Population Structure of the Dog Snapper, Lutjanus jocu (Bloch & Schneider, 1801), an Important Fishery Resource in the North of Bahia, Brazil: Influence of Habitat Suitability, Larvae Retention, and Fishing Pressure
Abstract
1. Introduction
2. Materials and Methods
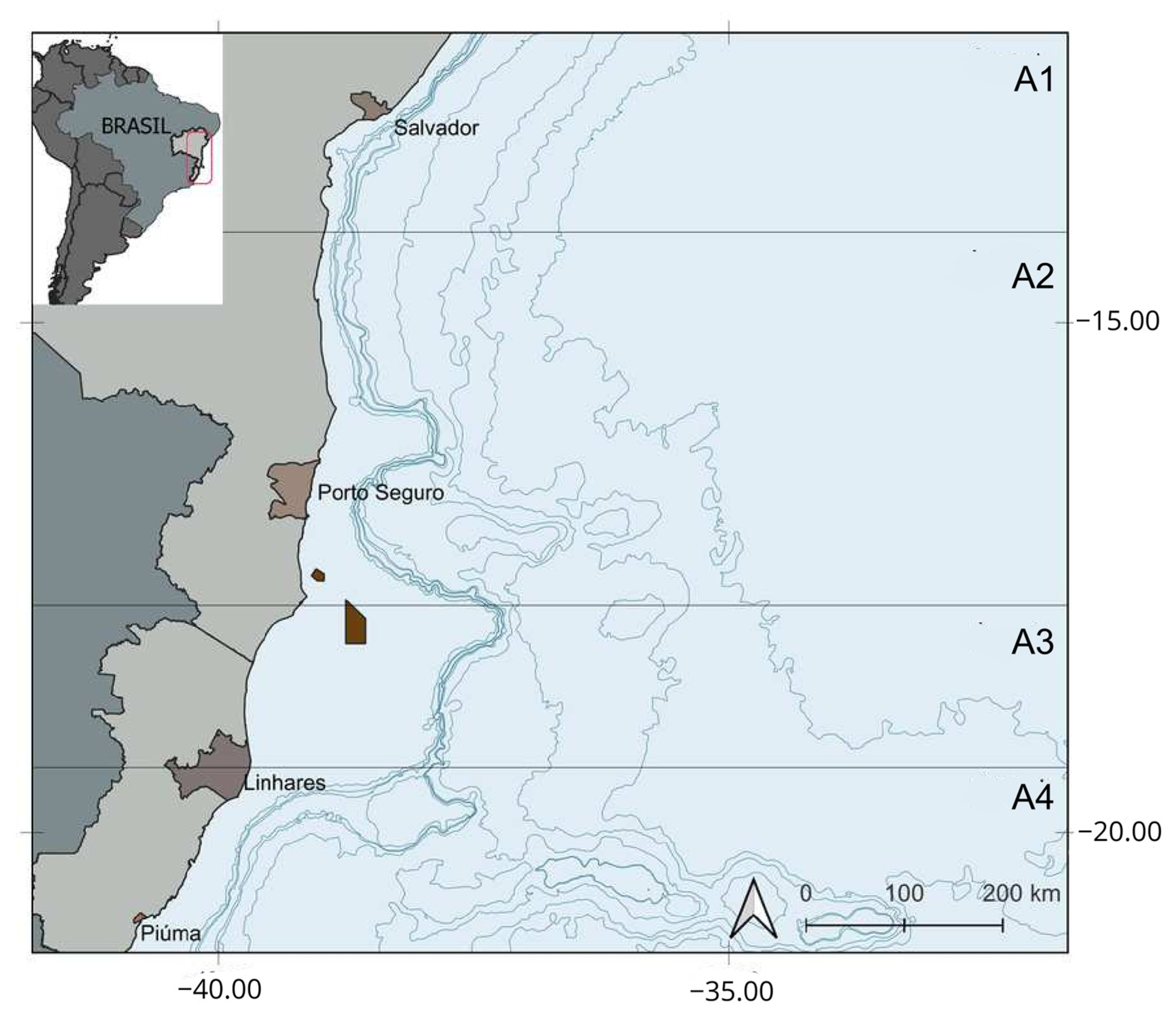
2.1. Sampling
2.2. Genetic Analyses
DNA Extraction, Amplification, and Sequencing
2.3. Data Analysis
3. Results
3.1. Genetic Variability
3.2. Genetic Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irfan, S.; Alatawi, A. Aquatic Ecosystem and Biodiversity: A Review. Open J. Ecol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Mar-Silva, A.F.; Diaz-Jaimes, P.; Doménguez-Mendonza, C.; Domínguez-Domínguez, O.; Val-diviezo-Riveira, J.; Espinoza-Herrera, E. Genomic assessment reveals signal of adaptive selection in populations of the Spotted rose snapper Lutjanus guttatus from the Tropical Eastern Pacific. PeerJ 2023, 11, 150–176. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Sampaio, I.; Schneider, H.; Gomes, G. Lack of spatial subdivision for the snapper Lutjanus purpureus (Lutjanidae-Perciformes) from southwest Atlantic based on multi-locus analyses. PLoS ONE 2016, 11, e0161617. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Capuli, E.; Garilao, C.; Pauly, D. The Species Table. Fish Base: Concepts Design and Data Sources; ICLARM: Los Baños, Philippines, 2000; pp. 76–85. [Google Scholar]
- Cruz, V.P.; Adachi, A.M.L.; Oliveira, P.H.; Ribeiro, G.S.; Paim, F.G.; Souza, B.C.; Rodrigues, A.S.; Vianna, M.; Delpiani, S.M.; de Astarloa, J.M.D.; et al. Genetic diversity in two threatened species of guitarfish (Elasmobranchii: Rhinobatidae) from the Brazilian and Argentinian coasts: An alert for conservation. Neotrop. Ichthyol. 2021, 19, e210012. [Google Scholar] [CrossRef]
- Veneza, I.; Silva, R.; Ferreira, C.; Mendonça, P.; Sampaio, I.; Evangelista-Gomes, G. Genetic connectivity and population expansion inferred from multilocus analysis in Lutjanus alexandrei (Lutjanidae–Perciformes), an endemic snapper from Northeastern Brazilian coast. PeerJ 2023, 11, e15973. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Barshis, D.J.; Traylor-Knowles, N.; Bay, R.A. Mechanisms of reef coral resistance to future climate change. Science 2014, 344, 895–898. [Google Scholar] [CrossRef]
- Gandra, M.; Assis, J.; Martins, M.R.; Abecasis, D. Reduced global genetic differentiation of exploited marine fish species. Mol. Biol. Evol. 2021, 38, 1402–1412. Available online: https://academic.oup.com/mbe/article/38/4/1402/6020262 (accessed on 1 August 2024). [CrossRef]
- Keenan, K.; McGinnity, P.; Cross, T.F.; Crozier, W.W.; Prodöhl, P.A. diveRsity: An R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol. Evol. 2013, 4, 782–788. [Google Scholar] [CrossRef]
