol2040027
Ordonio MB, Zaki RM, Elkordy AA. Dendrimers-Based Drug Delivery System: A Novel Approach in Addressing Parkinson’s Disease. Future Pharmacology. 2022; 2(4):415-430. https://doi.org/10.3390/futurepharmacol2040027
Chicago/Turabian StyleOrdonio, Michaella B., Randa Mohammed Zaki, and Amal Ali Elkordy. 2022. "Dendrimers-Based Drug Delivery System: A Novel Approach in Addressing Parkinson’s Disease" Future Pharmacology 2, no. 4: 415-430. https://doi.org/10.3390/futurepharmacol2040027
APA StyleOrdonio, M. B., Zaki, R. M., & Elkordy, A. A. (2022). Dendrimers-Based Drug Delivery System: A Novel Approach in Addressing Parkinson’s Disease. Future Pharmacology, 2(4), 415-430. https://doi.org/10.3390/futurepharmacol2040027







