Abstract
Peptides are highly potent biological active compounds with excellent selectivity and binding, but they have some drawbacks (e.g., low stability in vivo because of the enzymatic degradation, and fast elimination). To overcome their drawbacks, various peptidomimetics have been gaining ground. Different modifications have been examined, such as the modification of peptide backbone. One such seemingly simple modification is the replacement of the CHα group by an N atom. These amino acid derivatives are called azaamino acids, and peptides containing azaamino acid are called azapeptides. This exchange results in both steric and electronic differences from the original amino acids, thus affecting the structure and biological activity of the modified peptide. In this review, the synthesis possibilities of azapeptides and the impact of azaamino acid incorporation on the structure and biological activity are presented through examples. Different synthetic solutions for azaamino acid introduction and the various routes to build in the side chain are summarized to illustrate the improvement of the field of azaamino acid chemistry. The influence of the altered electronic and steric properties of N-atom on the structure is described, too. Finally, some examples are given with potent biological activity.
1. Introduction
Peptides may have high biological activity and can be a good basis to identify the best constructs with an optimized structure [1]. The improved peptide synthesis and the high variability of 20 natural amino acids with very divergent physical–chemical properties are a good tool to identify potential candidates. As peptides have many drawbacks, the next step is the improvement of the pharmacological properties of peptide candidates, and the next generation is their peptidomimetic. In these derivatives, the original structure of peptides should be preserved to retain the high efficacy and selectivity with enhanced pharmacokinetic and pharmacological properties. Peptides can be modified at their backbone or in their side chain [2]. Modification of the backbone may influence the structure of the peptidomimetics [3] and in vivo stability [4], whereas the altered side chain may have an effect not only on the structure, but also the interaction with the target molecule [5]. To replace the peptide bond with other chemical bonds, intensive synthetic works are needed to prepare the building blocks and the peptide mimicry. Incorporation of non-natural amino acids into a peptide sequence may have the advantage that more and more non-natural amino acids become commercially available. The combination of side-chain and backbone modifications may result in a synergistic effect. One such modification is the introduction of azaamino acid(s) into the sequences [6]. In these amino acids the CHα group is replaced by an N atom (Figure 1), and thus they are semicarbazide as amino acid surrogates. This “small” modification may have a large effect on the backbone geometry and on the structure of the peptide and thus on its activity and stability [7]. For example, the altered electronic and steric effect of Nα resulted in reverse biological functions for azapeptides. Enzyme substrates become inhibitors if azaamino acid is inserted at the P1 position [8,9,10]. Furthermore, modifying the side chain of azaamino acids and thus the introduction of non-natural azaamino acids may increase the applicability of this modification to study the structure–activity relationship [11,12,13,14]. In case of azapeptide synthesis, hydrazine derivatives should be used to incorporate the azaamino acid to replace amino acids in the sequence. Thus, in the synthesis of azapeptides the combination of peptide and hydrazine chemistry is necessary. There are many strategies to build in the azaamino acid residue into a peptide [7,15], but sometimes they cannot be used. The altered steric and electronic properties of Nα-alkylated hydrazine derivatives result in the introduction of azaamino acid and the coupling of amino acid to the azaamino acid being more difficult than in the case of peptide synthesis. This means that the success of one strategy depends on the sequence of the azapeptide, which makes azapeptide synthesis challenging. The altered geometry around the N-atom may force the peptidomimetic into a well-defined structure [16], or it may destroy the structure of the parent peptide [17]. In this brief review, different synthetic strategies are presented, incorporating the newest results. The effects of azaamino acids on the structure of peptides and biologically active examples is summarized.
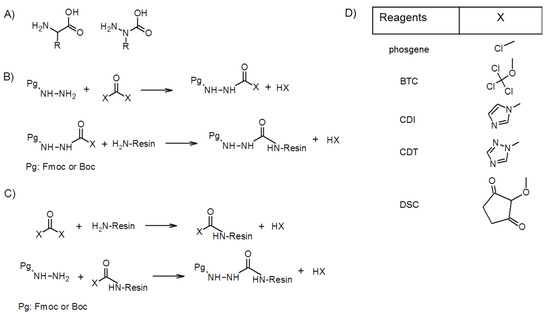
Figure 1.
Coupling strategy for azapeptide synthesis. (A) The general structure of an amino acid and azaamino acid; (B) the protected hydrazide is activated and coupled to the amino group on the resin; (C) the amino group on the resin is activated and reacted with the protected hydrazine; (D) the reagents can be used for azaamino acid introduction. After coupling, the Fmoc or Boc protecting group can be removed by piperidine and TFA, respectively, and the next amino or azaamino acid can be coupled. BCT: bis-(trichloromethyl)carbonate; CDI: carbonyldiimidazole; CDT: 1,1′-Carbonyl-di-(1,2,4-triazole); DSC: using N,N’-disuccinimidyl carbonate.
2. Synthesis of Azapeptides
The synthesis of azapeptides is based on peptide and hydrazine chemistry. Although there are many synthetic pathways to build in the azaamino acid residue and to carry out peptide synthesis, unfortunately azapeptide synthesis has remained challenging. The incorporation of an azaamino acid into a peptide can be done by the coupling of a hydrazine derivative in the presence of a carbonyl group source. The side chain of the azaamino acid can be built in before or after the coupling of the hydrazine derivative.
2.1. Incorporation of N-Alkylated Hydrazine Derivatives
The introduction of N-alkylated hydrazine derivative in the presence of a source of an activated carbonyl group can be done in two directions. The activated carbonyl group can be attached to the hydrazine or to the N-terminal amino group on the resin (Figure 1). The formation of azaamino acid using properly protected hydrazine derivative can be carried out with different sources of an activated carbonyl group (Figure 1). Carbonyldiimidazole (CDI) is a frequently used reagent to activate Fmoc-NH-NH2 as Fmoc-azaGly-imidazolide. This activation of Fmoc-NH-NH2 can be carried out in DMF at room temperature. Then this active form of azaGly is added to the free amino groups on the resin and the coupling is done for a longer amount of time (e.g., overnight at room temperature) [16,18,19].
