Abstract
The usage of nanoparticles became inevitable in medicine and other fields when it was found that they could be administered to hosts to act as oxidants or antioxidants. These oxidative nanoparticles act as pro-oxidants and induce oxidative stress-mediated toxicity through the generation of free radicals. Some nanoparticles can act as antioxidants to scavenge these free radicals and help in maintaining normal metabolism. The oxidant and antioxidant properties of nanoparticles rely on various factors including size, shape, chemical composition, etc. These properties also help them to be taken up by cells and lead to further interaction with cell organelles/biological macromolecules, leading to either the prevention of oxidative damage, the creation of mitochondrial dysfunction, damage to genetic material, or cytotoxic effects. It is important to know the properties that make these nanoparticles act as oxidants/antioxidants and the mechanisms behind them. In this review, the roles and mechanisms of nanoparticles as oxidants and antioxidants are explained.
1. Introduction
Nanoparticles are defined as materials with an overall size between 1 and 100 nanometres [1,2,3]. Their size, chemical reactivity, energy absorption, biological mobility, strength, surface area, sensitivity, and stability make them distinctive from bulk materials. These nanoparticles have recently emerged as major players in contemporary medicine, serving as anything from gene carriers for targeted cell distribution to contrast agents in medical imaging [1,4]. Based on the materials they are made of, these nanomaterials can be broadly classified as a metallic and non-metallic nanoparticles. Metallic nanoparticles (MNPs) are the nanoparticles that comprise inorganic metal, metal oxide cores, or metal-associated particles. Some of the commonly known metallic nanoparticles are aluminium [5], gold [6], iron [7], copper [8], silver [9], cerium [10], manganese [11], zinc [12], titanium oxide [13], nickel [14], quantum dots [15], etc. [16]. Non-metallic nanoparticles include ceramic-based nanoparticles, carbon-based nanoparticles, silica-based nanoparticles, and biological macromolecule-derived nanoparticles [16,17]. These nanoparticles are used in various applications including in the treatment of wastewater, for example, in heavy metal removal, as antibacterial agents, antioxidant agents, anticancer agents, drug delivery self-oxidation, etc. [18,19,20,21,22,23,24,25]. In general, antioxidants are compounds that prevent molecules from oxidizing, which can result in the production of free radicals. Eventually, polymerization and other chain reactions will occur. A number of antioxidants, including glutathione, mycothiol, uric acid, bilirubin, albumin, bacillithiol, and superoxide dismutase, serve as protective mechanisms against oxidative stress. Antioxidants work to counter oxidants in some way. Natural or synthetic substances known as antioxidants can prevent or delay the cellular damage caused by oxidants such as reactive oxygen species (ROS), reactive nitrogen species (RNS), unstable ions and molecules, etc. [26,27]. ROS are free radicals comprising reactive oxygen ions and peroxides and are produced in the metabolism of any living system. ROS have harmful effects on biomolecules such as DNA, RNA, protein, and lipids, and cause most pathological diseases in humans [28]. As a result, excessive ROS production damages cells and tissues. When this ROS level increases, the weakening of antioxidant protection leads to oxidative stress [29] and damage to macromolecules including DNA, causing mutations and promoting tumour growth. Nanoparticles act as oxidants and tend to have a negative impact on living systems via oxidative stress. Some nanoparticles act as antioxidants to nullify the action of oxidants. An antioxidant molecule donates electrons to free radicals and neutralize them to limit the damage to the body [30]. Antioxidants are obtained through foods, and most known antioxidants are plant-derived products [31]. When nanoparticles are incorporated with these antioxidant metabolites, this enhances the antioxidant properties, and these nanoparticles are referred to as nano-antioxidants. Antioxidant properties have been reported in metal and metal oxide nanoparticles, carbon nanotubes, cerium oxide nanoparticles, gold nanoparticles, copper nanoparticles, and several forms of polymer-loaded antioxidant nanoparticles [32]. Some oxide nanoparticles have inherent physicochemical properties that allow them to scavenge reactive nitrogen and oxygen species and mimic an antioxidant molecule that has been shown to be effective in treating a variety of ailments brought on by oxidative stress [33,34,35,36].
From the previous paragraph, it is clear that nanoparticles can act as oxidants and antioxidants. In this review, the mechanisms of metal and non-metal nanoparticles as oxidants and their potential negative effects, as well as the mechanisms of antioxidant activity of nanoparticles and their advantages, have been discussed in detail.
2. Mechanism of Nanoparticles as Oxidants
The imbalance caused due to increased pro-oxidants and lessened antioxidants within the cell is referred to as cellular oxidative stress [37,38,39]. One of the most prevalent types of oxidative stress is the generation of reactive oxygen species, such as the superoxide radical (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (OH), nitric oxide (HNO), and peroxynitrite (NO3) [38]. The characteristics that contribute to the generation of nanoparticle-induced ROS are as follows: the active surface of the nanoparticle, the size of the nanoparticle, photoactivation, toxins, metal ion dissolution, and nanoparticle interactions with biomolecules (Figure 1). Nanotoxicity is heavily influenced by oxidative stress, as depicted in Figure 2 [37]. ROS production within cells after the cellular entry of nanoparticles (NP) and the intracellular release of nanoparticles leads to increased ROS levels in the mitochondria, decreased ATP levels causing a flux in the tricarboxylic acid (TCA) cycle, and lowered cardiolipin, one of the important phospholipids required for the functioning of mitochondria [40,41], thus leading to mitochondrial dysfunction. Important contributors to NP-induced ROS include the reactive surface of NP with pro-oxidant functional groups, surface redox activation on transition metal-based NPs, and interactions between particles and cells [42,43].
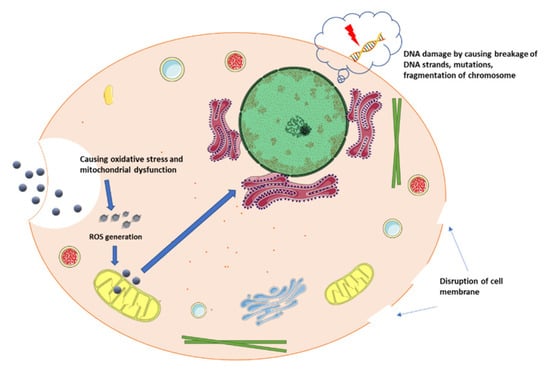
Figure 1.
Mechanism of nanoparticles as oxidants (inspired from Khanna et al. [37]).
