The Role of Oxidative Stress in Autism Spectrum Disorder: A Narrative Literature Review
Abstract
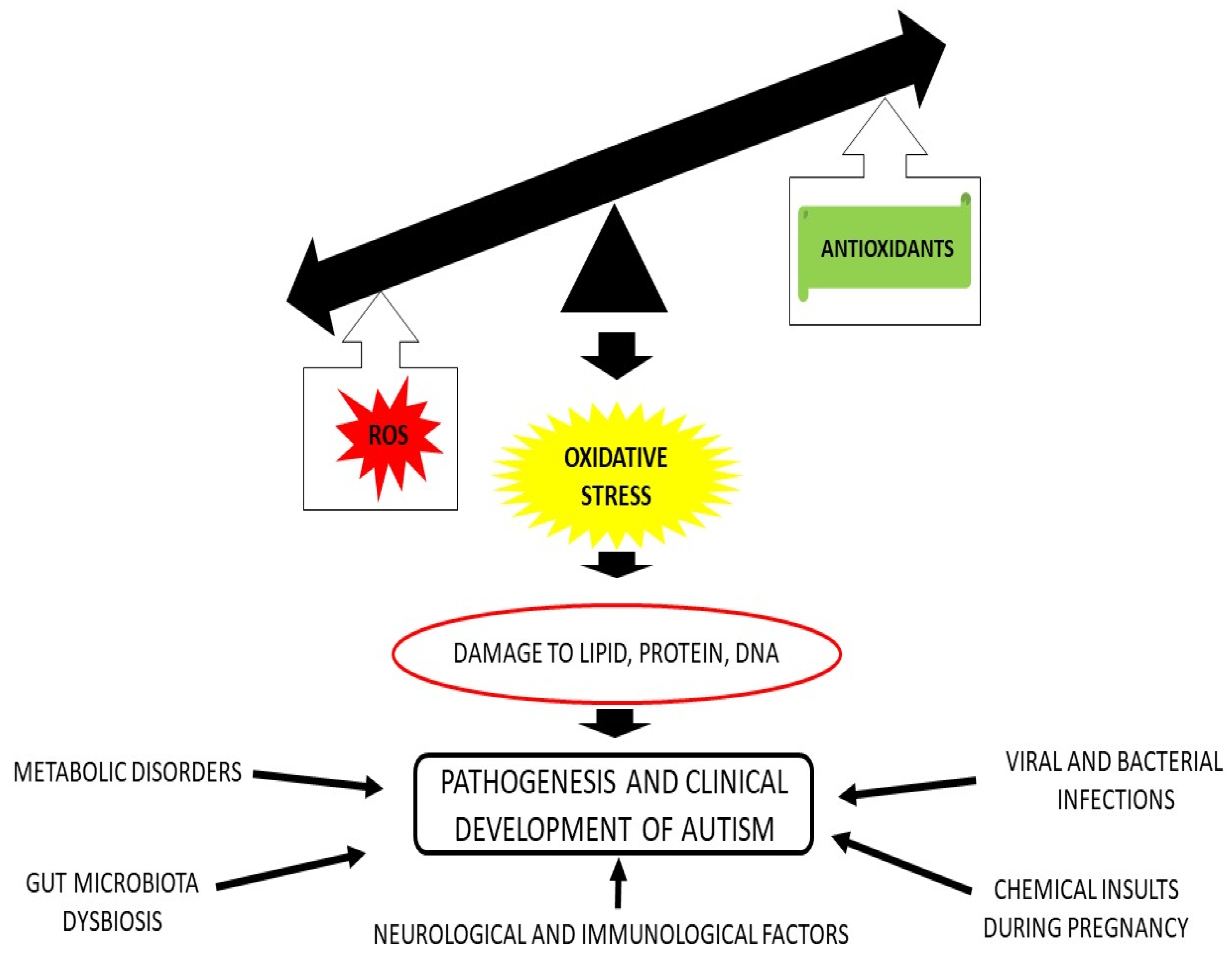
1. Introduction
2. ROS and RNS
ROS and RNS Production in ASD
3. Endogenous Enzymatic Defense
3.1. Polymorphism in Endogenous Enzymes
3.2. Endogenous Non Enzymatic Antioxidants
4. Exogenous Antioxidants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourgeron, T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Yankowitz, L.D.; Yerys, B.E.; Herrington, J.D.; Pandey, J.; Schultz, R.T. Dissociating regional gray matter density and gray matter volume in autism spectrum condition. NeuroImage Clin. 2021, 32, 102888. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rubini, E.; Baijens, I.M.M.; Horánszky, A.; Schoenmakers, S.; Sinclair, K.D.; Zana, M.; Dinnyés, A.; Steegers-Theunissen, R.P.; Rousian, M. Maternal One-Carbon Metabolism during the Periconceptional Period and Human Foetal Brain Growth: A Systematic Review. Genes 2021, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Nakatsu, T.; Kitaoka, T.; Kyotani, S. Assessment of oxidative stress in autism spectrum disorder using reactive oxygen metabolites and biological antioxidant potential. PLoS ONE 2020, 15, e0233550. [Google Scholar] [CrossRef]
- Burke, S.L.; Cobb, J.; Agarwal, R.; Maddux, M.; Cooke, M.S. How Robust is the Evidence for a Role of Oxidative Stress in Autism Spectrum Disorders and Intellectual Disabilities? J. Autism Dev. Disord. 2021, 51, 1428–1445. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Bolotta, A.; Battistelli, M.; Falcieri, E.; Ghezzo, A.; Manara, M.C.; Manfredini, S.; Marini, M.; Posar, A.; Visconti, P.; Abruzzo, P.M. Oxidative Stress in Autistic Children Alters Erythrocyte Shape in the Absence of Quantitative Protein Alterations and of Loss of Membrane Phospholipid Asymmetry. Oxidative Med. Cell. Longev. 2018, 2018, 6430601. [Google Scholar] [CrossRef]
- Ciccoli, L.; De Felice, C.; Paccagnini, E.; Leoncini, S.; Pecorelli, A.; Signorini, C.; Belmonte, G.; Guerranti, R.; Cortelazzo, A.; Gentile, M.; et al. Erythrocyte Shape Abnormalities, Membrane Oxidative Damage, and β-Actin Alterations: An Unrecognized Triad in Classical Autism. Mediat. Inflamm. 2013, 2013, 432616. [Google Scholar] [CrossRef]
- Giacometti, G.; Ferreri, C.; Sansone, A.; Chatgilialoglu, C.; Marzetti, C.; Spyratou, E.; Georgakilas, A.G.; Marini, M.; Abruzzo, P.M.; Bolotta, A.; et al. High predictive values of RBC membrane-based diagnostics by biophotonics in an integrated approach for Autism Spectrum Disorders. Sci. Rep. 2017, 7, 9854. [Google Scholar] [CrossRef]
- Bolotta, A.; Visconti, P.; Fedrizzi, G.; Ghezzo, A.; Marini, M.; Manunta, P.; Messaggio, E.; Posar, A.; Vignini, A.; Abruzzo, P.M. Na+, K+-ATPase activity in children with autism spectrum disorder: Searching for the reason(s) of its decrease in blood cells. Autism Res. 2018, 11, 1388–1403. [Google Scholar] [CrossRef]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative Stress and Erythrocyte Membrane Alterations in Children with Autism: Correlation with Clinical Features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H. Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxidative Med. Cell. Longev. 2021, 2021, 6685204. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Melnyk, S.; Jernigan, S.; Pavliv, O.; Trusty, T.; Lehman, S.; Seidel, L.; Gaylor, D.W.; Cleves, M.A. A functional polymorphism in the reduced folate carrier gene and DNA hypomethylation in mothers of children with autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153, 1209–1220. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3293. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Caito, S.W.; Aschner, M. Mitochondrial Redox Dysfunction and Environmental Exposures. Antioxid. Redox Signal. 2015, 23, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder—Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef] [PubMed]
- Carducci, F.; Ardiccioni, C.; Fiorini, R.; Vignini, A.; Di Paolo, A.; Alia, S.; Barucca, M.; Biscotti, M.A. The ALA5/ALA6/ALA7 repeat polymorphisms of the glutathione peroxidase-1 (GPx1) gene and autism spectrum disorder. Autism Res. 2022, 15, 215–221. [Google Scholar] [CrossRef]
- Ming, X.; Johnson, W.G.; Stenroos, E.S.; Mars, A.; Lambert, G.H.; Buyske, S. Genetic variant of glutathione peroxidase 1 in autism. Brain Dev. 2010, 32, 105–109. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Durocher, F.; Edwards, S.M.; Hamoudi, R.; Jackson, R.A.; Ardern-Jones, A.; Murkin, A.; Dearnaley, D.P.; Kirby, R.; Houlston, R.; et al. Association between the GCG polymorphism of the selenium dependent GPX1 gene and the risk of young onset prostate cancer. Prostate Cancer Prostatic Dis. 2002, 5, 189–192. [Google Scholar] [CrossRef]
- Hardie, L.J.; Briggs, J.A.; Davidson, L.A.; Allan, J.M.; King, R.F.; Williams, G.I.; Wild, C.P. The effect of hOGG1 and glutathione peroxidase I genotypes and 3p chromosomal loss on 8-hydroxydeoxyguanosine levels in lung cancer. Carcinogenesis 2000, 21, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, S.; Kotejarai, Z.; Goldgar, D.; Houlston, R.; Frazerwilliams, M.; Ahern, R.; Henk, J.; Gore, M.; Rhysevans, P.; Archer, D. Association between polymorphisms of the gene and second primary tumours after index squamous cell cancer of the head and neck. Oral Oncol. 2005, 41, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.P.; Gong, Y.; Grant, P.J.; Wild, C.P. Glutathione peroxidase 1 genotype is associated with an increased risk of coronary artery disease. Coron. Artery Dis. 2003, 14, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Zoroglu, S.S.; Armutcu, F.; Ozen, S.; Gurel, A.; Sivasli, E.; Yetkin, O.; Meram, I. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Yorbik, O.; Sayal, A.; Akay, C.; Akbiyik, D.I.; Sohmen, T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 341–343. [Google Scholar] [CrossRef]
- Tórsdóttir, G.; Hreidarsson, S.; Kristinsson, J.; Snaedal, J.; Jóhannesson, T. Ceruloplasmin, superoxide dismutase and copper in autistic patients. Basic Clin. Pharmacol. Toxicol. 2005, 96, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Kovač, J.; Macedoni Lukšič, M.; Trebušak Podkrajšek, K.; Klančar, G.; Battelino, T. Rare single nucleotide polymorphisms in the regulatory regions of the superoxide dismutase genes in autism spectrum disorder. Autism Res. 2014, 7, 138–144. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Nasyrova, R.F.; Moskaleva, P.V.; Vaiman, E.E.; Shnayder, N.A.; Blatt, N.L.; Rizvanov, A.A. Genetic Factors of Nitric Oxide’s System in Psychoneurologic Disorders. Int. J. Mol. Sci. 2020, 21, 1604. [Google Scholar] [CrossRef]