Instead of CDI, its triazol derivative 1,1′-carbonyl-di-(1,2,4-triazole) (CDT) can be used in the same conditions [20]. A well-known and commonly used reagent is phosgene or its equivalents (di and triphosgene). Nα-alkyl carbazates were transformed into Fmoc azaamino acid chlorides by treating with phosgene in toluene. Their DCM solution was then added to the peptide-resin [21,22]. Bis-(trichloromethyl)carbonate (BTC) as the activating agent as a safer alternative to phosgene was applied to form azaamino acylchloride, too [17,18,23]. In a study, the coupling efficacy of different activating reagents (phosgene, diphosgene, BTC, CDI, CDT) was compared [24]. In THF none of them gave a good yield, but CDT was very efficient in NMP for a longer reaction time at RT or 30 min at 60 °C. It should be noticed that phosgene and its derivative were not tested in DCM, the commonly used solvent in their reaction. Less reactive derivative can be formed by using N,N’-disuccinimidyl carbonate (DSC). The activated carbazate is more stable because of its decreased reactivity, but active enough to form an amide bond with the free amino group of resin-bound peptide [11]. In these examples, Fmoc-protected hydrazine derivatives were activated before the coupling. There are some examples when the amino group on the resin was first activated as carbamoyl derivatives (X-CO-NH-peptide-resin) and then reacted with the hydrazine derivatives. To do so, DSC and the unprotected hydrazine was used to build up the azaGly moiety on the resin [25]. CDI was also used in this manner on resin [26] or in solution [27]. For these reactions the side chain of the azaamino acid should be built into the protected hydrazine before the coupling. Typical synthesis for the alkylated hydrazine derivatives is the reaction of protected hydrazine and the proper aldehyde followed by reduction via a hydride source or catalytic hydrogenation—for example, Boc-protected azaanalouge of Phe, Val, Ala, Leu, Ile, Trp, Tyr, Asp, Asn, Glu, Gln, and Pro [28,29,30] and Fmoc-protected azaderivatives of Ala, Leu, Val, Gly, Phe, Tyr, Trp, Lys, Orn, Arg, and Asp [31] were prepared using this route. Direct alkylation of protected hydrazine was used to synthesize 2-(3,5-dimethoxyphenyl)propan-2-yloxycarbonyl (Ddz)-protected azaaspartic acid derivative [32,33,34].
If the side chain is not introduced before the coupling of azamino acid building blocks, the low reactivity of Nα-alkylated hydrazine derivatives may be avoided. In this synthetic route, the side chain of azaamino acid is formed on the resin after the introduction of semicarbazone into the peptide sequence–submonomer synthesis of azapeptides.
2.2. Submonomer Synthesis of Azapeptides
Although the above-mentioned synthetic strategy, the activation of protected N-alkylated hydrazine derivatives, is a suitable process to build azaamino acids into a peptide sequence, it has some drawback. The formation of side products, hydantoin, oxadiazalone, or symmetric urea, may decrease the yield of the synthesis (Figure 2). Bourguet et al. introduced benzophenone as a suitable protecting group of semicarbazone in the synthesis of azaGly-containing azapeptide (Figure 2) [33]. The hydrazone protection surmounts to the formation of hydantoin or oxadiazalone formation and is suitable to synthesize azaGly-containing dipeptide in solution. For a good yield the activation was crucial; using phosgene or CDI as an activator the main product was symmetric urea. With p-nitrophenyl chloroformate activation this side reaction was avoided (Figure 2). The hydrolysis of semicarbazone resulted in azaGly-containing azadipeptides. The benzophenone protection made possible the regioselective modification of the Nα atom in the semicarbazone before the hydrolysis in solution or on the resin submonomer azapeptide synthesis [35]. However, conditions (1N HCl in THF) used in the solution phase were not useful for semicarbazone deprotection in the solid phase [33]; transimination with NH2OH·HCl in pyridine at 60 °C for 12 h was successfully used [35]. The azapeptide derivatives of the growth hormone-releasing peptide GHRP-6 were synthesized successfully using submonomer azapeptide synthesis and a conventional solid-phase peptide synthesis with the Fmoc/tBu strategy. The p-nitrophenyl chloroformate activation was also used to build in the benzophenone hydrazone into peptide sequences of chromobox homolog 7 ligands [21].
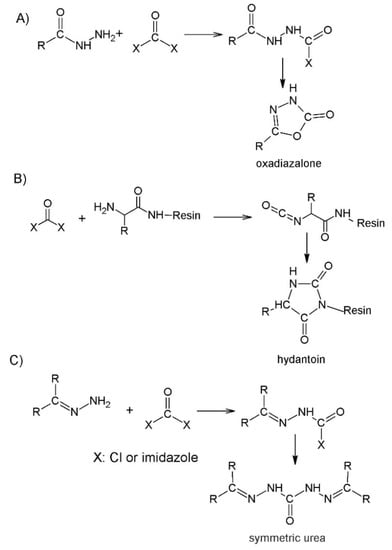
Figure 2.
Possible side reactions during the azaamino acid coupling. The formation of side products—oxadiazalone (A), hydantoin (B) and symmetric urea (C).
Using DSC instead of p-nitrophenyl chloroformate in the activation of benzophenone hydrazone, the formation of symmetric urea can be avoided, too, and the purification is simpler because of the missing p-nitrophenol as a side product (Figure 2) [36]. This activation was used also in the synthesis of cyclic azapeptides [34], cluster of differentiation 36 receptor modulator azapeptides [37], azapeptidomimetics of angiotensin 1–7 [38], and C-terminal azapeptides [39].
After the incorporation of semicarbazone, its Nα-atom can be modified by introducing the side chain of the azaamino acid in solution or on the resin. In the first examples this modification was done by alkylation using halogenated molecules [21,36]. Nowadays the chemistry of this modification is extended, and many kinds of reactions have been tested [15]. Recently, on-resin alkylation by alcohols using a Mitsunobu reaction was reported [38]. When the side chain is built in the benzophenone group can be removed by NH2OH·HCl in pyridine [39]. When the Mitsunobu reaction was used the protecting group was o-nitro-benzaldehyde. This semicarbazone was stable against NH2OH solution; thus, its cleavage was done in a two-step process. First, the electron-withdrawing NO2 group was reduced, then the amino derivative was cleaved by NH2OH [38]. If the protection of semicarbazide or semicarbazone is removed, the next amino acid can be coupled. Unfortunately, coupling to the azaamino acid in the sequences has some difficulties.
