Abstract
Vaccination against SARS-CoV-2 is an important and essential strategy to combat the 2019 coronavirus disease (COVID-19) pandemic. Vaccination has shown to be effective in reducing the spread of SARS-CoV-2, reducing the chances of becoming infected and developing severe COVID-19, and reducing hospitalization and mortality rates. However, the vaccinations against SARS-CoV-2 are accompanied by undesirable side effects which may be in part responsible for a reduction in the willingness to become vaccinated. At this moment (June 2022), 24.3% of the US adult population (18+ years old) is not fully vaccinated against SARS-CoV-2, and 49.5% did not receive their follow-up booster vaccination. The most important motives for refusing vaccination are the unknown long-term side effects and the known acute side effects of vaccination. Here, we discuss the importance of recognizing the impact of this reactogenicity on individuals’ willingness to vaccinate and how the development of effective and safe medicines that prevent or mitigate the unwanted side effects of the vaccination may help to increase the willingness to vaccinate.
1. Vaccination against SARS-CoV-2
The 2019 coronavirus disease (COVID-19) has resulted in more than 6.31 million deaths worldwide and more than 1.01 million deaths in the United States alone [1]. An essential step to ending this pandemic is vaccination against SARS-CoV-2. The vaccines that are currently approved by the US Food and Drug Administration (FDA) include Spikevax (Moderna), Comirnaty (Pfizer), and the Janssen COVID-19 Vaccine (Janssen) [2,3,4]. The aim of vaccination against SARS-CoV-2 is to prevent the development of (severe) COVID-19, thereby reducing the hospitalization and mortality rates. Dye [5] evaluated the effectivity of vaccination among adults, 18+ years old, who had received at least one dose of vaccine. In this study, the COVID-19 incidence and mortality rates in countries with very low (0–9%), low (10–39%), medium (40–69%), and high (>70%) vaccination levels were compared. During the primary half of 2021, when the Alpha variant of SARS-CoV-2 was dominant, the COVID-19 mortality rate was reduced by 60%, 75%, and 81% in countries with low, medium, and high vaccination coverage, compared with countries that had a very low vaccination level. The reductions in new COVID-19 cases were 57%, 70%, and 80%, respectively. The impact of mortality was similar during the second half of 2021, when the Delta variant of SARS-CoV-2 became dominant in the US, with smaller effects, however, on incidence [6]. Next to the protection developed through a previous infection with SARS-CoV-2, vaccination is an important tool to achieve herd immunity, i.e., a sufficient level of immunity among the population to prevent the further spread of the virus. A German study reported that with the SARS-CoV-2 Omicron variant, more than 90% of the population needed to be vaccinated in order to achieve herd immunity [7].
2. Willingness to Become Vaccinated
The effectiveness of vaccination programs depends on the willingness of individuals to become vaccinated. However, doubts and worries can negatively influence this willingness and may lead to vaccine hesitancy and/or even rejection [8]. In the case of the COVID-19 vaccines, these worries may concern either unknown long-term side effects or known potential acute side effects. The concerns are further fueled by the fact that Spikevax and Comirnaty use the relative new messenger RNA technology, which is largely unknown to the general public, and the notion that the FDA has only approved emergency-use authorization to use these vaccines after an exceptionally short development phase.
Indeed, the data of the US Centers of Disease Control and Prevention reveal a decrease in the percentages of vaccination among the US population while the COVID-19 pandemic progresses [9], with only 50.5% of the adult population (18+ years old) having received a booster vaccination. At present (June 2022), 24.3% of the US adult population (18+ years old) is not fully vaccinated against SARS-CoV-2, and 49.5% did not receive a booster (see Figure 1).
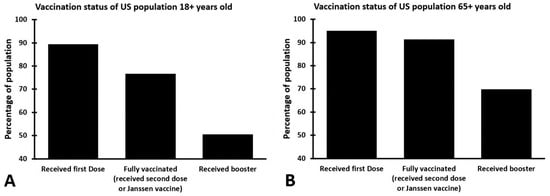
Figure 1.
Percentages of the US population that received COVID-19 vaccinations. Shown are the percentages of those who are 18+ years old (A) and those who are 65+ years old (B) that received one or more COVID-19 vaccinations (reported on 12 June 2022). The data are from reference [9].