- Kim, H.W.; Cho, S.C.; Kim, J.W.; Cho, I.H.; Kim, S.A.; Park, M.; Cho, E.J.; Yoo, H.J. Family-based association study between NOS-I and -IIA polymorphisms and autism spectrum disorders in Korean trios. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Goin-Kochel, R.P.; Porter, A.E.; Peters, S.U.; Shinawi, M.; Sahoo, T.; Beaudet, A.L. The MTHFR 677C-->T polymorphism and behaviors in children with autism: Exploratory genotype-phenotype correlations. Autism Res. 2009, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Chen, H.; Liu, B.; Ji, W.; Yang, C. Methylenetetrahydrofolate reductase polymorphisms C677T and risk of autism in the Chinese Han population. Genet. Test. Mol. Biomark. 2012, 16, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Faber, S.; Fahrenholz, T.; Wolle, M.M.; Kern, J.C., II; Pamuku, M.; Miller, L.; Jamrom, J.; Kingston, H.M.S. Chronic exposure to xenobiotic pollution leads to significantly higher total glutathione and lower reduced to oxidized glutathione ratio in red blood cells of children with autism. Free Radic. Biol. Med. 2019, 134, 666–677. [Google Scholar] [CrossRef]
- Chen, L.; Shi, X.J.; Liu, H.; Mao, X.; Gui, L.N.; Wang, H.; Cheng, Y. Oxidative stress marker aberrations in children with autism spectrum disorder: A systematic review and meta-analysis of 87 studies (N = 9109). Transl. Psychiatry 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry. 2012, 2, e134. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, C.; Singh, A. Coenzyme Q10 a mitochondrial restorer for various brain disorders. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 2197–2222. [Google Scholar] [CrossRef]
- Raizner, A.E. Coenzyme Q10. Methodist DeBakey Cardiovasc. J. 2019, 15, 185–191. [Google Scholar] [CrossRef]
- Crane, F.L.; Löw, H.; Sun, I.; Navas, P.; Gvozdjáková, A. Plasma membrane coenzyme Q: Evidence for a role in autism. Biol. Targets Ther. 2014, 8, 199–205. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Physiological relevance of food antioxidants. Adv. Food Nutr. Res. 2020, 93, 205–250. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Natural Antioxidants: A Novel Therapeutic Approach to Autism Spectrum Disorders? Antioxidants 2020, 9, 1186. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Head, B. Vitamin E: How much is enough, too much and why! Free Radic. Biol. Med. 2021, 177, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Duconge, J.; Miranda-Massari, J.R.; Gonzalez, M.J.; Jackson, J.A.; Warnock, W.; Riordan, N.H. Pharmacokinetics of vitamin C: Insights into the oral and intravenous administration of ascorbate. Puerto Rico Health Sci. J. 2008, 27, 7–19. [Google Scholar]