2.3. Coupling the Next Amino Acid to Azaamino Acid on Resin
In case of azaGly this coupling does not require specific activation. DIC-HOBt coupling reagent was used successfully [40]. As the semicarbazide has decreased nucleophilicity, the structure of the group on the Nα-atom has high influence on the coupling efficiency to the azaamino acid. Symmetrical anhydride, formed by the DIC method [21], BTC activation [38], and the mixed anhydride method using isobutyl-chloroformate [16], was applied to synthesize azapeptide. In recent studies the efficiency of coupling reagents and the influence of the side chain of both the coupled amino acid and the azaamino acid on the resin was studied [41,42,43]. When Fmoc-Ala-OH was coupled to azaAla on resin, COMU, PyOXIM, and HATU (Scheme 1A) were the best coupling reagents with more than 93% yield. In this coupling both the amino acid and the reagents were used in 10-fold excess [41]. Next, the effect of the side chain in the Fmoc-AA-OH was studied [42]. It turned out that the COMU can result in high conversion at proper reaction time. A 6.5- to 28-fold longer amount of time was necessary for the Val and Ile coupling, because their side chain reduced the reaction rate of the coupling (Scheme 1B). It was also noticed that the side chain of azaamino acid controlled the rate and the yield of the reaction [43]. When Fmoc-Ala-OH was coupled to different azadipeptides using COMU, the azaGly dipeptide reactivity was comparable with the reaction of natural amino acids (Scheme 1C). However, in the case of azaVal, no tripeptide was detected. These results indicate that there is no common method for an efficient and good yield of azapeptide synthesis; if the yield were enhanced the reaction time and coupling reagent(s) would be optimized.
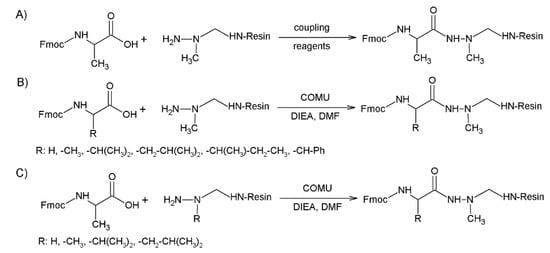
Scheme 1.
Probing the efficiency of amino acid coupling to the azaamino acid in the sequence. (A) Using Fmoc-Ala-OH and azaAla-resin different coupling reagents were examined; (B) using COMU as an efficient reagent the effect of the side reaction was measured; (C) using Fmoc-Ala-OH the steric influence of the side chain of azaamino acid was explored.
3. The Effect of Azaamino Acid Substitution on the Peptide Structure
Azapeptides predominantly have β-turn conformations. Their conformational properties are explained by the lone-pair−lone-pair repulsion of the adjacent hydrazide nitrogen atoms. In these β-turns, two H-bonds were proposed if azaAla or azaAsn are in the (i + 2) position [44,45]. One of these two H-bonds is between a C=O (i) and N-H (i + 3) group, but the other is unusual, between the Namide-H (i + 3) and Namide (i + 2) groups. This H-bond should be weak because of the delocalization of the lone pair of Namide and its perpendicular orientation. However, its role in the stabilization of protein structure and stability was proven by experimental and theoretical studies [46]. Recently, this H-bond and its importance was studied in model N-methylated azapeptides [47]. The results showed increased stability of the structure of azapeptides and their N-methylated derivatives. Calculations and structure determination supported that the azaamino acid in the i + 2 position induce stable β-turn formation [48]. Recently, the effect of azaamino acid side chain in a dipeptide Ac-Pro-azaXaa-NH-Me on the conformation, its stability, and its cis- trans isomerization of amide bonds preceding the azaamino residue were predicted in the gas phase and water by calculations [49]. In case of azaAsn and azaAsp the side-chain H-bonding was of high importance to stabilize the β-trun motif. The polypeptide chains in collagen had lefthanded polyproline type-II helical conformation. The backbone modification of peptides mainly destroyed the triple helix formation. In contrast, when azaGly was introduced into the sequences a hyperstable triple helix was formed [16]. This modification increased the number of H-bonds and caused stabilization of the PPII structure of collagen. It was proven that short collagen peptides (12-mer) can form a triple helix when all Gly are substituted by azaGly [50]. Not only was the azaGly substitution tolerated, but the achiral azaPro could also mimic the L-Pro in the triple helix of collagen [6], whereas the D-Pro inhibited the formation of a triple helix [51]. The structural basis of the azaGly effect on the triple helix stability was given with an atomic-resolution crystal structure of collagen-containing aza-glycine [52]. The N-N lone pair repulsion destabilized the non-triple helical conformations, whereas the anomeric effect stabilized the triple helical conformations. The increasing number of azaamino acids in a peptide may result in higher structural stability. More azaPhe was introduced into oligomers (2α:1aza ratio, Boc-(Phe-azaPhe-Ala)2-OMe) and it was noticed that the oligomers adopted a C=O(i)···H–N(i + 2) hydrogen-bonded helical conformation [53]. Cyclisation of these oligomers resulted in cyclic azapeptides that retained the ability to form a β-turn structure and could self-assemble into a supramolecular construction [54]. In another case, using an azaamino acid spacer and a macrocycle scaffold resulted in hemispheres that could form well-defined dimeric capsules with side chains inside their cavities [27]. Peptides with consecutive azaamino acids (aza-aza-α) adopted 10-membered β-turn conformations with the tendency to form helical structures [55]. However, the azaamino acid substitution may force peptides into a β-turn and triple helical form of collagen, which destroys the β-harpin formation [17]. This effect was dependent on the side chain of the azaamino acid. AzaVal was more detrimental to the stability of β-harpin than the azaGly residue. These results show that the azaamino acid substitution has the potential to change or to stabilize the structure of peptides and thus enhance their biological activity. The dynamic chirality of Nα also has high potential to modulate the binding ability of azapeptides [56].