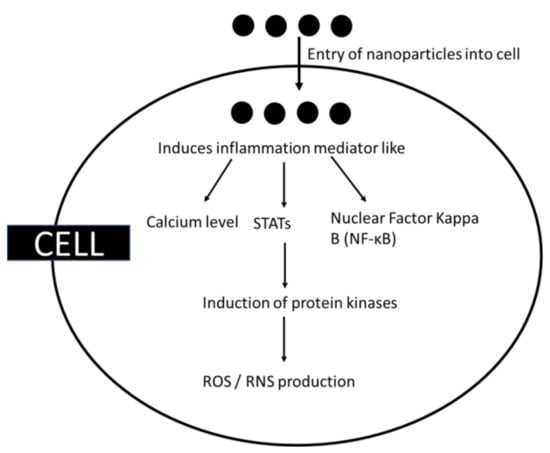
Figure 2.
ROS generation by metal nanoparticles.
Where positively charged nanoparticles interact electrostatically with negatively charged groups on the membrane surface, the plasma membrane is prone to rupture. By interacting with anionic and cations, gold nanoparticles with either a negative or positive charge modify the membrane’s surface charge, producing a net surface charge [44,45]. In the rupture of the endothelial cell membrane, the form of the nanoparticles is also crucial since needle-shaped nanoparticles are more likely to cause harm than sphere- or flat-shaped nanoparticles [46,47]. Nanoparticles are able to diffuse more easily at elevated temperatures since their surface-area-to-volume ratio is high. It is because of this property that nanoparticles can sinter at lower temperatures than larger particles [48]. The formation of reactive groups on the particle surface is caused by the structural/chemical imperfections of particles. When these reactive groups react with oxygen molecules, it leads to the production O2−, followed by the Fenton reaction, where more ROS is produced [49]. The Fenton reaction, or a Fenton-like reaction, is influenced by NPs, and involves the dissociation of metals ions from NPs. Dissociated metal ions can inhibit cellular enzymes, disrupt membrane structure, interfere with electron-shuttling processes, lower redox potential, lower mitochondrial membrane potential, and again increase intracellular ROS. Furthermore, NPs have been shown to generate intracellular ROS by disrupting electron transfer, raising the ratio of NADP+/NADPH, and interfering with mitochondrial function. The above processes yield superoxide anions, which can then combine with nitric oxide radical (NO) to form reactive nitrogen species (RNSs), such as peroxynitrite anion, nitrogen oxide radical, nitrate anion, carbonate anion, etc. These free radicals can kill cells by damaging proteins, lipids, and nucleic acids, and lead NP-induced genotoxicity, oncogenesis, multidrug resistance, aging, etc. [50,51,52,53,54,55]. Many studies have been conducted to prove that NPs could cause ROS-mediated toxicity in vitro and in vivo [56].
Ingestion, inhalation, and injection are the three basic routes through which nanoparticles may enter the body. Through inhalation, nanoparticles settle down on the mucous, causing inflammatory responses and oxidative stress in the lungs and damaging the epithelial cells, thus releasing interleukins responsible for inflammation. This causes diseases such as asthma, metal fume fever, fibrosis, carcinogenesis, etc. [57]. The use of nanoparticles in consumer products and biomedical applications has led to the ingestion of nanoparticles that then reach the gastrointestinal tract. Then, nanoparticles enter the blood and other organs, interacting with gastrointestinal tract mucosa and affecting the luminal components, mucosa, and microbiome of the gastrointestinal tract [58]. The injection of nanoparticles mostly happens while using NPs for drug delivery or as contrast agents. This leads to hemolysis, platelet activation, and platelet aggregation [59]. Once they enter the bloodstream, they may produce unfavourable biological outcomes in a wide range of organs considered secondary sites of contact. They have been shown to spread to a variety of organs, including the kidneys, brain, spleen, liver, and heart. As the kidneys play an important role in xenobiotic elimination, renal clearance can eliminate NPs absorbed in systemic circulation. The physicochemical properties of NPs play a vital role in translocation, and their migration to remote areas is a major concern for toxicity [60]. As they enter the organs, they induce ROS production, oxidise proteins, and damage DNA, which can be detected by carbonyls and estimating 8-hydroxy-2′-deoxyguanosine. In most instances, oxidative stress blocks antioxidant enzymes, including catalase, superoxide dismutase, and glutathione peroxidase, and depletes non-enzymatic antioxidants such as glutathione, vitamin E, and vitamin C [61,62].
2.1. Metal-Based Nanoparticles as Oxidants
Metal nanoparticles can drive redox processes, resulting in the continuous endogenous generation of ROS in a positive feedback loop, causing major genotoxicity. The chronic oxidative stress that occurs following exposure to metal NPs is mediated by the upregulation of inflammatory mediators such as nuclear factor kappa B (NF-κB), signal transducer and activator of transcription (STATs), mitochondrial dysfunction, and higher intracellular calcium levels [63] (Figure 3). They can either directly or indirectly activate the mitogen-activated protein kinase pathways leading to the production of reactive free radicals [64,65]. As mentioned earlier, the physiochemical properties of metal NPs such as size, configuration, composition, shape, surface area, functionalization, charge, magnetic property, etc., have a direct/indirect and significant impact on the induction of oxidative stress [66] and lead to genotoxicity or cellular toxicity. NPs are directly responsible for causing ROS to form when they are in the acidic environment of lysosomes. NPs have been proven to cause damage to the DNA strands and change gene expression [64,65]. It was reported that ROS generation caused by hydrophilic NPs such as titanium oxide can lead to cancerous growth. In one study, titanium NPs was found to cause benign mouse fibrosarcoma that later became malignant [65].
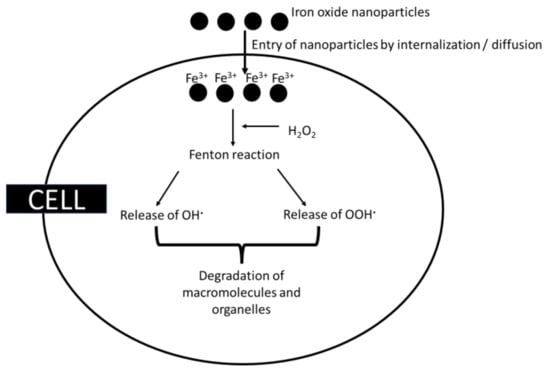
Figure 3.
Oxidative damage caused by iron oxide nanoparticles.
