Abstract
Intraspecific variations in the morphological traits of juveniles and adults of the Brazilian silverside, Atherinella brasiliensis, from three estuarine habitats were studied to understanding whether their morphology interacts with their dietary composition and habitat structure. For each individual, fourteen morphological measurements and eight functional traits were recorded related to food acquisition and locomotion. The highest abundance of A. brasiliensis was recorded in mudflats, which were often associated with a greater number of juveniles. Overall, 392 A. brasiliensis stomachs were examined, and their diet comprised mainly zooplankton organisms, followed by insects and benthic crustaceans. Among the morphological measures, our data revealed that in vegetated habitats (seagrass and riparian vegetation), individuals showed a higher oral gape surface and caudal peduncle and fed predominately on epibiotic or benthic fauna, while for individuals that had bigger eyes in unvegetated habitats (mudflat), this facilitated the ingestion of zooplankton and diatoms. Furthermore, a greater relative body height recorded in unvegetated habitats enhanced swimming performance and was linked to the effects of the lowest habitat structure. The results highlight the significant effects of morphological variation on juvenile and adult food acquisition and swimming ability.
1. Introduction
In tropical estuaries, fish species use multiple habitats during their life cycle, where the larvae and juveniles are abundant within the structurally complex habitats found in shallow areas, while adults occur in deep waters where habitat conditions tend to be much more homogeneous [1,2,3,4] There is a dependency in the use of estuaries during early life stages, often linked to the protection of predators and the provision of the largest amount of food resources available to fish species [5,6,7]. These characteristics have been mainly used to assess the value of a mosaic of estuarine habitat types recognised as nursery areas and particularly for vegetated and structured habitats such as seagrass [8,9,10], saltmarshes [7], and mangroves [11,12]. However, many fish species are highly dependent on unvegetated habitats that they use for nursery grounds, such as mudflats [13,14] and beaches [15,16]. While not functioning as a nursery, other vegetated habitats are also important sources of food and shelter for juvenile and adult fishes, such as riparian vegetation [17]. However, in the last few years, there has been an increase in studies that have emphasised the value of estuarine habitats for the ichthyofauna that use these environments [1,5,18,19].
Ecological morphology, or ecomorphology, is considered a useful tool for understanding the interrelationships between morphological variations that occur during ontogenetic phases and food resources used by fishes in different estuarine habitats [20]. Some authors suggest that the body shape of fishes and their way of life are closely correlated and are a good predictor of ecology [21,22,23]. For example, fish species that shift to other resources during ontogeny show modifications in body characteristics, and thus, show the large influence and relevance of ecological fitness and changes in feeding modes to optimise their competitive ability [24,25]. In particular, the study of fish ecomorphology has been fully integrated with the concept of trophic plasticity [26,27].
The Brazilian silverside, Atherinella brasiliensis (Quoy & Gaimard, 1854) (Atheriniformes: Atherinopsidae), is a common estuarine resident and generalist species and is abundant in estuaries and other nearshore shallow water ecosystems [28,29], such as beaches [15,16], coastal lagoons [30,31], and bays [32,33]. Furthermore, A. brasiliensis has shown its importance, having a key structuring role in estuarine food webs [34,35], because generalist species are able to feed on and exploit prey from multiple basal nutrient sources [36]. Generally, zooplankton, insects, and diatoms form the principal diet of Brazilian silversides in estuaries [37].
An improved understanding of how ecomorphology affects diet and prey choice in fishes is important for gaining fundamental knowledge on the range of prey sizes available in the environment [38,39] and to predict the natural dynamics of estuarine systems [22]. Intraspecific variations in morphological characteristics may generate a differential use of habitats by fish populations and can provide interesting information about the functional meaning of phenotypic characteristics [23]. Many researchers have reported changes in juvenile fish feeding between vegetated and unvegetated habitats, and most of these studies have shown that they are highly associated with environmental prey availability [13,32,40] or fish body size [27,41,42].