4. Biological Activity of Azapeptides
4.1. Enzyme Inhibitors
Cysteine proteases are enzymes that are involved in various physiological processes, yet they are involved in the pathogenesis of several diseases. Cysteine protease inhibitors based on azapeptides were reported to bind effectively and specifically to such proteases. One instance is the azapeptide analogue of cathepsin B inhibitor, which demonstrated high specificity to cathepsin B (500 folds over cathepsin K (Z-Arg-Leu-His-Agly-Ile-Val-OMe), Ki (nM): 0.55 and 0.0011 on cathepsin K and cathepsin B, respectively, Table 1) [57] In addition, the inhibitors were shown to adopt β-turn conformation, enabling specific interaction with cathepsin B, and subsequently efficient inhibition. Another cysteine protease that was targeted in a similar approach is calpain, in which the native substrate was modified with azaglycine, resulting in a specific calpain inhibitor [40] The synthesized azapeptide inhibitors were shown to be selective to m-calpain by 20 fold over cathepsin B (Ac-Thr-Ser-Leu-Agly-Ser-Pro-Pro-Pro-Ser-NH2) Ki (µM): 3.5 and 69.9 on m-calpain and cathepsin B, respectively). The conformation of azapeptides and the substrate peptide was very similar and did not show any difference between good and poor inhibitor azapeptides. These inhibitors inhibit the enzyme because of the slower hydrolysis of the enzyme–azapeptidyl bond, which is formed when the enzyme cleaves the amide bond next to the azaamino acid residue. In these constructs the azaamino acid moiety behaves as a warhead. In other type of enzyme inhibitors the azaamino acid in the P1 position just replaces the native amino acid to increase the binding. For inhibition a warhead is used to inhibit the enzyme activity. Some azapeptidyl aldehyde or ketones were developed with good inhibitory activity against different enzymes [58]: Z-Leu-Leu-azaLeu-CHO as a 20S proteasome inhibitor (IC50: 9.02 µM), Z-Asp-Glu-Val-azaAsp-COMe as a caspase-3 inhibitor (IC50: 7.74 µM), Z-Ile-Glu-Thr-azaAsp-COMe as a caspase-6 inhibitor (IC50: 9.08 µM), and Z-Ala-Ala-azaAsn-COBn as legumains inhibitor (IC50: 22.23 and 17.82 µM).

Table 1.
Azapeptides with different biological activity.
Azadipeptide nitriles are members of a class of efficient inhibitors of human cysteine cathepsins [59,65]. Both N atoms in the azapeptide should be alkylated because of the synthetic aspect [59]. Although the N-methylation on the P2-P1 amide bond is generally disadvantageous for inhibitory activity, it did not worsen the inhibitory effect. These modifications (N-methylation, aza analogue) together increased the proteolytic stability of peptides and increased their activity, which was more pronounced than the activity of peptide-based inhibitors. The most potent inhibitor was the Z-Phe-(N-Me)azaAla-nitril (Ki (nM): 0.074, 0.22, 0.022 on cathepsin L, S, and K, respectively). In another study, azadipeptide nitriles were also very active against cathepsin B1 (SmCB1) from Schistosoma mansoni (Z-Phe-(N-Me)azahPhe-nitril, Ki (nM): 4.8; hPhe: homophenylalanine) [60]. As in the other cases, the azaanalogues showed a higher inhibitory effect than dipeptide nitriles. In a recent study, azatri- and azatetrapeptide nitriles were studied against the SARS-CoV-2 main protease and were found to be effective (Z-Abu-Tle-Phe-azaGln-nitril, kinac/Ki: 37,500; Abu: 2-aminobutyric acid, Tle: tert-leucine) [61].
Continuously activated protein kinase B (PKB/Akt) is associated with many types of human cancer. ATP mimetic PKB inhibitors are not selective enough. Peptides that may bind to the substrate-binding site may inhibit the binding of its substrate proteins and thus can be promising drugs as PKB-selective inhibitors. One such a peptide is Arg-Pro-Arg-Nva-Tyr-Dap-Hol (PTR6154; Nval, norvaline; Dap, 2,3-diaminopropionic acid; Hol, homoleucine) [66]. This peptide was modified using an aza scan; every amino acid was substituted by its aza-analogue. Some of these azapeptides showed inhibitory activity, but they were less effective than the PTR6154 (IC50: 0.94 and 13.7 µM for PTR6154 and H-Arg-azaPro-Arg-Nva-Tyr-Dap-Hol-NH2, respectively [62]. In the case of another peptide, Arg-Pro-Arg-Nle-Tyr-Dap-Nle-OH identified by immunoblot assay as a promising Akt inhibitor, an azaGly scan was carried out [25]. An extra azaGly was inserted in every position in the original sequence. Some of these azapeptides were very active, better than the positive control (Akt-in [67]) and the original sequence (Arg-Pro-azaGly-Arg-Nle-Tyr-Dap-Nle-NH2 and azaGly-Arg-Pro-Arg-Nle-Tyr-Dap-Nle-NH2). The azaGly derivatives were more stable in human serum and their structure was very similar to the structure of the original sequence. Comparing the results of azaamino acid substitutions, it can be concluded that azaGly introduction is tolerated in many positions and has no effect on the solution phase conformation, but results in improved serum stability and biological activity.
When the N-terminal Ala was changed with azaAla in a short N-terminal Smac-derived pentapeptide analogue Ala-Val-Pro-Phe-Tyr-NH2, the azapeptides had higher stability towards aminopeptidases and the same binding ability to the target protein XIAP BIR3 [68].
As azaamino acid has a high tendency to form β-turn, the insertion can result in a β-turn conformation of the azapeptide. Sometimes this conformation does not fit with the binding and the azapeptide has lower activity than the original peptide [69].
4.2. Receptor Ligands
The unique structure of azapeptides enables further possible treatments—for instance, cluster of differentiation 36 receptor (CD36), a scavenger receptor correlated with type 2 diabetes and cardiovascular disease [70,71]. Growth hormone-releasing peptides (GHRP) provide protection for normal cardiac function, which is performed by binding with CD36 [72]. GHRP-6 derived modulators show efficient binding affinity to CD36, providing cardioprotective activity. Regardless, one issue with GHRP-based modulators is the lack of selectivity [73]. Alternatively, azapeptides provide an additional window of opportunity to bind more specifically to CD36 [63]. In part this is related to the structural effects influenced by azaamino acids such as β-turns, enabling the overall structure to adopt a favorable conformation for interaction with CD36 [73]. The best derivative (Ala-D-Trp-Ala-AzaPhe-D-Phe-Lys-NH2) had affinity towards the CD36 receptor (IC50 binding (M): 7.58 × 10−6), but it could not bind to the ghrelin receptor, previously known as GHS-R1a (IC50 binding (M) > 10−5). Its cardioprotective effect was studied in vivo and it was proven that the adiponectin level was enhanced during this process [74]. It had anti-atherosclerotic properties in mice [75] and it showed a protective effect against subretinal inflammation and photoreceptor degeneration in vivo [76]. Cyclic azapeptide derivatives may enhance the potential of these construct as CD36 modulators and anti-inflammatory molecules [77]. Further modification of azaPhe residue in the Ala-D-Trp-Ala-AzaPhe-D-Phe-Lys-NH2 sequence resulted in efficient molecules, too (H-His-D-Trp-Ala-aza(4-n-propoxy)Phe-D-Phe-Lys-NH2 IC50 binding (µM): 1.65) [37].