2.1.1. Iron Nanoparticles as Oxidants
Through the Fenton reaction, iron nanoparticles produce redox-active iron and hydroxyl ions in the presence of hydrogen peroxide (refer to Equation (1)) [67,68], leading to the release of hydroxyl radicals. A particular illustration of the Fenton reaction is the Haber–Weiss reaction. In order to create a reactive species that can oxidise a wide range of organic substrates, hydrogen peroxide and ferrous salts must react. If Fe2+ can be produced from Fe3+ again, iron may have catalytic properties [69]. Superoxide radicals also liberate Fe2+ from ferritin, an intracellular iron-storage protein, or other proteins with [4Fe-4S]2+ clusters that favour the Fenton reaction. Furthermore, NADH triggers the Fenton reaction by reloading Fe2+ from Fe3+ [67].
Fe2+ + H2O2 → Fe3+ + OH· + OH−
Furthermore, ferrous iron can be converted to ferric iron, which can then react with superoxide radicals to restart the reaction. Additionally, ferric iron can form superoxide radicals when it reacts with peroxide radicals [67,70]. A variety of diseases, including neurodegenerative diseases, cardiovascular disorders, and cancers, have been linked to these compounds in the body. During ferrite-based ROS-mediated cancer therapy, these iron oxide nanoparticles are converted by hydrogen peroxide to produce highly toxic hydroxyl free radicals, which leads to tumour cell death in the acidic microenvironment of the tumour [70,71]. These free radicals can also damage biological macromolecules and the organelles of cells [72] (Figure 4). A positive feedback loop formed by iron accumulation, oxidative stress, and protein aggregation was found to produce toxicity in the cells [73,74]. It was found that citrate-coated iron oxide nanoparticles have protein oxidation [75].Various proteins, including α-synuclein are found to aggregate as a result of iron accumulation and oxidative stress, which might lead to Alzheimer’s and Parkinson’s diseases [76]. Ahamed et al. [77] reported that iron nanoparticles induced cytotoxicity by identifying higher levels of LDH, and defence in the antioxidant system was observed due to the generation of ROS caused by iron accumulation. As a result of ROS, permeabilization of the outer mitochondrial membrane releases soluble proteins into the cytosol where caspase activation occurs, resulting in apoptosis. Van den Bos et al. [78] found that dextran-coated SPIONs show lipid peroxidation, which is dose-dependent, but in lower amounts these iron oxides are not particularly toxic [79].
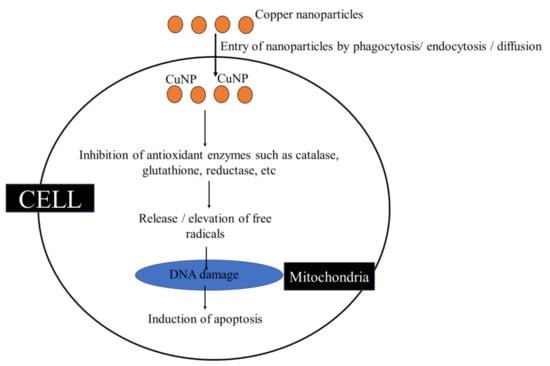
Figure 4.
Oxidative damage caused by copper nanoparticles.
2.1.2. Copper Nanoparticles as Oxidants
Copper nanoparticles (CuNP) are widely used as they possess various bioactivity, including antimicrobial activity [80]. They do induce ROS generation where the biological responses are dependent on the amount of ROS released, the type of cellular pathways, etc. Copper nanoparticles act as pro-oxidants, meaning that they induce ROS production or inhibit antioxidants, thereby increasing oxidative stress [81]. The intake of CuNP by immune cells is through phagocytosis, endocytosis, passive diffusion, etc. Passive uptake does take place by direct interaction by modulating the NLRP3 inflammasome. Copper nanoparticles interact with cell surface receptors and activate intracellular pathways including the MAPK pathway, TLR4 pathway, and lectin pathway for the entry and induction of inflammation [81,82]. Fahmy et al. [83] recorded the inhibitory action of copper nanoparticles against cellular antioxidant enzymes such as catalase and glutathione reductase and increase the glutathione peroxidase activity. This suggests that copper nanoparticles are not only able to generate reactive oxygen species, but also inhibit the antioxidant defences of cells (Figure 5). Fahmy et al. [83] also found that co-treating CuNP with antioxidant resveratrol increased the life of cells. The cytotoxicity of Cu2+ was found to be caused by DNA damage and apoptosis-mediated cell death [83,84]. Similarly, a study by Zhou et al. [85] observed that, on exposure to copper nanoparticles, there was a higher apoptotic rate. It was also recorded that copper nanoparticles administered by intranasal instillation are tend to accumulate in the liver by entering into the mucosa, encountering severe damage. This results in the release of ROS from liver cells, triggering an oxidative stress response that leads to ER stress and cell apoptosis [86]. There was an increased ROS production leading to genotoxicity and cancer formation in CuNPs treated with Mytilus galloprovincialis [87].
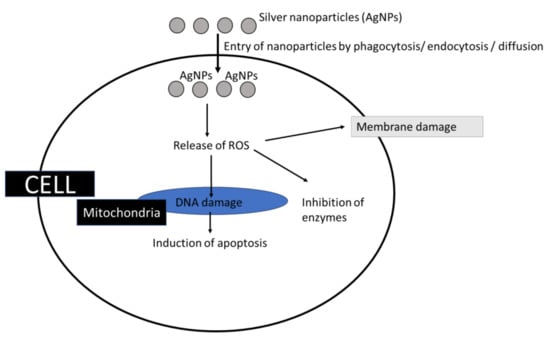
Figure 5.
Role of silver nanoparticles as oxidants.
2.1.3. Silver Nanoparticles as Oxidants
Silver nanoparticles (AgNPs) possess unique properties and can be used in various biomedical and environmental fields, as well as healthcare-related products, etc., for their antimicrobial properties [88,89],
These characteristics are attributed to their higher ability to induce ROS. In addition to interacting with the cell membrane, AgNPs internalize and enter the cell through diffusion or membrane damage [90]; mostly, Ag+ dissociates from the nanoparticle core and stimulates ROS production [86,91,92]. The ROS produced are cytotoxic (even the AgNPs are directly cytotoxic) and the genotoxic effects mostly lead to apoptosis/membrane damage/enzyme inhibition etc. (Figure 6). When the silver nanoparticles react with membrane proteins containing sulfur, enzymes and proteins bound to the membrane become inactive. By attacking the membrane, silver nanoparticles can disrupt the respiratory chain and reduce energy production. They also attack unsaturated fatty acids on cell membranes and alter membrane fluidity, thereby impairing cell function by destroying membrane permeability and integrity [93,94]. It is reported that silver nanoparticles could cause apoptosis by the activation of caspase in-vivo [95].