The present study aims to determine to what extent dietary compositions and habitat use of juvenile and adult Brazilian silversides are influenced by changes in morphological variation. We expect that structural variation in habitats mediates effects on traits related to performance aspects, foraging efficiency, and swimming for Brazilian silverside in estuaries [25,43]. Specifically, we hypothesised that in vegetated habitats (Seagrass and Riparian vegetation), individuals of A. brasiliensis would have a “deep body shape” for an improved ability to manoeuver, detect, and capture prey in these shallow habitats.
2. Materials and Methods
2.1. Study Area
The Mamanguape river estuary is located on the north coast of Paraíba state in northeastern Brazil and is part of the Environmental Protection Area (Área de Proteção Ambiental—APA) of Barra de Mamanguape (6°43′02″ S 35°67′46″ W) (Figure 1). The estuary presents approximately 25 km of extension, constituting an area of 16,400 ha, and its mouth is partially closed by a coastal reef line that act as a natural barrier protecting the entry of waves from oceanic waters [44]. There is a well-preserved forest mangrove in the area that extends by 6000 ha, which grows around the primary channel and tidal creek, in addition to Atlantic Forest remnants [45]. Endangered species, such as the seahorse, Hippocampus reidi Ginsburg, 1933, and the West Indian manatee, Trichechus manatus Linnaeus, 1978, inhabit this estuary [46]. The regional climate is classified by Köppen as As-type (hot and humid) [47]. The average rainfall recorded in the area is between 1750 and 2000 mm annually, and the average temperature is approximately 24–26 °C.
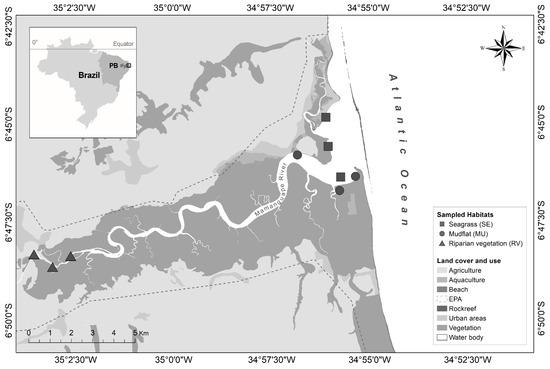
Figure 1.
Mamanguape river estuary, northeast Brazil. Habitats: seagrass (■), mudflat (●), and riparian vegetation (▲). Delimitation of the Environmental Protection Area of Barra de Mamanguape is represented by the dashed outline in light gray.
Three habitats were sampling along an estuarine salinity gradient and distinct physiographic features (Figure 1): (1) seagrass: subtidal beds formed by the species Halodule wrightii Ascherson (1868), Halophila decipiens Ostenfeld (1902), and Halophila baillonis Ascherson (1874), with a sandy substrate that forms sandbars exposed during low tide [10,48], 2018); (2) mudflat: unvegetated tidal bottoms found in protected estuaries characterized by a lower influence of waves and fine sediment (somewhat muddy) and which extend as far as 1 km during low tide [13,49]; and (3) riparian vegetation: vegetated area covered by shoreland and freshwater emergent aquatic plants and sandy sediment [44]. Both seagrass and mudflat habitats were delimited in the euhaline estuary region, while riparian vegetation habitat was delimited in the oligohaline estuary region.
2.2. Sampling
Samplings were carried out during three rainy-season months (June to August 2015) and three dry-season months (October and November 2015, and January 2016). Fish were collected with a beach seine net (10 m long; 1.5 m height; stretched mesh size of 5 mm), which was hauled parallel to the shore for approximately 30 m and to a maximum depth of 1.5 m during low tide. The sampling unit was standardized with 3 replicates in an effort to capture individuals that use the area for feeding, separated 50 m apart to minimize the influence on the following hauls. The sample design contained a total of 162 samples (3 habitats × 3 samples × 3 replicates × 6 months). The collected fish were anaesthetized on ice and stored in 70% alcohol. In the laboratory, all fishes were identified, counted, measured (total length, mm), and weighed (total weight; g) and then fixed in 10% formaldehyde and stored in 70% alcohol for conservation.