The αvβ3, αvβ5, and α5β1 integrins play important roles in human tumor metastasis and tumor-induced angiogenesis. Molecules that may inhibit their interaction with natural ligands can be antiangiogenic compounds. One such compound is cilengitide (c(RGDf(NMe)V)), a cyclic peptide derivative. Its azaamino acid-substituted derivatives showed reduced or no activity [64]. Only in the case of the azaGly-substituted derivative (c[Arg-azaGly-Asp-D-Phe-Val, IC50 binding to αvβ3 (nM) > 5.7]) was some activity retained.
5. Conclusions
The modification of peptides with azaamino acids is a promising method for the development of efficient peptidomimetics. These derivatives may have increased stability and biological activity over peptides containing natural amino acids. However, the replacement of individual amino acids by an azaamino acid analogue is not always applicable. Most results show that the incorporation of azaGly is well tolerated in terms of biological activity. The preparation of such derivatives is increasingly facilitated by the numerous synthetic approaches that have been developed and are described in this review. In conclusion, the azaGly scan or incorporation of azaamino acids can help to improve the pharmacokinetic and pharmacological properties of peptide-based drugs.
Author Contributions
Conceptualization, Z.B.; writing—original draft preparation, Z.B., K.T. and M.Y.; writing—review and editing, Z.B., K.T. and M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was completed in the ELTE Thematic Excellence Programme supported by the Hungarian Ministry for Innovation and Technology, termed Szint+, and was funded by the Stipendium Hungaricum Scholarship of the Tempus Public Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox—Transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef]
- Avan, I.; Dennis Hall, C.; Katritzky, A.R. Peptidomimetics via modifications of amino acids and peptide bonds. Chem. Soc. Rev. 2014, 43, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.M.; Cabalteja, C.C.; Horne, W.S. Peptide Backbone Composition and Protease Susceptibility: Impact of Modification Type, Position, and Tandem Substitution. Chembiochem 2016, 17, 712–718. [Google Scholar] [CrossRef]
- Frey, V.; Viaud, J.; Subra, G.; Cauquil, N.; Guichou, J.F.; Casara, P.; Grassy, G.; Chavanieu, A. Structure-activity relationships of Bak derived peptides: Affinity and specificity modulations by amino acid replacement. Eur. J. Med. Chem. 2008, 43, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.J.; Moreland, W.T.; Laubach, G.D. N- [2-Isopropyl-3-(-aspartyl-l-arginyl)-carbazoyl]-l-tyrosyl-l-valyl-l-histidyl-l-prolyl-l-phenylalanine, an Isostere of Bovine Angiotensin II. J. Am. Chem. Soc. 1963, 85, 4040–4041. [Google Scholar] [CrossRef]
- Proulx, C.; Sabatino, D.; Hopewell, R.; Spiegel, J.; García Ramos, Y.; Lubell, W.D. Azapeptides and their therapeutic potential. Future Med. Chem. 2011, 3, 1139–1164. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.C.; Carroll, D.L. Reaction of acyl carbazates with proteolytic enzymes. Biochem. Biophys. Res. Commun. 1975, 67, 639–644. [Google Scholar] [CrossRef]
- Magrath, J.; Abeles, R.H. Cysteine Protease Inhibition by Azapeptide Esters. J. Med. Chem. 1992, 35, 4279–4283. [Google Scholar] [CrossRef]
- Powers, J.C.; Boone, R.; Carroll, D.L.; Gupton, B.F.; Kam, C.M.; Nishino, N.; Sakamoto, M.; Tuhy, P.M. Reaction of azapeptides with human leukocyte elastase and porcine pancreatic elastase. New inhibitors and active site titrants. J. Biol. Chem. 1984, 259, 4288–4294. [Google Scholar] [CrossRef]
- Chingle, R.; Mulumba, M.; Chung, N.N.; Nguyen, T.M.D.; Ong, H.; Ballet, S.; Schiller, P.W.; Lubell, W.D. Solid-Phase Azopeptide Diels-Alder Chemistry for Aza-pipecolyl Residue Synthesis to Study Peptide Conformation. J. Org. Chem. 2019, 84, 6006–6016. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Beckmann, A.M.; Dudic, A.; Li, T.; Sellier, R.; Bartz, U.; Gütschow, M. 3-Cyano-3-aza-β-aminoAcid Derivativesas Inhibitors of Human Cysteine Cathepsins. ACS Med. Chem. Lett. 2014, 5, 1076. [Google Scholar] [CrossRef]
- Miyata, K.; Narita, A.; Fujisawa, R.; Roppongi, M.; Ito, S.; Shingo, T.; Oba, T. Synthesis of boronophenylalanine-like aza-amino acids for boron-containing azapeptide precursors. Tetrahedron Lett. 2020, 61, 152585. [Google Scholar] [CrossRef]
- Boeglin, D.; Xiang, Z.; Sorenson, N.B.; Wood, M.S.; Haskell-Luevano, C.; Lubell, W.D. Aza-scanning of the potent melanocortin receptor agonist Ac-His-D-Phe-Arg-Trp-NH. Chem. Biol. Drug Des. 2006, 67, 275–283. [Google Scholar] [CrossRef]
- Chingle, R.; Proulx, C.; Lubell, W.D. Azapeptide Synthesis Methods for Expanding Side-Chain Diversity for Biomedical Applications. Acc. Chem. Res. 2017, 50, 1541–1556. [Google Scholar] [CrossRef]
- Zhang, Y.; Malamakal, R.M.; Chenoweth, D.M. Aza-Glycine Induces Collagen Hyperstability. J. Am. Chem. Soc. 2015, 137, 12422–12425. [Google Scholar] [CrossRef]