- Aschenbrenner, A.; Hackradt, C.W.; Ferreira, B.P. Spatial variation in density and size structure indicate habitat selection throughout life stages of two southwestern Atlantic snappers. Mar. Environ. Res. 2016, 113, 49–55. [Google Scholar] [CrossRef]
- Menezes, R.; Giglio, V.J.; Albuquerque, C.Q.; Rosa, R.S. A review of the dog snapper (Lutjanus jocu) along the Brazilian province: Distributional records, ecology, fisheries and conservation. Ocean. Coast Manag. 2022, 220, 106094. [Google Scholar] [CrossRef]
- Medeiros, P.R. Distribuição e Uso de Habitat por Peixes Recifais e sua Relação com a Complexidade Ambiental no Arquipélago Fernando de Noronha, Nordeste do Brazil. Ph.D. Thesis, Universidade Federal da Paraíba, João Pessoa, Brazil, 2011. [Google Scholar]
- Bezerra, I.M.; Hostim-Silva, M.; Teixeira, J.L.S.; Hackradt, C.W.; Félix-Hackradt, F.C.; Schiavetti, A. Spatial and temporal patterns of spawning aggregations of fish from the Epinephelidae and Lutjanidae families: An analysis by the local ecological knowledge of fishermen in the Tropical Southwestern Atlantic. Fish Res. 2021, 239, 105937. [Google Scholar] [CrossRef]
- Lutz, Í.; Martins, K.; Cardoso, B.; Miranda, A.; Costa, J.L.; Silva, I.; Evangelista-Gomes, G. Connectivity and high genetic diversity in populations of the dog snapper Lutjanus jocu (Lutjanidae: Perciformes) from the South Western Atlantic, recovered with multilocus analysis. Environ. Biol. Fishes 2024, 107, 1121–1135. [Google Scholar] [CrossRef]
- Verba, J.T.; Stow, A.; Bein, B.; Pennino, M.G.; Lopes, P.F.M.; Ferreira, B.P.; Mortier, M.; Lima, Q.M.S.; Pereira, R.J. Low population genetic structure is consistent with high habitat connectivity in a commercially important fish species (Lutjanus jocu). Mar. Biol. 2023, 170, 5. [Google Scholar] [CrossRef]
- de Souza, A.S.; Dias, E.A., Jr.; Perez, M.F.; Cioffi, M.d.B.; Bertollo, L.A.C.; Garcia-Machado, E.; Vallinoto, M.N.S.; Manoel, G.P.; Molina, W.F. Phylogeography and historical demography of two sympatric Atlantic snappers: Lutjanus analis and L. jocu. Front. Mar. Sci. 2019, 6, 545–552. [Google Scholar] [CrossRef]
- Schmidt, C.; Karachaliou, E.; Vandergast, A.G.; Crandall, E.D.; Falgout, J.; Hunter, M.E.; Garroway, C.J. A survey of mammal and fish genetic diversity across the global protected area network. Conserv. Lett. 2025, 18, e13092. [Google Scholar] [CrossRef]
- Shuzitski, K.; McCartney, M.A.; Burton, M.L. Population connectivity among Dry Tortugas, Florida, and Caribbean populations of mutton snapper (Lutjanus annalis), inferred from multiple microsatellite loci. Fish. Bull. 2009, 107, 501–509. [Google Scholar]