2.3. Morphological Traits
Fourteen morphological traits were measured based on [23], [32], and [50] (Figure 2). For each individual, the following measurements were taken: total length (TL, mm) and standard length (SL, mm). A numeric vernier caliper (0.1 mm precision) was used to measure body height (BH), body width (BW), head length (HL), head height (HH), relative eye height (ERH), pectoral fin length (PFL), pectoral fin width (PFW), caudal peduncle length (CPL), caudal peduncle height (CPH), caudal peduncle width (CPW), mouth width (WM), and mouth height (HM).
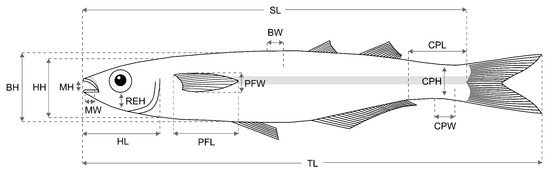
Figure 2.
A representative species, Atherinella brasiliensis, with fourteen (14) morphological traits: total length (TL), standard length (SL), body height (BH), body width (BW), head height (HH), head length (HL), relative eye height (REH), mouth height (HM), mouth width (WM), pectoral fin length (PFL), pectoral fin width (PFW), caudal peduncle height (CPH), caudal peduncle length (CPL), and caudal peduncle width (CPW).
Morphological measurements were transformed following the method of [51] (Table 1). These measurements led to the estimation of 8 ecomorphological indices related to position in the water column, locomotion, and food acquisition (Table 1) [23,32,52,53,54]. All individuals were subsequently grouped into two size-based classes according to length at first maturity (L50 = 80 mm): juveniles (<79 mm) and adults (≥80 mm) [55].

Table 1.
Ecomorphological indices with respective formulae, functions, and ecological meaning. Formulae include the following measurements: standard length (SL), body height (BH), body width (BW), head length (HL), head height (HH), relative eye height (ERH), pectoral fin length (PFL), pectoral fin width (PFW), caudal peduncle length (CPL), caudal peduncle height (CPH), caudal peduncle width (CPW), mouth width (WM), and mouth height (HM).
2.4. Diet Analysis
To analyze the diet of each individual, the volume percentage (%V) of different food items was measured [56]. The volumes of each item were measured in similar way to [57] and analyzed by displacement methods. Each item was evenly compressed between two glass plates, and the volume was recorded on a Petri dish with a 100 grid points. Prey items and fragments falling upon each of the 100 grid points were counted, and the volume was recorded. The total volume of each item was obtained by summing individual volumes across all samples. The volumetric proportion of each item was then calculated on the basis of the total volume of food eaten per consumer. Although the volumes of both unidentifiable materials were also calculated, these were not considered valid dietary categories and were not included in subsequent dietary analysis.
Food items were identified to the lowest possible taxonomic level and subsequently grouped into the following taxonomic categories: plant material (algae debris and plant); zooplankton (Calanoida, Cyclopoida, and Harpacticoida copepods, Bivalvia and Gastropoda larvae, Decapoda larvae, fish eggs, and invertebrates); insects; polychaeta; fish (fish and scales); mollusca (Bivalvia, Gastropoda, and Scaphopoda); decapoda (crabs and shrimp); benthic or epibenthic crustaceans (Amphipods, Caprella amphipods, Gammaridea amphipods, Cirripedia, Cumacea, Isopoda, Mysidacea, Ostracoda, and Tanaidacea); infaune (Foraminifera, Nematoda, Sipuncula, and Trematoda); and phytobenthos (diatoms) [13,32] (see Supplementary Materials).