- Mcmechen, M.A.; Willis, E.L.; Gourville, P.C.; Proulx, C.; Mosberg, H.; Sawyer, T.; Haskell-Luevano, C. Aza-Amino Acids Disrupt β-Sheet Secondary Structures. Molecules 2019, 24, 1919. [Google Scholar] [CrossRef]
- Kasznel, A.J.; Harris, T.; Porter, N.J.; Zhang, Y.; Chenoweth, D.M. Aza-proline effectively mimics L-proline stereochemistry in triple helical collagen. Chem. Sci. 2019, 10, 6979–6983. [Google Scholar] [CrossRef]
- Galibert, M.; Wartenberg, M.; Lecaille, F.; Saidi, A.; Mavel, S.; Joulin-Giet, A.; Korkmaz, B.; Brömme, D.; Aucagne, V.; Delmas, A.F.; et al. Substrate-derived triazolo- and azapeptides as inhibitors of cathepsins K and S. Eur. J. Med. Chem. 2018, 144, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Melton, S.D.; Brackhahn, E.A.E.; Orlin, S.J.; Jin, P.; Chenoweth, D.M. Rules for the design of aza-glycine stabilized triple-helical collagen peptides. Chem. Sci. 2020, 11, 10638–10646. [Google Scholar] [CrossRef] [PubMed]
- Traoré, M.; Gignac, M.; Doan, N.D.; Hof, F.; Lubell, W.D. Aza-amino acid scanning of chromobox homolog 7 (CBX7) ligands. J. Pept. Sci. 2017, 23, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Ahsanullah; Chingle, R.; Ohm, R.G.; Chauhan, P.S.; Lubell, W.D. Aza-propargylglycine installation by aza-amino acylation: Synthesis and Ala-scan of an azacyclopeptide CD36 modulator. Pept. Sci. 2019, 111, e24102. [Google Scholar] [CrossRef]
- André, F.; Marraud, M.; Tsouloufis, T.; Tzartos, S.J.; Boussard, G. Triphosgene: An efficient carbonylating agent for liquid and solid-phase aza-peptide synthesis. Application to the synthesis of two aza-analogues of the AChR MIR decapeptide. J. Pept. Sci. 1997, 3, 429–441. [Google Scholar] [CrossRef]
- Melton, S.D.; Smith, M.S.; Chenoweth, D.M. Incorporation of Aza-Glycine into Collagen Peptides. J. Org. Chem. 2020, 85, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shrivastava, R.; Singh, G.; Ali, R.; Sankar Ampapathi, R.; Bhadhuria, S.; Haq, W. AzaGly-Appended Peptidomimetics Structurally Related to PTR6154 as Potential PKB/Akt Inhibitors. Chembiochem 2017, 18, 1061–1065. [Google Scholar] [CrossRef]
- Ahn, I.A.; Kim, S.W.; Ro, S. Solid phase synthesis of azapeptides using an automatic synthesizer. Mol. Divers. 1998, 4, 23–24. [Google Scholar] [CrossRef]
- Jȩdrzejewska, H.; Szumna, A. Peptide-based capsules with chirality-controlled functionalized interiors—Rational design and amplification from dynamic combinatorial libraries. Chem. Sci. 2019, 10, 4412–4421. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.S.; Morley, J.S. Polypeptides. Part XIII. Preparation of α-aza-amino-acid (carbazic acid) derivatives and intermediates for the preparation of α-aza-peptides. J. Chem. Soc. Perkin Trans. 1 1975, 1, 1712–1720. [Google Scholar] [CrossRef]
- Melendez, R.E.; Lubell, W.D. Aza-amino acid scan for rapid identification of secondary structure based on the application of N-Boc-aza1-dipeptides in peptide synthesis. J. Am. Chem. Soc. 2004, 126, 6759–6764. [Google Scholar] [CrossRef] [PubMed]
- Frochot, C.; Vanderesse, R.; Driou, A.; Linden, G.; Marraud, M.; Cung, M.T. A solid-phase synthesis of three aza-, iminoaza- and reduced aza-peptides from the same precursor. Lett. Pept. Sci. 1997, 4, 219–225. [Google Scholar] [CrossRef]
- Boeglin, D.; Lubell, W.D. Aza-amino acid scanning of secondary structure suited for solid-phase peptide synthesis with Fmoc chemistry and aza-amino acids with heteroatomic side chains. J. Comb. Chem. 2005, 7, 864–878. [Google Scholar] [CrossRef]
- Freeman, N.S.; Hurevich, M.; Gilon, C. Synthesis of N’-substituted Ddz-protected hydrazines and their application in solid phase synthesis of aza-peptides. Tetrahedron 2009, 65, 1737–1745. [Google Scholar] [CrossRef]
- Bourguet, C.B.; Sabatino, D.; Lubell, W.D. Benzophenone semicarbazone protection strategy for synthesis of aza-glycine containing aza-peptides. Biopolymers 2008, 90, 824–831. [Google Scholar] [CrossRef]
- Zhang, J.; Mulumba, M.; Ong, H.; Lubell, W.D. Diversity-Oriented Synthesis of Cyclic Azapeptides by A3-Macrocyclization Provides High-Affinity CD36-Modulating Peptidomimetics. Angew. Chemie Int. Ed. 2017, 56, 6284–6288. [Google Scholar] [CrossRef]
- Sabatino, D.; Proulx, C.; Klocek, S.; Bourguet, C.B.; Boeglin, D.; Ong, H.; Lubell, W.D. Exploring side-chain diversity by Submonomer solid-phase aza-peptide synthesis. Org. Lett. 2009, 11, 3650–3653. [Google Scholar] [CrossRef]
- Garcia-Ramos, Y.; Lubell, W.D. Synthesis and alkylation of aza-glycinyl dipeptide building blocks. J. Pept. Sci. 2013, 19, 725–729. [Google Scholar] [CrossRef]
- Chignen Possi, K.; Mulumba, M.; Omri, S.; Garcia-Ramos, Y.; Tahiri, H.; Chemtob, S.; Ong, H.; Lubell, W.D. Influences of Histidine-1 and Azaphenylalanine-4 on the Affinity, Anti-inflammatory, and Antiangiogenic Activities of Azapeptide Cluster of Differentiation 36 Receptor Modulators. J. Med. Chem. 2017, 60, 9263–9274. [Google Scholar] [CrossRef]
- Dai, C.; Ma, J.; Li, M.; Wu, W.; Xia, X.; Zhang, J. Diversity-oriented submonomer synthesis of azapeptides mediated by the Mitsunobu reaction. Org. Chem. Front. 2019, 6, 2529–2533. [Google Scholar] [CrossRef]