- Renshaw, M.A.; Karlsson, S.; Gold, J.R. Isolation and characterization of microsatellites in lane snapper (Lutjanus synagris), mutton snapper (Lutjanus analis), and yellowtail snapper (Ocyurus chrysurus). Mol. Ecol. 2007, 7, 1084–1087. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1471-8286.2007.01785.x (accessed on 1 August 2024). [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Rosenberg, N.A.; Donnelly, P. Association mapping in structured populations. Am. J. Hum. Genet. 2000, 67, 170–181. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grunwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2014, e281. [Google Scholar] [CrossRef]
- Jost, L. GST and its relatives do not measure differentiation. Mol. Ecol. 2008, 17, 4015–4026. Available online: https://pubmed.ncbi.nlm.nih.gov/19238703/ (accessed on 1 August 2024). [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. Available online: https://www.jstor.org/stable/2408641 (accessed on 1 August 2024). [CrossRef]
- Jombart, T.; Ahmed, I. Adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Goudet, J.; Raymond, M.; De Meeûs, T.; Rousset, F. Hierfstat: Estimating hierarchical F-statistics. In R Package Version; R-Project: Vienna, Austria, 2022; pp. 5–7. [Google Scholar]
- Prichard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Francis, R.M. Pophelper: An R package and web app to analyze and visualize population structure. Mol. Ecol. Resour. 2017, 17, 27–32. [Google Scholar] [CrossRef]
- Jombat, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 94, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Chessel, D.; Dufour, A.B.; Thioulous, J. The ade4 package-I-One-table methods. R News 2004, 4, 5–10. [Google Scholar]
- Garza, J.C.; Williamson, E.G. Detection of reduction in population size using data from microsatellite loci. Mol. Ecol. 2001, 10, 305–318. Available online: https://pubmed.ncbi.nlm.nih.gov/11298947/ (accessed on 1 August 2024). [CrossRef]
- Allendorf, F.W.; Luikart, G. Conservation and the Genetics of Populations; Blackwell Publishing: Hoboken, NJ, USA, 2007; Volume 1, pp. 1–642. [Google Scholar]
- Hedgecock, D.; Pudovkin, A.I. Sweepstakes reproductive success in highly fecund marine fish and shellfish: A review and commentary. Bull. Mar. Sci. 2011, 87, 971–1002. Available online: https://www.ingentaconnect.com/content/umrsmas/bullmar/2011/00000087/00000004/art00013;jsessionid=6tdjmv3mi72b.x-ic-live-01 (accessed on 1 August 2024). [CrossRef]
- Luikart, G.; Allendorf, F.W.; Cornuet, J.M.; Sherwin, W.B. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 1998, 89, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Veneza, I.; Sampaio, I.; Araripe, J.; Schneider, H.; Gomes, G. High Levels of Genetic Connectivity among Populations of Yellowtail Snapper, Ocyurus chrysurus (Lutjanidae–Perciformes), in the Western South Atlantic Revealed through Multilocus Analysis. PLoS ONE 2015, 10, e0122173. [Google Scholar] [CrossRef] [PubMed]