2.5. Statistical Analyses
One-way analysis of variance (with a significance level set at p < 0.05) was used to compare fish abundance (number of individuals/hauls) between habitats, and two-way analysis of variance (with a significance level set at p < 0.05) was used to compare functional traits between habitats (three levels: seagrass, mudflat, and riparian vegetation) and size classes (two levels: juvenile and adult). All data (functional traits and fish abundance) were previously log-transformed using log10(x + 1), where x is the raw value, to address the assumptions of normality and homogeneity of variance in the parametric analyses. A post hoc Tukey’s HSD test followed the ANOVA procedures every time the null hypothesis was rejected at α = 0.05 [58]. ANOVA was performed using the open-access R software version 4.2.2 [59].
In order to determine the relative role of the functional traits of juvenile and adults of A. brasiliensis in relation to feeding categories availability in three habitats, we used distance-based linear models (DistLMs) [60,61], using the Akaike information criterion (AIC) to select the best explanatory model. AIC is a goodness-of-fit measure that favours smaller residual error in the model, and its values do not continue to improve with increases in the number of predictor variables in the model [62]. Distance-based redundancy analysis (dbRDA) was used to evaluate the influence of predictor variables (morphological traits) on the results of prey abundance in each habitat. The dbRDA is considered as the most robust equivalent of redundancy analysis (RDA) when data imposes the use of the Bray–Curtis distance [60]. A food resource matrix was built containing the volume of food items for each size classes, and a secondary matrix included the functional traits. Functional traits were log-transformed (log(x + 1), and feeding categories data were square-root-transformed. All multivariate analyses were performed using the statistical package PRIMER v6 + PERMANOVA [62,63].
3. Results
3.1. Distribution and Abundance
A total of 3969 individuals (n = 863 in seagrass, n = 2270 in mudflats, and n = 836 in riparian vegetation) were caught. During the study, the highest abundance was recorded in mudflats, with the ANOVA test (p < 0.001) indicating significant spatial differences (Figure 3).
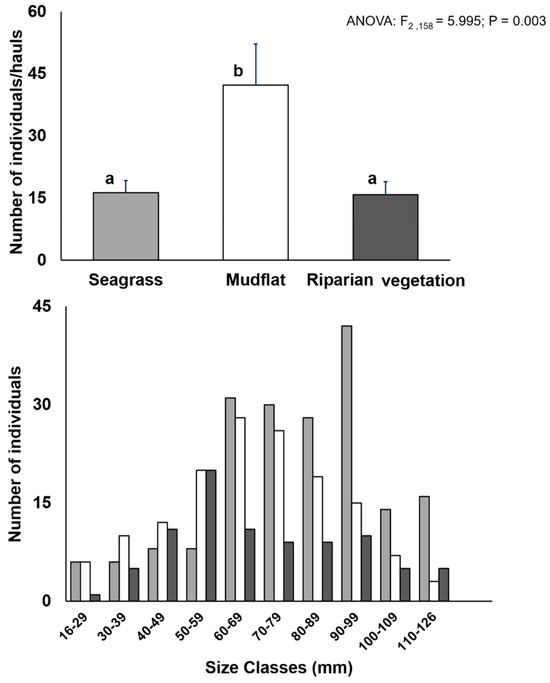
Figure 3.
(Upper): spatial variation in number of individuals (CPUE average ± SE); (lower): size distributions of Atherinella brasiliensis in the estuarine habitats (seagrass, mudflat, and riparian vegetation). Different superscript letters indicate significant difference (p < 0.05) compared by ANOVA followed by Tukey’s test.
The distribution of size classes showed juveniles and adults ranging in size from 16–126 mm in total length; the size of A. brasiliensis differed among sites. The smallest individuals (juveniles) showed the highest abundance in mudflats, whereas the largest individuals were mainly in seagrass (Figure 3).