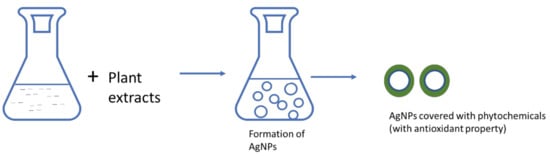
Figure 6.
Silver nanoparticles with antioxidant property.
2.1.4. Other Metal/Non-Metal Nanoparticles as Oxidants
The particle surface of a nanoparticle has a major role in ROS generation [96]. SiO• and SiO2• are the radicals seen on quartz nanoparticles which are believed be involving in formations of hydroxyl and oxygen radicals [97,98]. Sometimes the reactants are present in the environment [99]. The reactive sites of nanoparticles such as Si either donate or accept electrons from molecular oxygen, leading to the formation of oxygen radicals which participate in the generation of other ROS [100]. The size of the particles plays a major role in the generation of ROS [97,98], and chromium and other metals such as vanadium NPs form ROS through Fenton reactions [101]. Metal-based NPs have been found to promote NF-κB pathways for ROS generation [102]. Carbon nanotubes induce ROS after being internalized; single-walled carbon nanotube-induced NADPH-mediated ROS leads to mitochondrial failure and pulmonary damage [103]. Carbon nanotubes can also cause genotoxic effects by interacting with DNA, causing aberrant cell development and, ultimately, leading to carcinogenesis and fibrogenesis [104]. The addition of carbon nanoparticles to lung surfactant enhanced the number of nicotinic coenzymes of NAD+, NADH, NADP+, and NADPH in the cell [105]. These are responsible for causing ROS generation and oxidative stress.
3. Mechanism of Nanoparticles as Antioxidants
Metallic nanoparticles, in particular, have been found to have enzyme-like antioxidant properties which can scavenge free radicals and lower ROS concentrations. Metallic NPs, such as magnetic, silver, and gold NPs, appear to offer promising potential for treating and preventing illnesses caused by excessive ROS production [106,107,108]. Nanotechnology, in conjunction with materials science, has significantly reduced free-radical generation during nanoparticle creation, and these nanoparticles are called nano-antioxidants [109]. Nano-antioxidants are nonorganic NPs functionalized with antioxidants or antioxidant enzymes for use as delivery systems for antioxidants, as well as NPs with intrinsic antioxidant properties. Significant antioxidant properties include superoxide dismutase, catalase, oxidase, and peroxidase-mimicking activity. Metallic NMs may have a significant antioxidative effects due to their ability to bounce between different multiple oxidation states [108]. The mechanism of nanoparticle antioxidant activity is unclear and yet to be discovered.
The nano-antioxidant properties of nanoparticles are mostly depending on the method by which they are synthesized, as there are numerous preparation techniques such as the solvent displacement method, supercritical fluid technology, emulsion or solvent evaporation, the templating technique, and the nanoprecipitation technique. The antioxidant activity of novel metal nanoparticles such as silver, gold, and the transition metal oxides of copper oxide and nickel oxide, is extensively employed and studied. Coupling/incorporating various phytochemicals into single or bimetallic combinational NPs enhances antioxidant activity. Antioxidant characteristics rely on chemical composition, nature, stability, surface-to-volume ratio, size, surface coating, and surface charge [51]. Certain oxide nanoparticles, because of their intrinsic physicochemical characteristics, can scavenge reactive nitrogen and oxygen species and mimic antioxidant molecules or antioxidant enzymes [52,53]. Another mechanism of nanoparticles for quenching free radicals depends on the ability of a nanomaterial to quench alkyl peroxyl radicals by converting them to hydroperoxides [54].
3.1. Silver Nanoparticles as Antioxidants
In an earlier section, the oxidant properties of silver nanoparticles that can inhibit cell growth by interfering with membrane proteins or signaling pathways were discussed. Furthermore, the ability of silver nanoparticles to interact with sulfur groups of proteins, especially on antioxidant enzymes, and interfere in antioxidant activity, was considered. A huge number of articles on the antioxidant activity of silver nanoparticles have been published in recent years [109]. Using nanoparticles as radical scavengers, for their redox potential, or as carriers for antioxidant compounds are developing fields of research in the science of oxidative stress [94]. The antioxidant properties of AgNPs might be depended on methods of preparation (Figure 6), but in most cases AgNPs are prepared using plant extracts [110,111]. These phytochemicals do help inform us on nanoforms and the antioxidant activity of AgNPs. Ansar et al. [112] synthesized Ag NPs from Brassica oleracea leaves with a good scavenging percent ranging from 60 to 80%. The abundance of surface-generated flavonoids and phenolics as capping agents could be the reason for antioxidant activity of these nanoparticles [110]. AgNPs mediated by aerial parts of Lavandula stoechas was reported to scavenge DPPH radicals of 75% at 25 mg/mL through their phytochemical constituents’ such as phenols, terpenoids and flavonoids [111].
3.2. Copper Nanoparticles as Antioxidants
As mentioned in the above section, CuNPs have oxidant activity; when they are green-synthesised, they possess antioxidant activity. In a study, greenly synthesized copper nanoparticles using avocado seeds have shown antioxidant activity of nearly 17% to 22% [113]. Similarly, Wu et al. [36] have recorded the maximum antioxidant activity of 21% for CuNPs produced using Cissus vitiginea, where the colour change from violet to yellow indicates that DPPH is decreased by donating hydrogen atoms with high scavenging activity [114]. In the above cases, the phytochemicals of the plant extract have a role in the enhancement of antioxidant activity. CuNPs were reported to increase plasma ferric-reducing ability and catalase activity, but interestingly decreased plasma Cu and ceruloplasmin levels [115]. The application of CuNPs on tomato leaves increased chlorophyll content, as well as enzymes such as ascorbate peroxidase, glutathione peroxidase, superoxide dismutase, and phenylalanine ammonia lyase, and non-enzymatic antioxidants such as vitamin C and glutathione. They also enhanced the quality of nutraceutical and commercial fruits by increasing non-enzymatic antioxidant components, including vitamin C, glutathione, flavonoids, firmness, total soluble solids, and titratable acidity [116]. At high concentrations, Cu-NPs also had a significant impact on the release of proinflammatory mediators from brain microvessel endothelial cells [117].