As defined in 2015 by the SAGE Working Group on Vaccine Hesitancy, vaccine hesitancy refers to a delay in acceptance or a refusal of vaccination despite the availability of vaccination services [10]. The 3C model proposed by the working group comprises complacency (perceiving the consequences of COVID-19 infection as low and therefore considering vaccination as an unnecessary preventive action), convenience (the availability and affordability of a vaccine), and confidence (e.g., trust in vaccine safety and those that deliver them) as key factors determining vaccine hesitancy. Indeed, in relation to vaccination against SARS-CoV-2, a recent study revealed that lack of vaccine confidence and complacency explained 38% and 21% of the variance in vaccine hesitancy [11]
A frequently reported reason for the resistance and fear against vaccination is the risk of experiencing side effects [12,13]. A representative American study revealed that 90% of those who reject vaccination fear possible side effects from the vaccine more than they fear COVID-19 itself [14]. In addition, European and US studies showed that fear of side effects was an important motive in refraining from becoming vaccinated [15,16,17].
3. Side Effects of Vaccination
The majority of vaccinated individuals will experience one or more undesirable side effects within the first week after vaccination. When evaluating the side effects experienced in the first seven days after vaccination with Spikevax, Comirnaty, and the Janssen COVID-19 Vaccine, as described in the prescribing information [2,3,4], it is evident that both local and systemic side effects are frequently experienced, including pain (up to 89.9%), fatigue (up to 67.8%), headache (up to 63.0%), myalgia (up to 61.7%), and arthralgia (up to 56.6%). These side effects may have a negative effect on wellbeing and daily functioning (e.g., job performance or driving) and thus on quality of life. Therefore, an effective treatment that prevents or reduces these side effects would be beneficial to those who receive a vaccination. In addition, such a treatment may convince those that previously rejected a vaccination to reconsider their decision.
4. Treatment or Prevention of Vaccination Side Effects
Individuals that will have a vaccination are usually informed that possible transient side effects disappear within a couple of days. In the US, the Immunization Action Coalition (IAC) advises vaccinated people who experience side the effects of vaccination to apply a cold compress to the injection site to ease local side effects such as pain and swelling [18]. Vaccinated individuals could also consider taking an OTC pain reliever to reduce pain at the injection site or headache or an antipruritic drug to reduce itch. There is currently no compelling evidence that antipyretic or analgesic medications have a relevant negative impact on vaccine efficacy [19,20].
Although there are existing treatments used against some of the side effects (e.g., painkillers), it would be advisable to have more effective treatments that are effective in reducing or preventing all side effects. Currently, the FDA has not approved any medication specifically for the purpose of reducing or preventing vaccination side effects. However, there is a clear need among vaccinated individuals for effective treatments for the side effects of vaccination. The studies described in the prescribing information of Spikevax, Comirnaty, and the Janssen COVID-19 Vaccine illustrate this need, as these reported the use of antipyretic and/or pain medication by up to 57.3% of the individuals that were vaccinated (see Figure 2).
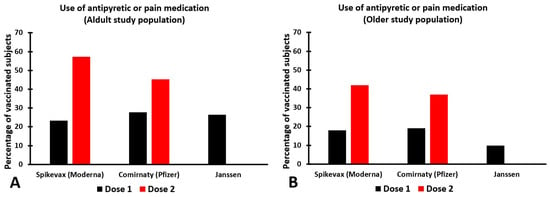
Figure 2.
The use of antipyretic or pain medication to treat SARS-CoV-2 vaccination side effects. The use of antipyretic or pain medication is within the first seven days after vaccination for adults (A) and older subjects (B). Note: across the studies, different age ranges were used for the samples of adult subjects (Spikevax: 18–64 years old; Comirnaty: 16–55 years old; Janssen: 18–59 years old) and elderly subjects (Spikevax: 65+ years old; Comirnaty: 56+ years old; Janssen: 60+ years old). The data are from the references [2,3,4].
Side effects of vaccination occur because the vaccine has triggered an immune response against SARS-CoV-2. Interestingly, the number of reported side effects is higher after the second dose [2,3,4]. This is likely due to the fact that the body recognizes the vaccine from the first vaccination and then produces a more robust immune response after the second vaccination. In line with this, the percentage of people that used antipyretic or pain medication after vaccination was much higher after the second dose than the first dose [2,3,4]. Surprisingly, there is no scientific data published showing to what extent the use of antipyretic or pain medication was actually effective in reducing or preventing vaccination side effects.