3.2. Diet
For the diet study of A. brasiliensis, the stomach contents of 423 individuals were analysed, of which 392 stomachs with prey were analysed and 31 stomachs were empty (7.3%). The diet of the species consisted of various prey items (see Supplementary Materials). In general, the diets comprised mainly zooplankton organisms, followed by insects and benthic crustaceans (Figure 4). The diet in the seagrass environment predominantly comprised zooplankton and benthic crustaceans, while zooplankton dominated in the diet in the mudflats. In the riparian vegetation, insects and benthic crustaceans were found in large volumes in the stomach contents (Figure 4).
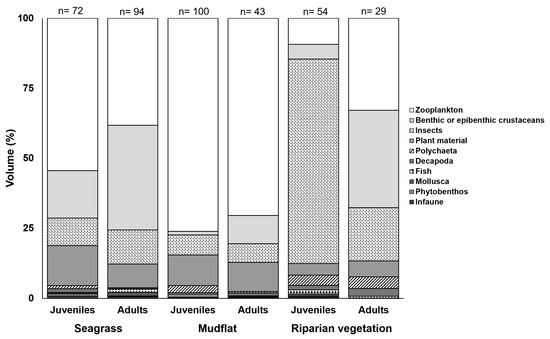
Figure 4.
Percentage volumetric contribution (%V) of the most important food items in the diet of Atherinella brasiliensis in relation to size classes in each estuarine habitat (seagrass, mudflat, and riparian vegetation). n = numbers at the top of each graph are the numbers of stomachs analyzed for each habitat.
In general, the juveniles selected zooplankton prey items in both seagrass and mudflat areas, whereas insect appeared to be of greater importance to juveniles in riparian vegetation areas (Figure 4). Adults of A. brasiliensis consumed insect and benthic crustaceans, especially in seagrass and riparian vegetation; the predation of zooplankton was higher in the mudflat habitat than in the other habitats (Figure 4).
3.3. Morphological Traits
Figure 5 shows the average morphological traits for A. brasiliensis between size classes and habitats. Only CI, RH, and OGS showed significant differences (p < 0.05) by size classes, whereas RH, OGS, CPCI, and ES differed by habitat. Overall, CI was highest in juveniles, while OGS and RH were highest in adults; by habitat, RH and CPCI were highest in riparian vegetation, while OGS was highest in mudflat and riparian vegetation, and ES was highest in mudflat (Figure 5). No significant differences in APRF, RPL, and RHL were shown between size classes and habitats (Figure 5).
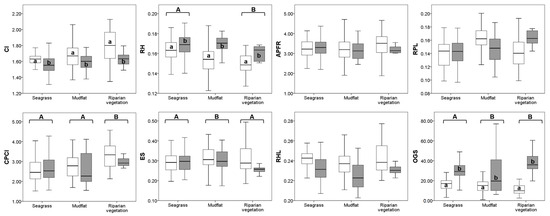
Figure 5.
Box-plot (minimum, 25% quantile, median, 75% quantile, and maximum) of ecomorphological indices of Atherinella brasiliensis collected in estuarine habitats (seagrass, mudflat, and riparian vegetation). Full variable names are listed in the Materials and Methods. Different superscript letters indicate significant differences (p < 0.05) compared by ANOVA followed by Tukey’s test. Capital letters: habitat comparisons; lowercase letters: size class comparisons. size classes: juveniles (☐) and adults (■).