- Verba, T.J.; Ferreira, C.E.; Pennino, M.G.; Hagberg, L.; Lopes, P.F.; Padovani, F.B.; Stow, A. Genetic structure of the threatened Gray Parrotfish (Sparisoma axillare) in the Southwestern Atlantic. Coral Reefs 2023, 42, 105–117. [Google Scholar] [CrossRef]
- Silva, J.C.; Soeth, M.; Hackradt, C.W.; Lima, A.; Félix-Hackradt, F.C. Daily age, growth rate, and pelagic larval duration of commercially important snapper species in Abrolhos National Marine Park. Fish Biol. 2023, 104, 1411–1422. [Google Scholar] [CrossRef]
- Rocha, L.A. Patterns of distribution and processes of speciation in Brazilian reef fishes. J. Biogeogr. 2003, 30, 1161–1171. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2699.2003.00900.x (accessed on 1 August 2024). [CrossRef]
- Luiz, O.J.; Madin, J.S.; Robertson, D.R.; Rocha, L.A.; Wirtz, P.; Floeter, S.R. Ecological traits influencing range expansion across large oceanic dispersal barriers: Insights from tropical Atlantic reef fishes. Proc. R. Soc. Lond. B 2012, 279, 1033–1040. [Google Scholar] [CrossRef]
- Evangelista-Gomes, F.L.; Santana, C.S.; Costa, J.M.; Lessa, R.P. Genetic diversity and population structure of Lutjanus analis (Cuvier, 1828) from the Brazilian coast using mitochondrial DNA. J. Fish Biol. 2020, 97, 1093–1105. [Google Scholar] [CrossRef]
- Pinheiro, H.T.; Rocha, L.A.; Macieira, R.M.; Carvalho-Filho, A.; Anderson, A.B.; Bender, M.G.; Di Dario, F.; Ferreira, C.E.L.; Figueiredo-Filho, J.; Francini-Filho, R.; et al. South-western Atlantic reef fishes: Zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Divers. Distrib. 2018, 24, 951–965. [Google Scholar] [CrossRef]
- Félix-Hackradt, F.C.; Hackradt, C.W.; Pérez-Ruzafa, Á.; García-Charton, J.A. Discordant patterns of genetic connectivity between two sympatric species, Mullus barbatus (Linnaeus, 1758) and Mullus surmuletus (Linnaeus, 1758), in south-western Mediterranean Sea. Mar. Environ. Res. 2013, 92, 23–34. [Google Scholar] [CrossRef]
- Rebouças, R.C.; Dominguez, J.M.; Avena, P.P.; Nunes, A.S.; Melo, L.C. Continental shelf habitats off a large South American metropolis: Salvador City, Eastern Brazil: Continental shelf habitats off a Brazil City. In Seafloor Geomorphology as Benthic Habitat; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 473–485. [Google Scholar] [CrossRef]
- Leão, Z.M.A.N.; Kikuchi, R.K.P.; Testa, V. Corals and coral reefs of Brazil. In Latin American Coral Reefs; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 9–52. [Google Scholar]
- Mello, U.C.P.; Summerhayes, J.P. Upper continental margin sedimentation off Brazil, Part IV: Salvador to Vitoria, Southeastern Brazil. Contrib. Sedimentol. 1975, 4, 78–116. [Google Scholar]
- Moura, R.L. Brazilian reefs as priority areas for biodiversity conservation in the Atlantic Ocean. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; Volume 2, pp. 917–920. [Google Scholar]