Currently, in the US there is no treatment registered for the prevention or reduction of vaccination side effects. However, a new drug, SJP-003, is being developed to prevent or reduce vaccination side effects [21]. SJP-003 is a combination of a non-steroid anti-inflammatory drug, NSAID (naproxen), and an antihistamine drug (fexofenadine). Both drugs are available over the counter (OTC) and have a long track record of safety and efficacy. Recent research showed that the combination of NSAID (indomethacin) and an antihistamine drug (ketotifen) had synergistic antiviral effects against SARS-CoV-2 [22]. It is therefore believed that SJP-003 may also be more effective in preventing or reducing the side effects of vaccination than when the drugs are taken alone. Case reports have been published suggesting the efficacy of SJP-003 in preventing and reducing side effects after receiving an influenza vaccination [21] and after receiving multiple travel vaccinations [23]. Future double-blind, placebo-controlled clinical trials should examine the efficacy of SJP-003 in the prevention of side effects associated with vaccination against SARS-CoV-2.
5. Discussion
Immune fitness, i.e., the inbuilt capacity to adapt to external health challenges by establishing, maintaining, and regulating an appropriate immune response, is a key factor in health and disease [24]. Recent publications revealed that in terms of pandemic preparedness, maintaining an adequate immune fitness is essential to reducing the presence and severity of SARS-CoV-2 symptoms [25,26], and adequate immune fitness may reduce the risk of becoming infected with SARS-CoV-2. Vaccination is another important strategy for reducing the number SARS-CoV-2 infections, hospitalizations, and mortality rates due to COVID-19. To achieve herd immunity, it is important that vaccination rates are high. Unfortunately, COVID-19 vaccinations have some potentially acute side effects that may reduce the willingness to become vaccinated, and there are currently no treatments approved by the FDA to reduce or prevent vaccination side effects. Although the use of antipyretic or pain medication to reduce vaccination side effects is common (see Figure 2), the reported data do not reveal to what extent the used medication was effective [2,3,4].
The availability of an effective and safe drug to prevent or minimize vaccination side effects would have several advantages. First of all, the individuals that receive a vaccination could benefit from such a medication by experiencing less or no vaccination side effects. This will increase the likelihood that they will also take follow-up boosters and other vaccinations. Secondly, individuals that currently hesitate to become vaccinated due to the fear of vaccination side effects might reconsider their decision. As such, an effective medicine could increase the willingness to become vaccinated among the general public. Preferably, such a medicine should be available OTC. If no prescription is needed, the time and effort of obtaining a safe and effective therapeutic would not pose a burden on the healthcare system. OTC drugs have a long-standing and well-known safety record, which will further enhance the willingness to use these drugs.
In conclusion, the availability of an effective and safe medicine that prevents acute vaccination side effects will ease the vaccination experience of those who become vaccinated and could increase the willingness to become vaccinated among those who now hesitate to become vaccinated.
Author Contributions
Conceptualization, P.K., J.B., J.G. and J.C.V.; writing—original draft preparation, P.K., J.B. and J.C.V.; writing—review and editing, P.K., J.B., J.G. and J.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Over the past 3 years, J.C.V. has acted as a consultant/advisor for KNMP, Mentis, Red Bull, Sen-Jam Pharmaceutical, and Toast! J.G. is a part-time employee of Nutricia Research and has received research grants from the Nutricia research foundation, Top Institute Pharma, Top Institute Food and Nutrition, GSK, STW, NWO, Friesland Campina, CCC, Raak-Pro, and EU. The other authors have no potential conflicts of interest to disclose.
References
- Our World in Data. Available online: https://ourworldindata.org/explorers/coronavirus-data-explorer (accessed on 12 June 2022).
- Prescribing Information Spikevax. Available online: https://www.fda.gov/media/155675/download (accessed on 12 June 2022).
- Prescribing Information Comirnaty. Available online: https://www.fda.gov/media/154834/download (accessed on 12 June 2022).
- Prescribing Information Janssen COVID-19 Vaccine. Available online: https://www.fda.gov/media/146304/download (accessed on 12 June 2022).
- Dye, C. The benefits of large scale COVID-19 vaccination. BMJ 2022, 377, o867. [Google Scholar] [CrossRef] [PubMed]
- Suthar, A.B.; Wang, J.; Seffren, V.; Wiegand, R.E.; Griffing, S.; Zell, E. Public health impact of COVID-19 vaccines in the US: Observational study. BMJ 2022, 377, e069317. [Google Scholar] [CrossRef] [PubMed]
- Eichner, M. Bericht über Die Erstellung Einer Strukturierten Literaturrecherche zur Rolle der Geimpften am SARS-CoV-2-Pandemiegeschehen; Epimos GmbH, Ed.; Ministerium für Soziales, Gesundheit und Integration Baden-Württemberg: Stuttgart, Germany, 2021. [Google Scholar]
- Siciliani, L.; Wild, C.; McKee, M.; Kringos, D.; Barry, M.M.; Barros, P.P.; De Maeseneer, J.; Murauskiene, L.; Ricciardi, W.; members of the Expert Panel on Effective Ways of Investing in Health. Strengthening vaccination programmes and health systems in the European Union: A framework for action. Health Policy 2020, 124, 511–518. [Google Scholar] [CrossRef] [PubMed]
- COVID Data Tracker. COVID-19 Vaccinations in the United States. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total (accessed on 12 June 2022).