3.4. Relationship Between Morphological Traits and Diet
The relationship between ecomorphological indices and feeding categories in both size classes were analysed with multiple regression analysis (DistLM). A marginal test identified RHL and ES as the variables that were the most strongly attributed to changes in the feeding categories for juveniles between habitats, explaining 6.33 and 6.27% of the total variation, respectively (Table 2). Samples from different habitats grouped distinctly along the dbRDA1 axis in the ordination (Figure 6A), which captured the main part of the variation explained by the model on the first axis (61.3% of the fitted and 7.8% of the total variation). ES was a strong predictor for positive values along dbRDA1, while OGS predicted negative values. The species showing the strongest influence on the observed patterns were benthic crustaceans and phytobenthos. These species showed a positive relationship to CPCI, ES, and RH and a negative relationship to OGS (Figure 6A). The second axis encompassed a considerably smaller share of the explained variation (32.4% of the fitted and 4.1% of the total variation). RH and CPCI were strong predictors of negative values along dbRDA2. The species showing the strongest influence on the observed patterns were insects and phytobenthos. These species showed a positive relationship to ES and OGS and negative relationships to RH and CPCI (Figure 6A).

Table 2.
Results of distance-based linear models (distLM) for comparison by size classes (juveniles and adults) of the ecomorphological indices of Atherinella brasiliensis from three estuarine habitats. Full variable names are listed in the Materials and Methods. Number in bold = p < 0.05.
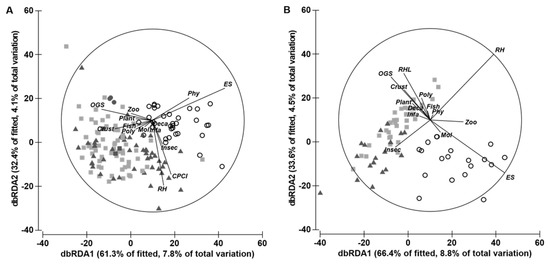
Figure 6.
Distance-based redundancy analysis (dbRDA) showing distribution by size class ((A) = juveniles and (B) = adults) for the ecomorphological indices of Atherinella brasiliensis and food groups in estuarine habitats: seagrass (■), mudflat (O), and riparian vegetation (▲). Variable names are listed in Table 1. Taxonomic categories: Crust = benthic or epibenthic crustaceans; Deca = decapoda; fish; Infa = infaune; Inse = insects; Plant = plant material; Mol = mollusca; Phy = phytobenthos; Poly = polychaeta, and Zoo = zooplankton.
We identified the most parsimonious model of adults of A. brasiliensis for a combination of eight morphological traits. The marginal test identified ES (7.38%) as the single variable that was most strongly attributed to changes in feeding categories for adults between habitats, followed by RH (6.58%). As Figure 6B shows, samples from different habitats were grouped along dbRDA1, with samples from vegetated habitats (seagrass and riparian vegetation) to the left and samples from mudflats further to the right, the latter also correlating positively with RH and ES and negatively with OGS and RHL. The food categories showing the strongest influence on the observed patterns were zooplankton, with a positive relationship to RH and ES, and insects and benthic crustacean, with a negative relationship to OGS and RHL (Figure 6B). With regard to dbRDA2, this axis encompassed a considerably smaller share of the explained variation (33.6% of the fitted and 4.5% of the total variation). The species showing the strongest influence on the observed patterns were benthic crustacea and polychaeta, with a positive relationship to OGS, RHL, and RH, and insects, with a negative relationship to ES (Figure 6B).
4. Discussion
Our results indicate that the effects of morphological variation in juveniles and adults of A. brasiliensis on food acquisition and swimming capacity are not similar between the three estuarine habitats. Among the morphological measures, we found that in vegetated habitats, juveniles and adults had a higher oral gape surface and caudal peduncle, whereas bigger eyes were of particular relevance for individuals registered in the unvegetated habitat, suggesting that these measures could be correlated with the use of the habitat and capture of prey in these shallow habitats. These findings are consistent with studies that found that the fishes in vegetated habitats had a wider mouth and strong caudal peduncle, aiding in food selection and better swimming performance [38]. It is worth noting that CI and RH were slightly higher for individuals registered in the unvegetated habitat, which presumably reflects streamlined body forms [64], and this suggests that fishes use specific strategies optimised for rapid steady swimming in this habitat [5,18,65]. The occurrence of intraspecific morphological variation in silversides has been investigated and was explained by phenotypic plasticity and environmental control of the phenotype [66]. Furthermore, phenotype differentiation has an important role in determining the functional differences in an organism in the ecosystem [23,67].