- Francini-Filho, R.B.; Coni, E.O.C.; Meirelles, P.M.; Amado-Filho, G.M.; Thompson, F.L.; Pereira-Filho, G.H.; Bastos, A.C.; Abrantes, D.P.; Ferreira, C.M.; Gibran, F.Z. Dynamics of coral reef benthic assemblages of the Abrolhos Bank, eastern Brazil: Inferences on natural and anthropo-genic drivers. PLoS ONE 2013, 8, e054260. [Google Scholar] [CrossRef] [PubMed]
- Negrão, F.; Lacerda, C.H.F.; Melo, T.H.; Bianchini, A.; Calderon, E.N.; Castro, C.B.; Sumida, P.Y.G. The first biological survey of the Royal Charlotte Bank (SW Atlantic) reveals a large and di-verse ecosystem complex. Estuar. Coast. Shelf Sci. 2021, 255, 107363. [Google Scholar] [CrossRef]
- Freitas, M.O.; Abilhoa, V.; Spach, H.L.; Minte-Vera, C.V.; Francini-Filho, R.B.; Kaufman, L.; Moura, R.L. Feeding ecology of two sympatric species of large-sized groupers (Perciformes: Epinephelidae) on Southwestern Atlantic coralline reefs. Neotrop. Ichthyol. 2017, 15, e160047. [Google Scholar] [CrossRef]
- França, A.R.; Olavo, G.; Rezende, S.M.; Ferreira, B.P. Spatio-temporal distribution of mutton snapper and dog snapper spawning aggregations in the South-west Atlantic. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 3, 1596–1610. [Google Scholar] [CrossRef]
- Kadison, E.; Nemeth, R.S.; Herzlieb, S.; Blondeau, J. Temporal and spatial dynamics of Lutjanus cyanopterus (Pisces: Lutjanidae) and L. jocu spawning aggregations in the United States Virgin Islands. Rev. Biol. Trop. 2006, 54, 69–78. [Google Scholar]
- Previero, M.; Minte-Vera, C.V.; Freitas, M.O.; Moura, R.L.; Tos, C.D. Age and growth of the dog snapper Lutjanus jocu (Bloch & Schneider, 1801) in Abrolhos Bank. Northeastern Brazil. Neotrop. Ichthyol. 2011, 9, 393–401. [Google Scholar] [CrossRef]
- Frédou, T.; Ferreira, B.P.; Letourneur, Y. Assessing the stocks of the primary snappers caught in Northeastern Brazilian reef systems. 1: Traditional modelling approaches. Fish. Res. 2009, 99, 90–96. [Google Scholar] [CrossRef]
- Nunes, J.d.A.C.d.C.; Medeiros, D.V.; Reis-Filho, J.A.; Sampaio, C.L.S.; Barros, F. Reef fishes captured by recreational spearfishing on reefs of Bahia State, northeast Brazil. Biota Neotrop. 2012, 12, 179–185. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Palumbi, S.R. Meta-analysis reveals lower genetic diversity in overfished populations. Mol. Ecol. 2014, 23, 29–39. [Google Scholar] [CrossRef]
- Kough, A.S.; Claro, R.; Lindeman, K.C.; Paris, C.B. Decadal analysis of larval connectivity from Cuban snapper (Lutjanidae) spawning aggregations based on biophysical modeling. Mar. Ecol. Prog. Ser. 2016, 550, 175–190. [Google Scholar] [CrossRef]
- Lessa, G.C.; Dominguez, J.M.; Bittencourt, A.C.; Brichta, A. The tides and tidal circulation of Todos os Santos Bay, Northeast Brazil: A general characterization. An. Acad. Bras. Ciências 2001, 73, 245–261. [Google Scholar] [CrossRef]