3.3. Iron Nanoparticles as Antioxidants
Iron oxide nanoparticles were reported to increase catalase activity, glutathione peroxidase, and ascorbate peroxidase by 225.08%, 223.04%, and 69.89%, respectively, in different tomato cultivars [118]. As like in the case of AgNPs and CuNPs, plant extract addition increased the antioxidant property of iron oxide nanoparticles. It was reported that iron nanoparticles synthesized using Blumea eriantha showed antioxidant activity with a percentage of 74.94% [119]. In a study, antioxidant capacity of iron nanoparticles synthesized using E. robusta leaves was found in a concentration-dependent manner [120].
3.4. Other Metallic/Non-Metallic Nanoparticles as Antioxidants
Fullerenes, as well as single-walled and multiwalled carbon nanotubes have been reported to have antioxidant properties and can be used preclinically to check for inflammatory arthritis and neurodegenerative diseases. Poly(ethylene glycol)-conjugated hydrophilic carbon clusters could act as immunomodulators that selectively target T cells to scavenge the intracellular oxygen radicals generated by antigen-stimulated T cells [121]. A study by Oliveira et al. [122] showed red propolis-embedded mesoporous silica nanoparticles to have good antioxidant activity, as the physicochemical integrity of the pharmacological characteristics is preserved as a carrier of isoflavonoids, flavonoids, and other chemicals present in red propolis extract.
Several forms of polymer-loaded antioxidant nanoparticles, metallic and metal oxide nanoparticles, and carbon nanotubes have also been shown to possess antioxidant properties [96]. Nano-antioxidants are capable of accessing, reacting, and responding as required to specific functions in targeted tissues. Nano-antioxidant therapy can be used in combination with conventional therapy to offer a viable treatment option for patients suffering from oxidative-stress-related conditions [123,124]. Due to their intrinsic physicochemical features, certain oxide nanoparticles can scavenge reactive nitrogen and oxygen species (RNS/ROS) and imitate the antioxidant molecule [125,126]. Nanomedicines can dramatically change the pharmacokinetic properties of antioxidant drugs while reducing their adverse effects. Furthermore, nanomaterials, including inorganic nanoparticles, possess inherent antioxidant capabilities which are demonstrated through direct reactions with ROS and/or emulating natural antioxidant enzymes, demonstrating significant ROS-scavenging potential. The development of ROS-scavenging or ROS-responsive antioxidative nanotherapies derived from organic materials, or inorganic/organic hybrid materials, has gained a lot of attention recently, with promising results in reducing oxidative damage in various animal models of various diseases [126]. Nanotechnology-based systems are now being used to prevent and treat aging-related pathological ailments such as Alzheimer’s and Parkinson’s diseases, as well as cardiovascular diseases, obesity, type 2 diabetes, and cancers [110].
4. Conclusions
The oxidation mechanisms of all nanoparticles are mostly ROS-mediated, and they can induce apoptosis in-vivo and in-vitro. The mechanism causing oxidative stress is slightly different amongst metal nanoparticles, and is dependent on size, surface chemistry, and shape of the nanoparticles. The antioxidant properties of the metal/non-metal nanoparticles are mostly rendered by the addition of plant extracts. Phytochemicals could nullify the oxidant nature of nanoparticles and give them antioxidant properties too.
Author Contributions
A.V.S., conceptualization, writing—original draft, reviewing, editing, and supervision.; S.P.R.S., writing—original draft, reviewing, and editing; R.D., writing—original draft, reviewing and editing; V.V.R., reviewing and editing; S.P., reviewing and editing; K.R., conceptualization, reviewing, editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anu Mary Ealia, S.; Saravana Kumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Samrot, A.V.; Shobana, N.; Jenna, R. Antioxidant Activity of Different Staged Ripened Fruit of Capsicum annuum and Its Green Synthesized Silver Nanoparticles. BioNanoScience 2018, 8, 632–646. [Google Scholar] [CrossRef]
- Samrot, A.V.; Cypriyana, P.J.; Saigeetha, S.; Selvarani, A.J.; Purayil, S.K.; Ponnaiah, P. Microbially synthesized silver nanoparticles: Mechanism and advantages—A review. Nanobiotechnol. Plant Prot. 2022, 2022, 439–478. [Google Scholar]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Rai, A.; Park, K.; Zhou, L.; Zachariah, M.R. Understanding the mechanism of aluminium nanoparticle oxidation. Combust. Theory Model. 2006, 10, 843–859. [Google Scholar] [CrossRef]
- Verma, H.N.; Singh, P.; Chavan, R.M. Gold nanoparticle: Synthesis and characterization. Vet. World 2014, 7, 72–77. [Google Scholar] [CrossRef]
- Carvell, J.; Ayieta, E.; Gavrin, A.; Cheng, R.; Shah, V.R.; Sokol, P. Magnetic properties of iron nanoparticle. J. Appl. Phys. 2010, 107, 103913. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Qin, B.; Xing, D.; Guo, Y.; Fan, R. Investigation of the Mending Effect and Mechanism of Copper Nano-Particles on a Tribologically Stressed Surface. Tribol. Lett. 2004, 17, 961–966. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Afeseh Ngwa, H.; Kanthasamy, A.; Gu, Y.; Fang, N.; Anantharam, V.; Kanthasamy, A.G. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol. 2011, 256, 227–240. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Le, T. Zinc Oxide Nanoparticle as a Novel Class of Antifungal Agents: Current Advances and Future Perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef]
- Burke, D.J.; Zhu, S.; Pablico-Lansigan, M.P.; Hewins, C.R.; Samia, A.C.S. Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol. Fertil. Soils 2014, 50, 1169–1173. [Google Scholar] [CrossRef]
- Zach, M.P.; Penner, R.M. Nanocrystalline nickel nanoparticles. Adv. Mater. 2000, 12, 878–883. [Google Scholar] [CrossRef]
- Kouwenhoven, L.; Marcus, C. Quantum dots. Phys. World 1998, 11, 35–40. [Google Scholar] [CrossRef]