The population inhabiting the mudflats demonstrated a close relationship between eye size and an increase in the consumption of zooplankton and phytobenthos items. Previous comparative studies of food resource use by juvenile fishes in this estuary found evidence that Brazilian silverside consumed a greater proportion of zooplankton items and diatoms [25,43]. It is possible that larger eyes induce sharper visual acuity, particularly for the detection of small and drifting prey [68]. Big eyes in fishes are also thought to be involved in the response to an increase in turbidity in this habitat, which makes it possible to capture prey [69]. Numerous studies point to effects the turbidity on reduced prey capture rates by juvenile fishes [70,71,72], especially for visually oriented predators such as Brazilian silverside [73,74]. Other characteristics that may influence the increased planktonic prey encounter rates include body pigmentation, image size and contrast, or the bioluminescent abilities from numerous taxa of zooplankton fauna [4,75,76]. This is thought to contribute to total visibility and predator–prey encounters [75,77] and capture rates, and it would be advantageous for A. brasiliensis to capture small prey in mudflats.
Oral gape surface in individuals was larger in structured habitats, such as riparian vegetation and seagrass areas, and may be due to many individuals exhibiting morphologies that are best suited for capturing available prey of a relatively large size (comparatively larger than zooplankton). Both habitats support epibiotic or benthic fauna that are dominated mainly by polychaeta, cumacea, isopoda, and amphipoda (see Supplementary Materials), which are important prey resources for all sizes of A. brasiliensis. Their protrusible jaws enable them to suck different prey that are located on regions of seagrass leaf or submerged riparian vegetation roots before biting down and swallowing [78,79]. The use of suction feeding is also expected in terms of the choice of prey and capture success by A. brasiliensis in vegetated habitats [80,81]. Our data support this scenario because these items formed a large portion of the diet during the study. Models of suction feeding predict that fish with a relatively larger mouth will probably increase water volume directed towards the predator’s mouth [82,83], and this appears to be clearly more efficient in feeding on invertebrate associated with leaf fronds in vegetated habitats [84]. In agreement, structured habitats in estuaries increase the surface area available for colonisation by epibionts and provide a greater quantity of food available to juvenile fishes [85,86].
Although the importance of suction feeding has been considered for relatively large-sized captured prey by A. brasiliensis, perhaps the use of ram feeding may also have implications for the capture of zooplankton items in estuarine vegetated habitats. We cannot discard this possibility due to the greater consumption of zooplankton prey types in these sites, particularly in the seagrass habitat. This can be illustrated clearly for the use of ram feeding to capture elusive prey, such as zooplankton organisms [87]. During ram feeding, fish employ a strategy of swimming with a wide-open mouth through the water while moving toward the prey. For example, ref. [88] found that reef fish species that feed on calanoid copepods feed by ram suction. A study by [89] suggested that fishes that feed predominately on elusive prey showed a high capture success when employing ram feeding. Thus, our results, based on stomach contents, suggest that A. brasiliensis may employ both ram and suction feeding in structured habitats.
Another important question that emerged from our diet analysis of individuals from riparian vegetation was that both juveniles and adults were shown to prey mainly upon insects (immature aquatic and adult life stages). Such specific interactions may be due to several factors: (1) the individuals widely consumed food resources which may be overabundant [90], (2) the habitat structure could allow for the establishment of a diverse aquatic insect community and supply of terrestrial insects for fishes [91]), and (3) dietary plasticity related to generalist and opportunistic feeding strategies documented for Brazilian silverside [25,37,43]. This suggests that riparian vegetation on the upper estuary may play an important role in producing prey for juvenile fishes. Overall, diets of juvenile fishes in riverine environments tend to be dominated by nonplanktonic invertebrates, such as benthic crustaceans and insect larvae [92]. Unfortunately, there is less knowledge concerning the value of riparian vegetation in the nursery functions of tropical estuaries. Our results strongly support the idea that the riparian vegetation provided valuable properties attractive for A. brasiliensis in the upper estuary based on the fact that this habitat showed a greater number of individuals and high food availability. These findings regarding riparian vegetation are significant, thus leading us to consider it as an essential habitat for juveniles of this species [93,94]. In fact, ref. [17] suggested that there are three approaches to quantifying the habitat value to fishes: the quality, quantity, and availability of food resources.