- Previero, M.; Gasalla, M.A. Risk assessment of small-scale reef fisheries off the Abrolhos Bank: Snappers and groupers under a multidimensional evaluation. Fish. Manag. Ecol. 2020, 27, 231–247. [Google Scholar] [CrossRef]
- Eggertsen, L.; Luza, A.L.; Cordeiro, C.A.; Dambros, C.; Ferreira, C.E.; Floeter, S.R.; Bender, M.G. Complexities of reef fisheries in Brazil: A retrospective and functional approach. Rev. Fish Biol. Fish. 2024, 34, 511–538. [Google Scholar] [CrossRef]


| Localities | SSA | PS | ABR | ES |
|---|---|---|---|---|
| n | 30.3 | 35.1 | 40 | 14.6 |
| A | 38 | 41 | 41 | 36 |
| Ar | 3.66 | 3.83 | 3.73 | 3.42 |
| Ap | 0 | 1 | 1 | 1 |
| HO | 0.65 | 0.59 | 0.62 | 0.60 |
| He | 0.64 | 0.63 | 0.61 | 0.58 |
| FIS | −0.01 | 0.06 | −0.02 | −0.03 |
| M-ratio | 0.80 | 0.69 | 0.81 | 0.82 |
| Source of Variation | Df | Sum Sq | Mean Sq | Sigma | % of Total |
|---|---|---|---|---|---|
| Between groups | 1 | 43.303 | 43.30 | 0.60 | 15.72 |
| Within groups | 2 | 26.116 | 13.05 | 0.35 | 9.12 |
| Within populations | 123 | 350.617 | 2.89 | 2.89 * | 75.15 |
| Total | 126 | 420.037 | 59.24 | 3.85 | 100 |
| Abrolhos | Espírito Santo | Porto Seguro | Salvador | |
|---|---|---|---|---|
| Abrolhos | 0.029 | 0.059 | 0.111 ** | |
| Espírito Santo | 0.012 | 0.092 ** | 0.103 ** | |
| Porto Seguro | 0.024 | 0.043 * | 0.112 ** | |
| Salvador | 0.075 * | 0.077 * | 0.074 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, G.C.; De Biasi, J.B.; Hackradt, C.W.; Félix-Hackradt, F.C. Population Structure of the Dog Snapper, Lutjanus jocu (Bloch & Schneider, 1801), an Important Fishery Resource in the North of Bahia, Brazil: Influence of Habitat Suitability, Larvae Retention, and Fishing Pressure. Coasts 2025, 5, 21. https://doi.org/10.3390/coasts5020021
Marques GC, De Biasi JB, Hackradt CW, Félix-Hackradt FC. Population Structure of the Dog Snapper, Lutjanus jocu (Bloch & Schneider, 1801), an Important Fishery Resource in the North of Bahia, Brazil: Influence of Habitat Suitability, Larvae Retention, and Fishing Pressure. Coasts. 2025; 5(2):21. https://doi.org/10.3390/coasts5020021
Chicago/Turabian StyleMarques, Glaciane Conceição, Juliana Beltramin De Biasi, Carlos Werner Hackradt, and Fabiana Cezar Félix-Hackradt. 2025. "Population Structure of the Dog Snapper, Lutjanus jocu (Bloch & Schneider, 1801), an Important Fishery Resource in the North of Bahia, Brazil: Influence of Habitat Suitability, Larvae Retention, and Fishing Pressure" Coasts 5, no. 2: 21. https://doi.org/10.3390/coasts5020021
APA StyleMarques, G. C., De Biasi, J. B., Hackradt, C. W., & Félix-Hackradt, F. C. (2025). Population Structure of the Dog Snapper, Lutjanus jocu (Bloch & Schneider, 1801), an Important Fishery Resource in the North of Bahia, Brazil: Influence of Habitat Suitability, Larvae Retention, and Fishing Pressure. Coasts, 5(2), 21. https://doi.org/10.3390/coasts5020021