- Doan, N.D.; Zhang, J.; Traoré, M.; Kamdem, W.; Lubell, W.D. Solid-phase synthesis of C-terminal azapeptides. J. Pept. Sci. 2015, 21, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Bánóczi, Z.; Tantos, Á.; Farkas, A.; Majer, Z.; Dókus, L.E.; Tompa, P.; Hudecz, F. New m-calpain substrate-based azapeptide inhibitors. J. Pept. Sci. 2013, 19, 370–376. [Google Scholar] [CrossRef]
- Arujõe, M.; Ploom, A.; Mastitski, A.; Järv, J. Comparison of various coupling reagents in solid-phase aza-peptide synthesis. Tetrahedron Lett. 2017, 58, 3421–3425. [Google Scholar] [CrossRef]
- Arujõe, M.; Ploom, A.; Mastitski, A.; Järv, J. Influence of steric effects in solid-phase aza-peptide synthesis. Tetrahedron Lett. 2018, 59, 2010–2013. [Google Scholar] [CrossRef]
- Troska, A.; Arujõe, M.; Mastitski, A.; Järv, J.; Ploom, A. Steric impact of aza-amino acid on solid-phase aza-peptide bond synthesis. Tetrahedron Lett. 2021, 69, 152973. [Google Scholar] [CrossRef]
- André, F.; Vicherat, A.; Boussard, G.; Aubry, A.; Marraud, M. Aza-peptides. III. Experimental structural analysis of aza-alanine and aza-asparagine-containing peptides. J. Pept. Res. 1997, 50, 372–381. [Google Scholar] [CrossRef]
- Benatalah, Z.; Aubry, A.; Boussard, G.; Marraud, M. Evidence for a β-turn in an azadipeptide sequence. Int. J. Pept. Protein Res. 1991, 38, 603–605. [Google Scholar] [CrossRef]
- Adhikary, R.; Zimmermann, J.; Liu, J.; Forrest, R.P.; Janicki, T.D.; Dawson, P.E.; Corcelli, S.A.; Romesberg, F.E. Evidence of an Unusual N–H···N Hydrogen Bond in Proteins. J. Am. Chem. Soc. 2014, 136, 13474–13477. [Google Scholar] [CrossRef]
- Baruah, K.; Sahariah, B.; Sakpal, S.S.; Deka, J.K.R.; Bar, A.K.; Bagchi, S.; Sarma, B.K. Stabilization of Azapeptides by N amide ···H–N amide Hydrogen Bonds. Org. Lett. 2021, 23, 4949–4954. [Google Scholar] [CrossRef]
- Lee, H.J.; Ahn, I.A.; Ro, S.; Choi, K.H.; Choi, Y.S.; Lee, K.B. Role of azaamino acid residue in beta-turn formation and stability in designed peptide. J. Pept. Res. 2000, 56, 35–46. [Google Scholar] [CrossRef]
- El Khabchi, M.; Lahlou, H.; El Adnani, Z.; Mcharfi, M.; Benzakour, M.; Fitri, A.; Benjelloun, A.T. Conformational preferences of Ac-Pro-azaXaa-NHMe (Xaa = Asn, Asp, Ala) and the effect of intramolecular hydrogen bonds on their stability in gas phase and solution. J. Mol. Model. 2021, 27, 368. [Google Scholar] [CrossRef]
- Zhang, Y.; Herling, M.; Chenoweth, D.M. General Solution for Stabilizing Triple Helical Collagen. J. Am. Chem. Soc. 2016, 138, 9751–9754. [Google Scholar] [CrossRef]
- Zhang, Y.; Malamakal, R.M.; Chenoweth, D.M. A Single Stereodynamic Center Modulates the Rate of Self-Assembly in a Biomolecular System. Angew. Chem. Int. Ed. Engl. 2015, 54, 10826–10832. [Google Scholar] [CrossRef]
- Harris, T.; Chenoweth, D.M. Sterics and Stereoelectronics in Aza-Glycine: Impact of Aza-Glycine Preorganization in Triple Helical Collagen. J. Am. Chem. Soc. 2019, 141, 18021–18029. [Google Scholar] [CrossRef]
- Zhou, Z.; Deng, C.; Abbas, C.; Didierjean, C.; Averlant-Petit, M.-C.; Bodiguel, J.; Vanderesse, R.; Jamart-Grégoire, B. Synthesis and Structural Characterization of 2:1 [α/Aza]-oligomers. Eur. J. Org. Chem. 2014, 2014, 7643–7650. [Google Scholar] [CrossRef]
- Ibrahim, M.I.A.; Zhou, Z.; Deng, C.; Didierjean, C.; Vanderesse, R.; Bodiguel, J.; Averlant-Petit, M.C.; Jamart-Grégoire, B. Impact of Cα-Chirality on Supramolecular Self-Assembly in Cyclo-2:1-[α/aza]-Hexamers (d/l-Phe-azaPhe-Ala)2. Europ. J. Org. Chem. 2017, 2017, 4703–4712. [Google Scholar] [CrossRef]
- Tonali, N.; Correia, I.; Lesma, J.; Bernadat, G.; Ongeri, S.; Lequin, O. Introducing sequential aza-amino acids units induces repeated β-turns and helical conformations in peptides. Org. Biomol. Chem. 2020, 18, 3452–3458. [Google Scholar] [CrossRef]
- Danelius, E.; Ohm, R.G.; Ahsanullah; Mulumba, M.; Ong, H.; Chemtob, S.; Erdelyi, M.; Lubell, W.D. Dynamic Chirality in the Mechanism of Action of Allosteric CD36 Modulators of Macrophage-Driven Inflammation. J. Med. Chem. 2019, 62, 11071–11079. [Google Scholar] [CrossRef]
- Wieczerzak, E.; Jankowska, E.; Rodziewicz-Motowidło, S.; Giełdoń, A.; Ła̧Giewka, J.; Grzonka, Z.; Abrahamson, M.; Grubb, A.; Brömme, D. Novel azapeptide inhibitors of cathepsins B and K. Structural background to increased specificity for cathepsin B. J. Pept. Res. 2005, 66, 1–11. [Google Scholar] [CrossRef]
- Corrigan, T.S.; Lotti Diaz, L.M.; Border, S.E.; Ratigan, S.C.; Kasper, K.Q.; Sojka, D.; Fajtova, P.; Caffrey, C.R.; Salvesen, G.S.; McElroy, C.A.; et al. Design, synthesis, and in vitro evaluation of aza-peptide aldehydes and ketones as novel and selective protease inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 1387–1402. [Google Scholar] [CrossRef]