- Khan, S.A. Metal nanoparticles toxicity: Role of physicochemical aspects. Met. Nanopart. Drug Deliv. Diagn. Appl. 2020, 1–11. [Google Scholar]
- Chen, Y.C.; Huang, X.C.; Luo, Y.L.; Chang, Y.C.; Hsieh, Y.Z.; Hsu, H.Y. Non-metallic nanomaterials in cancer theranostics: A review of silica- and carbon-based drug delivery systems. Sci. Technol. Adv. Mater. 2013, 14, 044407. [Google Scholar] [CrossRef]
- Samrot, A.V.; Suvedhaa, B.; Sahithya, C.S.; Madankumar, A. Purification and Utilization of Gum from Terminalia Catappa, L. for Synthesis of Curcumin Loaded Nanoparticle and Its In Vitro Bioactivity Studies. J. Clust. Sci. 2018, 29, 989–1002. [Google Scholar] [CrossRef]
- Samrot, A.V.; Burman, U.; Philip, S.A.; Shobana, N.; Chandrasekaran, K. Synthesis of curcumin loaded polymeric nanoparticles from crab shell derived chitosan for drug delivery. Inform. Med. Unlocked 2018, 10, 159–182. [Google Scholar] [CrossRef]
- Samrot, A.V.; Senthilkumar, P.; Rashmitha, S.; Veera, P.; Sahithya, C.S. Azadirachta indica influenced biosynthesis of super-paramagnetic iron-oxide nanoparticles and their applications in tannery water treatment and X-ray imaging. J. Nanostruct. Chem. 2018, 8, 343–351. [Google Scholar] [CrossRef]
- Samrot, A.V.; Shobana, N.; Durga Sruthi, P.; Sahithya, C.S. Utilization of chitosan-coated superparamagnetic iron oxide nanoparticles for chromium removal. Appl. Water Sci. 2018, 8, 192. [Google Scholar] [CrossRef]
- Samrot, A.V.; Angalene, J.; Roshini, S.M.; Raji, P.; Stefi, S.M.; Preethi, R.; Selvarani, A.J.; Madankumar, A. Bioactivity and Heavy Metal Removal Using Plant Gum Mediated Green Synthesized Silver Nanoparticles. J. Clust. Sci. 2019, 30, 1599–1610. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Pachiyappan, S.; Kumar, S.S. Surface-Engineered Super-Paramagnetic Iron Oxide Nanoparticles for Chromium Removal. Int. J. Nanomed. 2019, 14, 8105–8119. [Google Scholar] [CrossRef] [PubMed]
- Justin, C.; Samrot, A.V.; Sahithya, C.S.; Bhavya, K.S.; Saipriya, C. Preparation, characterization and utilization of coreshell super paramagnetic iron oxide nanoparticles for curcumin delivery. PLoS ONE 2018, 13, e0200440. [Google Scholar] [CrossRef] [PubMed]
- Geetha, N.; Prabhavathi, G.; Ayeshamariam, A.; Beevi, A.H.; Punithavelan, N.; Uthiram, C.; Jayachandran, M. Review on a Nanomaterials Mechanisms-Induced Oxidative Stress and Toxicity. J. Powder Metall. Min. 2017, 6, 185. [Google Scholar]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Azeez, L.; Lateef, A.; Adebisi, S.A. Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl. Nanosci. 2017, 7, 59–66. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or Foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2017, 58, 2491–2507. [Google Scholar] [CrossRef]
- Bennet Rohan, D.; Raji, P.; Divya Kumar, M.; Kripu Sharma, V.; Keerthana, D.; Karishma, S.; Antony, V.; Thirumurugan, R.; Purayil, S.K.; Ponnaiah, P.; et al. Green Synthesis and Antibacterial Activity Studies of Silver Nanoparticles from the Aqueous Extracts of Euphorbia hirta. J. Pure Appl. Microbiol. 2020, 14, 301–306. [Google Scholar]
- Ge, X.; Cao, Z.; Chu, L. The Antioxidant Effect of the Metal and Metal-Oxide Nanoparticles. Antioxidants 2022, 11, 791. [Google Scholar] [CrossRef]
- Dutta, D.; Mukherjee, R.; Ghosh, S.; Patra, M.; Mukherjee, M.; Basu, T. Cerium Oxide Nanoparticles as Antioxidant or Pro-oxidant Agents. ACS Appl. Nano Mater. 2022, 5, 1690–1701. [Google Scholar] [CrossRef]
- Milanezi, F.G.; Meireles, L.M.; de Christo Scherer, M.M.; de Oliveira, J.P.; da Silva, A.R.; de Araujo, M.L.; Endringer, D.C.; Fronza, M.; Guimarães, M.C.; Scherer, R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm. J. 2019, 27, 968–974. [Google Scholar] [CrossRef]
- Devi, R.S.; Jeevitha, M.; Preetha, S.; Rajeshkumar, S. Free Radical Scavenging Activity of Copper Nanoparticles Synthesized from Dried Ginger. J. Pharm. Res. Int. 2020, 32, 1–7. [Google Scholar] [CrossRef]
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158. [Google Scholar] [CrossRef]
- Khanna, P.; Ong, C.; Bay, B.; Baeg, G. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Tee, J.K.; Ong, C.N.; Bay, B.H.; Ho, H.K.; Leong, D.T. Oxidative stress by inorganic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 414–438. [Google Scholar] [CrossRef]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2020, 55, 331–342. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, N.; Zhu, M.; Lu, J.; Zhong, H.; Xue, X.; Guo, S.; Li, M.; Wei, X.; Tao, Y.; et al. TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: A proteomic and metabolomic insight. Redox Biol. 2018, 15, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Madl, A.K.; Plummer, L.E.; Carosino, C.; Pinkerton, K.E. Nanoparticles, Lung Injury, and the Role of Oxidant Stress. Annu. Rev. Physiol. 2014, 76, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Ozoemena, K.I.; Tshikhudo, R.T.; Moutloali, R.M. Monolayer-protected clusters of gold nanoparticles: Impacts of stabilizing ligands on the heterogeneous electron transfer dynamics and voltammetric detection. Langmuir 2010, 26, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; Bundschuh, M.; Klingelhofer, D.; Groneberg, D.A. Gold nanoparticles: Recent aspects for human toxicology. J. Occup. Med. Toxicol. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Doshi, N.; Mitragotri, S. Needle-shaped polymeric particles induce transient disruption of cell membranes. J. R. Soc. Interface 2010, 7, S403–S410. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Miranda, O.R.; Thompson, M.A.; Pabelick, C.M.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Prakash, Y.S.; Mukherjee, P. Effect of Nanoparticle Surface Charge at the Plasma Membrane and Beyond. Nano Lett. 2010, 10, 2543–2548. [Google Scholar] [CrossRef]
- Naito, M.; Yokoyama, T.; Hosokawa, K.; Nogi, K.B. Chapter 3-Characteristics and Behavior of Nanoparticles and Its Dispersion Systems. In Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 109–168. [Google Scholar]