- Meguid, N.A.; Anwar, M.; Bjørklund, G.; Hashish, A.; Chirumbolo, S.; Hemimi, M.; Sultan, E. Dietary adequacy of Egyptian children with autism spectrum disorder compared to healthy developing children. Metab. Brain Dis. 2017, 32, 607–615. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Llopis-González, A.; Zazpe-García, I.; Marí-Sanchis, A.; Morales-Suárez-Varela, M. Nutritional status of children with autism spectrum disorders (ASDs): A case-control study. J. Autism Dev. Disord. 2015, 45, 203–212. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Valachovicova, M.; Mislanova, C.; Hudecova, Z.; Sustrova, M.; Ostatnikova, D. Plasma concentrations of selected antioxidants in autistic children and adolescents. Bratisl. Lek. Listy 2009, 110, 247–250. [Google Scholar]
- Allen, L.H. Vitamin B-12. Adv. Nutr. 2012, 3, 54–55. [Google Scholar] [CrossRef]
- Bala, K.A.; Doğan, M.; Kaba, S.; Mutluer, T.; Aslan, O.; Doğan, S.Z. Hormone disorder and vitamin deficiency in attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASDs). J. Pediatr. Endocrinol. Metab. 2016, 29, 1077–1082. [Google Scholar] [CrossRef]
- Zhang, Y.; Hodgson, N.W.; Trivedi, M.S.; Abdolmaleky, H.M.; Fournier, M.; Cuenod, M.; Do, K.Q.; Deth, R.C. Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia. PLoS ONE 2016, 11, e0146797. [Google Scholar] [CrossRef]
- Gevi, F.; Belardo, A.; Zolla, L. A metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165859. [Google Scholar] [CrossRef] [PubMed]
- University of Louisville. Double-Blind, Placebo-Controlled, Crossover Study of Glutathione, Vitamin C and Cysteine in Children with Autism and Severe Behavior Problems. ClinicalTrials.gov; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT00889538 (accessed on 12 December 2022).
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Jardim, F.R.; de Rossi, F.T.; Nascimento, M.X.; da Silva Barros, R.G.; Borges, P.A.; Prescilio, I.C.; de Oliveira, M.R. Resveratrol and Brain Mitochondria: A Review. Mol. Neurobiol. 2018, 55, 2085–2101. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Bobermin, L.D.; Souza, D.G.; Rodrigues, M.D.; de Assis, A.M.; Wajner, M.; Gonçalves, C.A.; Souza, D.O.; Quincozes-Santos, A. Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochim. Biophys. Acta 2016, 1862, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Membrino, V.; Di Paolo, A.; Alia, S.; Papiri, G.; Vignini, A. The Role of Oxidative Stress in Autism Spectrum Disorder: A Narrative Literature Review. Oxygen 2023, 3, 34-44. https://doi.org/10.3390/oxygen3010004
Membrino V, Di Paolo A, Alia S, Papiri G, Vignini A. The Role of Oxidative Stress in Autism Spectrum Disorder: A Narrative Literature Review. Oxygen. 2023; 3(1):34-44. https://doi.org/10.3390/oxygen3010004
Chicago/Turabian StyleMembrino, Valentina, Alice Di Paolo, Sonila Alia, Giulio Papiri, and Arianna Vignini. 2023. "The Role of Oxidative Stress in Autism Spectrum Disorder: A Narrative Literature Review" Oxygen 3, no. 1: 34-44. https://doi.org/10.3390/oxygen3010004
APA StyleMembrino, V., Di Paolo, A., Alia, S., Papiri, G., & Vignini, A. (2023). The Role of Oxidative Stress in Autism Spectrum Disorder: A Narrative Literature Review. Oxygen, 3(1), 34-44. https://doi.org/10.3390/oxygen3010004