- Löser, R.; Frizler, M.; Schilling, K.; Gütschow, M. Azadipeptide Nitriles: Highly Potent and Proteolytically Stable Inhibitors of Papain-Like Cysteine Proteases. Angew. Chem. Int. Ed. 2008, 47, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Jílková, A.; Horn, M.; Fanfrlík, J.; Küppers, J.; Pachl, P.; Řezáčová, P.; Lepšík, M.; Fajtová, P.; Rubešová, P.; Chanová, M.; et al. Azanitrile Inhibitors of the SmCB1 Protease Target Are Lethal to Schistosoma mansoni: Structural and Mechanistic Insights into Chemotype Reactivity. ACS Infect. Dis. 2021, 7, 189–201. [Google Scholar] [CrossRef]
- Breidenbach, J.; Lemke, C.; Pillaiyar, T.; Schäkel, L.; Al Hamwi, G.; Diett, M.; Gedschold, R.; Geiger, N.; Lopez, V.; Mirza, S.; et al. Targeting the Main Protease of SARS-CoV-2: From the Establishment of High Throughput Screening to the Design of Tailored Inhibitors. Angew. Chem. Int. Ed. 2021, 60, 10423–10429. [Google Scholar] [CrossRef]
- Freeman, N.S.; Tal-Gan, Y.; Klein, S.; Levitzki, A.; Gilon, C. Microwave-Assisted Solid-Phase Aza-peptide Synthesis: Aza Scan of a PKB/Akt Inhibitor Using Aza-arginine and Aza-proline Precursors. J. Org. Chem. 2011, 76, 3078–3085. [Google Scholar] [CrossRef]
- Proulx, C.; Picard, É.; Boeglin, D.; Pohankova, P.; Chemtob, S.; Ong, H.; Lubell, W.D. Azapeptide analogues of the growth hormone releasing peptide 6 as cluster of differentiation 36 receptor ligands with reduced affinity for the growth hormone secretagogue receptor 1a. J. Med. Chem. 2012, 55, 6502–6511. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.; Mas-Moruno, C.; Kessler, H.; Lubell, W.D. Cyclic aza-peptide integrin ligand synthesis and biological activity. J. Org. Chem. 2012, 77, 5271–5278. [Google Scholar] [CrossRef]
- Frizler, M.; Lohr, F.; Furtmann, N.; Kläs, J.; Gütschow, M. Structural optimization of azadipeptide nitriles strongly increases association rates and allows the development of selective cathepsin inhibitors. J. Med. Chem. 2011, 54, 396–400. [Google Scholar] [CrossRef]
- Litman, P.; Ohne, O.; Ben-Yaakov, S.; Shemesh-Darvish, L.; Yechezkel, T.; Salitra, Y.; Rubnov, S.; Cohen, I.; Senderowitz, H.; Kidron, D.; et al. A Novel Substrate Mimetic Inhibitor of PKB/Akt Inhibits Prostate Cancer Tumor Growth in Mice by Blocking the PKB Pathway. Biochemistry 2007, 46, 4716–4724. [Google Scholar] [CrossRef]
- Hiromura, M.; Okada, F.; Obata, T.; Auguin, D.; Shibata, T.; Roumestand, C.; Noguchi, M. Inhibition of Akt Kinase Activity by a Peptide Spanning the βA Strand of the Proto-oncogene TCL1. J. Biol. Chem. 2004, 279, 53407–53418. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Tikhonova, I.G.; Martin, L.L.; Walker, B. Smac-derived Aza-peptide As an Aminopeptidase-resistant XIAP BIR3 Antagonist. Protein Pept. Lett. 2015, 22, 836–843. [Google Scholar] [CrossRef]
- Randolph, J.T.; Zhang, X.; Huang, P.P.; Klein, L.L.; Kurtz, K.A.; Konstantinidis, A.K.; He, W.; Kati, W.M.; Kempf, D.J. Synthesis, antiviral activity, and conformational studies of a P3 aza-peptide analog of a potent macrocyclic tripeptide HCV protease inhibitor. Bioorg. Med. Chem. Lett. 2008, 18, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2022, 118, 115–129. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Angin, Y.; Steinbusch, L.K.M.; Schwenk, R.W.; Luiken, J.J.F.P. CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 71–77. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Guillen Nieto, G.; Lopez-Mola, E.; Herrera-Martinez, L. Growth hormone releasing peptide-6 (GHRP-6) and other related secretagogue synthetic peptides: A mine of medical potentialities for unmet medical needs. Integr. Mol. Med. 2016, 3, 616–623. [Google Scholar] [CrossRef]
- Proulx, C.; Zhang, J.; Sabatino, D.; Chemtob, S.; Ong, H.; Lubell, W.D. Synthesis and Biomedical Potential of Azapeptide Modulators of the Cluster of Differentiation 36 Receptor (CD36). Biomedicines 2020, 8, 241. [Google Scholar] [CrossRef]
- Huynh, D.N.; Bessi, V.L.; Menard, L.; Piquereau, J.; Proul, C.; Febbraio, M.; Lubell, W.D.; Carpentier, A.C.; Burelle, Y.; Ong, H.; et al. Adiponectin has a pivotal role in the cardioprotective effect of CP-3(iv), a selective CD36 azapeptide ligand, after transient coronary artery occlusion in mice. FASEB J. 2018, 32, 807–818. [Google Scholar] [CrossRef]
- Frégeau, G.; Sarduy, R.; Elimam, H.; Esposito, C.L.; Mellal, K.; Ménard, L.; Leitão da Graça, S.D.; Proulx, C.; Zhang, J.; Febbraio, M.; et al. Atheroprotective and atheroregressive potential of azapeptide derivatives of GHRP-6 as selective CD36 ligands in apolipoprotein E-deficient mice. Atherosclerosis 2020, 307, 52–62. [Google Scholar] [CrossRef]
- Mellal, K.; Omri, S.; Mulumba, M.; Tahiri, H.; Fortin, C.; Dorion, M.F.; Pham, H.; Garcia Ramos, Y.; Zhang, J.; Pundir, S.; et al. Immunometabolic modulation of retinal inflammation by CD36 ligand. Sci. Rep. 2019, 9, 12903. [Google Scholar] [CrossRef]
- Ohm, R.G.; Mulumba, M.; Chingle, R.M.; Ahsanullah; Zhang, J.; Chemtob, S.; Ong, H.; Lubell, W.D. Diversity-Oriented A 3-Macrocyclization for Studying Influences of Ring-Size and Shape of Cyclic Peptides: CD36 Receptor Modulators. J. Med. Chem. 2021, 64, 9365–9380. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).