- Ganguly, R.; Singh, A.K.; Kumar, R.; Gupta, A.; Pandey, A.K.; Pandey, A.K. Nanoparticles as Modulators of Oxidative Stress. Nanotechnol. Mod. Anim. Biotechnol. 2019, 29–35. [Google Scholar]
- Yu, S.; Zhang, H.; Zhang, S.; Zhong, M.; Fan, H. Ferrite Nanoparticles-Based Reactive Oxygen Species-Mediated Cancer Therapy. Front. Chem. 2020, 9, 651053. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Li, C.W.; Li, L.L.; Chen, S.; Zhang, J.X.; Lu, W.L. Antioxidant Nanotherapies for the Treatment of Inflammatory Diseases. Front. Bioeng. Biotechnol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2018, 15, 4–33. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Sonwani, S.; Madaan, S.; Arora, J.; Suryanarayan, S.; Rangra, D.; Mongia, N.; Vats, T.; Saxena, P. Inhalation Exposure to Atmospheric Nanoparticles and Its Associated Impacts on Human Health: A Review. Front. Sustain. Cities 2021, 3, 690444. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 1–2. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, Y.; Liu, J.; Feng, X.; Zhou, T.; Shao, L. Is Neurotoxicity of Metallic Nanoparticles the Cascades of Oxidative Stress? Nanoscale Res. Lett. 2016, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Najafi, M.; Samadian, H.; Barabadi, H.; Azarnezhad, A.; Ahmadi, A. Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chem.-Biol. Interact. 2019, 321, 108814. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, M.; Sil, P.C. Nanotoxicity: Oxidative Stress Mediated Toxicity of Metal and Metal Oxide Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Onuma, K.; Sato, Y.; Ogawara, S.; Shirasawa, N.; Kobayashi, M.; Yoshitake, J.; Yoshimura, T.; Iigo, M.; Fujii, J.; Okad, F. Nano-Scaled Particles of Titanium Dioxide Convert Benign Mouse Fibrosarcoma Cells into Aggressive Tumor Cells. Am. J. Pathol. 2009, 175, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Karimi, H.; Khazaei, M. Review on Metal-based Nanoparticles: Role of ROS in Renal Toxicity. Chem. Res. Toxicol. 2020, 33, 2503–2514. [Google Scholar] [CrossRef]
- Ševců, A.; El-Temsah, Y.S.; Joner, E.J.; Černík, M. Oxidative Stress Induced in Microorganisms by Zero-valent Iron Nanoparticles. Microbes Environ. 2011, 26, 271–281. [Google Scholar] [CrossRef]
- Chen, H.Y. Why the Reactive Oxygen. Species of the Fenton Reaction Switches from Oxoiron(IV) Species to Hydroxyl Radical in Phosphate Buffer Solutions? A Computational Rationale. ACS Omega 2019, 4, 14105–14113. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82, 969–974. [Google Scholar] [CrossRef]
- He, F.; Zhang, M.; Qian, T.; Zhao, D. Transport of carboxymethyl cellulose stabilized iron nanoparticles in porous media: Column experiments and modeling. J. Colloid Interface Sci. 2009, 334, 96–102. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2019, 15, 115. [Google Scholar] [CrossRef]
- Li, X.; Elliott, D.W.; Zhang, W. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Mounsey, R.B.; Teismann, P. Chelators in the Treatment of Iron Accumulation in Parkinson’s Disease. Int. J. Cell Biol. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef]
- Stroh, A.; Zimmer, C.; Gutzeit, C.; Jakstadt, M.; Marschinke, F.; Jung, T.; Pilgrimm, H.; Grune, T. Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free. Radic. Biol. Med. 2004, 36, 976–984. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Majeed Khan, M.A.; Ali, D.; Alarafi, S. Iron Oxide Nanoparticle-induced Oxidative Stress and Genotoxicity in Human Skin Epithelial and Lung Epithelial Cell Lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef]
- Van Den Bos, E.J.; Wagner, A.; Mahrholdt, H.; Thompson, R.B.; Morimoto, Y.; Sutton, B.S.; Judd, R.M.; Taylor, D.A. Improved efficacy of stem cell labeling for magnetic resonance imaging studies by the use of cationic liposomes. Cell Transplant. 2003, 12, 743–756. [Google Scholar] [CrossRef]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Perreault, F.; Melegari, S.P.; da Costa, C.H.; de Oliveira Franco Rossetto, A.L.; Popovic, R.; Matias, W.G. Genotoxic effects of copper oxide nanoparticles in Neuro 2A cell cultures. Sci. Total Environ. 2012, 441, 117–124. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, J.J.; Zhou, Y.T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual Enzyme-like Activities of Iron Oxide Nanoparticles and Their Implication for Diminishing Cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- David, C.A.; Owen, A.; Liptrott, N.J. Determining the relationship between nanoparticle characteristics and immunotoxicity: Key challenges and approaches. Nanomedicine 2016, 11, 1447–1464. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. Vitr. 2009, 23, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.W.; Liu, T.; Verdile, G.; Bishop, G.; Haasl, R.J.; Smith, M.A.; Perry, G.; Martins, R.N.; Atwood, C.S. Copper Induces Apoptosis of Neuroblastoma Cells Via Post-Translational Regulation of the Expression of Bcl-2-family Proteins and the tx Mouse is a Better Model of Hepatic than Brain Cu Toxicity. Int. J. Clin. Exp. Med. 2008, 1, 76–88. [Google Scholar] [PubMed]
- Zhou, L.; Cheng, G.; Xu, C.; Liu, H.; Wang, Y.; Li, N.; Fan, X.; Zhu, C.; Xia, W. Copper Nanoparticles Induce Oxidative Stress via the Heme Oxygenase 1 Signaling Pathway in vitro Studies. Int. J. Nanomed. 2020, 16, 1565–1573. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.; Liu, X.; Yang, H.; Fang, Y.; Tian, L.; Li, K.; Nie, H.; Zhang, W.; Shi, Y.; et al. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J. Hazard. Mater. 2020, 401, 123349. [Google Scholar] [CrossRef]
- Ruiz, P.; Katsumiti, A.; Nieto, J.A.; Bori, J.; Jimeno-Romero, A.; Reip, P.; Arostegui, I.; Orbea, A.; Cajaraville, M.P. Short-term effects on antioxidant enzymes and long-term genotoxic and carcinogenic potential of CuO nanoparticles compared to bulk CuO and ionic copper in mussels Mytilus galloprovincialis. Mar. Environ. Res. 2015, 111, 107–120. [Google Scholar] [CrossRef]