- MacDonald, N.E.; the SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Gerretsen, P.; Kim, J.; Caravaggio, F.; Quilty, L.; Sanches, M.; Wells, S.; Brown, E.E.; Agic, B.; Pollock, B.G.; Graff-Guerrero, A. Individual determinants of COVID-19 vaccine hesitancy. PLoS ONE 2021, 16, e0258462. [Google Scholar] [CrossRef] [PubMed]
- Moving the Needle: Promoting Vaccination Uptake across the Life Course. Available online: https://www.rsph.org.uk/our-work/policy/vaccinations/moving-the-needle-promoting-vaccination-uptake-across-the-life-course.html (accessed on 23 March 2022).
- Fournet, N.; Mollema, L.; Ruijs, W.L.; Harmsen, I.A.; Keck, F.; Durand, J.Y.; Cunha, M.P.; Wamsiedel, M.; Reis, R.; French, J.; et al. Under-vaccinated groups in Europe and their beliefs, attitudes and reasons for non-vaccination; two systematic reviews. BMC Public Health 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- The Economist/YouGov poll. July 10–13, 2021. Available online: https://docs.cdn.yougov.com/w2zmwpzsq0/econTabReport.pdf (accessed on 23 March 2022).
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes toward a potential SARS-CoV-2 vaccine: A survey of U.S. adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Böhme, S.; Varghese, N.E.; Sabat, I.; Barros, P.P.; Brouwer, W.; van Exel, J.; Schreyögg, J.; Stargardt, T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur. J. Health Econ. 2020, 21, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and receptivity for COVID-19 vaccines: A rapid systematic review. Vaccines 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- The Immunization Action Coalition (IAC). Medical Management of Vaccine Reactions in Adults in a Community Setting. Available online: https://www.immunize.org/catg.d/p3082.pdf (accessed on 12 June 2022).
- Saleh, E.; Moody, M.A.; Walter, E.B. Effect of antipyretic analgesics on immune responses to vaccination. Hum. Vaccin. Immunother. 2016, 12, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.E.; Dhar, A.; Petruschke, R.; Locht, C.; Buchy, P.; Low, J.G. Use of analgesics/antipyretics in the management of symptoms associated with COVID-19 vaccination. NPJ Vaccines 2022, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Iversen, J.; Dahl, T.A. Methods and Compositions to Inhibit Adverse Effects Associated with Vaccinations. U.S. Patent 10,874,653 B2, 29 December 2020. Available online: https://patents.google.com/patent/US10874653B2/en?oq=10874653 (accessed on 12 June 2022).
- Kiani, P.; Scholey, A.; Dahl, T.A.; McMann, L.; Iversen, J.M.; Verster, J.C. In-vitro assessment of the antiviral activity of ketotifen, indomethacin and naproxen, alone and in combination, against SARS-CoV-2. Viruses 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Kiani, P.; Iversen, J.; Scholey, A.; Verster, J.C. The efficacy of the combination of naproxen and fexofenadine (SJP-003) to prevent or reduce side effects of receiving multiple travel vaccines: A case report. Vaccines 2022, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Wilod Versprille, L.J.F.; van de Loo, A.J.A.E.; Mackus, M.; Arnoldy, L.; Sulzer, T.A.L.; Vermeulen, S.A.; Abdulahad, S.; Huls, H.; Baars, T.; Scholey, A.; et al. Development and validation of the Immune Status Questionnaire (ISQ). Int. J. Environ. Res. Public. Health 2019, 16, 4743. [Google Scholar] [CrossRef] [PubMed]
- Kiani, P.; Balikji, J.; Kraneveld, A.D.; Garssen, J.; Bruce, G.; Verster, J.C. Pandemic preparedness: The importance of adequate immune fitness. J. Clin. Med. 2022, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Kiani, P.; Mulder, K.E.W.; Balikji, J.; Kraneveld, A.D.; Garssen, J.; Verster, J.C. Pandemic preparedness: Maintaining adequate immune fitness by attaining a normal, healthy bodyweight. J. Clin. Med. 2022, 11, 3933. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).