- Stalin Dhas, T.; Sowmiya, P.; Parthasarathy, K.; Natarajan, A.; Narendrakumar, G.; Kumar, R.; Samrot, A.V.; Riyaz, S.U.; Ganesh, V.K.; Karthick, V.; et al. In vitro antibacterial activity of biosynthesized silver nanoparticles against gram negative bacteria. Inorg. Nano-Met. Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Fabrega, J.; Fawcett, S.R.; Renshaw, J.C.; Lead, J.R. Silver nanoparticle impact on bacterial growth: Effect of pH, concentration, and organic matter. Environ. Sci. Technol. 2009, 43, 7285–7290. [Google Scholar] [CrossRef]
- Docea, A.O.; Calina, D.; Buga, A.M.; Zlatian, O.; Paoliello, M.M.; Mogosanu, G.D.; Streba, C.T.; Popescu, E.L.; Stoica, A.E.; Bîrcă, A.C.; et al. The Effect of Silver Nanoparticles on Antioxidant/Pro-Oxidant Balance in a Murine Model. Int. J. Mol. Sci. 2020, 21, 1233. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2018, 39, 16–26. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: Growth inhibition, cell injury, oxidative stress and internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar] [CrossRef]
- Mao, B.H.; Chen, Z.Y.; Wang, Y.J.; Yan, S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef]
- Vallyathan, V.; Shi, X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 1997, 105 (Suppl. 1), 165–177. [Google Scholar]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Klei, L.R.; Barchowsky, A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am. J. Physiol. 2001, 280, L442–L449. [Google Scholar] [CrossRef] [PubMed]
- Nilewski, L.; Li, G.; Sikkema, W.K.; Kent, T.A.; Tour, J.M. Carbon nanoparticles and oxidative stress: Could an injection stop brain damage in minutes? Nanomedicine 2015, 10, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Rasras, S.; Kalantari, H.; Rezaei, M.; Dehghani, M.A.; Zeidooni, L.; Alikarami, K.; Dehghani, F.; Alboghobeish, S. Single-walled and multiwalled carbon nanotubes induce oxidative stress in isolated rat brain mitochondria. Toxicol. Ind. Health 2019, 35, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Thakur, A.K. The Toxicological Mechanisms of Environmental Soot (Black Carbon) and Carbon Black: Focus on Oxidative Stress and Inflammatory Pathways. Front. Immunol. 2017, 8, 763. [Google Scholar] [CrossRef]
- Mohammad, G.; Vijendra, K.M.; Pandey, H.P. Antioxidant properties of some nanoparticle may enhance wound healing in t2dm patient. Dig. J. Nanomater. Biostruct. 2008, 3, 159–162. [Google Scholar]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Lushchak, O.; Zayachkivska, A.; Vaiserman, A. Metallic Nanoantioxidants as Potential Therapeutics for Type 2 Diabetes: A Hypothetical Background and Translational Perspectives. Oxidative Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, V.K.; Das, S.K.; Mishra, N.; Bisht, L.; Joshi, A.; Sharma, N. Characterization and Anti-Cancerous Effect of Putranjiva roxburghii Seed Extract Mediated Silver Nanoparticles on Human Colon (HCT-116), Pancreatic (PANC-1) and Breast (MDA-MB 231) Cancer Cell Lines: A Comparative StudY. Int. J. Nanomed. 2020, 15, 573–585. [Google Scholar] [CrossRef]
- Ansar, S.; Tabassum, H.; Aladwan, N.S.; Naiman Ali, M.; Almaarik, B.; AlMahrouqi, S.; Abudawood, M.; Banu, N.; Alsubki, R. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci. Rep. 2020, 10, 18564. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Rinitha, G. Nanostructural characterization of antimicrobial and antioxidant copper nanoparticles synthesized using novel Persea americana seeds. OpenNano 2018, 3, 18–27. [Google Scholar] [CrossRef]
- Samrot, A.V.; Raji, P.; Selvarani, J.A.; Angalene, L.A.; Sruthi, D.P.; Paulraj, P.; Iyappan, P.A. Handbook on Phytochemical Extraction, Screening and Its In-Vitro Bioactivity Assays; Publisher SARAS Publications: Nagercoil, India, 2019; ISBN 978-93-86519-60-3. [Google Scholar]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in Wistar rats. Pharmacol. Rep. 2019, 71, 509–516. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of Selenium and Copper Nanoparticles on Yield, Antioxidant System, and Fruit Quality of Tomato Plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef]
- Trickler, W.J.; Lantz, S.M.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J. Effects of copper nanoparticles on rat cerebral microvessel endothelial cells. Nanomedicine 2012, 7, 835–846. [Google Scholar] [CrossRef]
- Aazami, M.A.; Rasouli, F.; Ebrahimzadeh, A. Oxidative damage, antioxidant mechanism and gene expression in tomato responding to salinity stress under in vitro conditions and application of iron and zinc oxide nanoparticles on callus induction and plant regeneration. BMC Plant Biol. 2021, 21, 597. [Google Scholar] [CrossRef]
- Chavan, R.R.; Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Urade, M.N. Characterization, antioxidant, antimicrobial and cytotoxic activities and biological screening of green synthesized silver and iron nanoparticles using alcoholic Blumea eriantha DC plant extract. Mater. Today Commun. 2020, 18, 43. [Google Scholar]
- Vitta, Y.; Figueroa, M.; Calderon, M.; Ciangherotti, C. Synthesis of Iron Nanoparticles from aqueous extract of Eucalyptus Robusta Sm and evaluation of antioxidant and antimicrobial activity. Mater. Sci. Energy Technol. 2019, 3, 97–103. [Google Scholar] [CrossRef]
- Huq, R.; Samuel, E.L.; Sikkema, W.K.; Nilewski, L.G.; Lee, T.; Tanner, M.R.; Khan, F.S.; Porter, P.C.; Tajhya, R.B.; Patel, R.S.; et al. Preferential uptake of antioxidant carbon nanoparticles by T lymphocytes for immunomodulation. Sci. Rep. 2016, 6, 33808. [Google Scholar] [CrossRef]
- Azevedo de MOliveira, L.F.; Teles da Silva, L.V.; do Nascimento, T.G.; de Almeida, L.M.; Calumby, R.J.; Nunes, Á.M.; de Magalhães Oliveira, L.M.; da Silva Fonseca, E.J. Antioxidant and antimicrobial activity of red propolis embedded mesoporous silica nanoparticles. Drug Dev. Ind. Pharm. 2020, 46, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.; Baek, K.H. Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights. Molecules 2021, 26, 7031. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Johnson, M.; Walker, M.; Riley, K.; Sims, C. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).