The results from this present study showed that a high prevalence of insects and epibiotic or benthic fauna in the diet of individuals collected in vegetated habitats can also be examined within the context of the influences of the caudal peduncle and relative height of the body. Individuals with high values of these morphological traits are generally associated with swimming capacity, such as swimming speed and manoeuvrability, which suggests that body design could reflect those features that maximise the prey intake and resource utilisation abilities of fishes [43]. Thus, we accept our hypothesis that a deeper body shape leads to increased locomotor performance because this feature maximises thrust and stability during rapid bouts of swimming activity accomplished by a large caudal peduncle. Caudal peduncle depth is also strongly related to swimming speed and manoeuvrability, confirming the importance of this variable to the swimming ability of fishes [39]. Specifically, relative height has a larger effect on manoeuvrability and in the exploitation of small spaces among structures in structured habitats [32,38]. In accordance with [76], insectivore fish species present a greater capacity for manoeuvrability in structured environments, such as the estuarine vegetated habitats in this study.
5. Conclusions
In conclusion, it was possible to assess intraspecific variation in the morphological traits in juveniles and adults of A. brasiliensis, where ES and RH were strongly attributed to changes in feeding categories for adults between habitats. These variations involve increases in the consumption of insects and epibiotic fauna that live in or on vegetated habitats (of high structural complexity) and are defined by a consistent relationship found between the mouth’s morphology and caudal peduncle. These traits are probably better for food acquisition and swimming ability. On the other hand, larger eyes improved the vision of individuals and effectively increased foraging efficiency on zooplankton on mudflats. Further, a higher relative height (RH) of the body recorded in those habitats enhanced swimming performance and was linked to effects of the lowest habitat structure. Examination of other habitats utilised by A. brasiliensis during its life cycle in estuaries will be required for further evidence for the extent and community consequences of intraspecific variation or in predator–prey dynamics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coasts5030022/s1, Volume percentage (V%) of food items diet of Atherinella brasiliensis in the three habitats (seagrass, mudflat and riparian vegetation) of the Mamanguape river estuary. E = eggs, P = pupa, L = larva, n.i. = not identified.
Author Contributions
Conceptualization, E.B.M.; methodology, E.B.M.; formal analysis, M.L.d.A.A.; writing—original draft preparation, E.B.M. and M.L.d.A.A.; writing—review and editing, A.L.M.P.; supervision, A.L.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted under SISBIO Collection of Species Permit number 24557-27/10/2010 issued by ICMBio, Brazilian Environmental Agency, and follows the ethics rules which regulate the scientific use of animals in Brazil (Federal Law 11.794 of 8 October 2008).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are cointained in this document and Supplementary Materials.
Acknowledgments
We thank everyone who contributed directly and indirectly to this work. This work was partially supported by CNPq, Brazilian National Agency for Scientific and Technological Development (Proc. 477663/2011-7), Programa de Pesquisa Ecológica de Longa Duração—PELD, o Conselho Nacional de Desenvolvimento Científico e Tecnológico (Chamada CNPq/CONFAP-FAPs/PELD nº 23/2024, Proc. 445968/2024-9, and the Fundo de Recursos Hídricos—CT-HIDRO (Chamada CNPq/MCTI/FNDCT/CT-Hidro nº 63/2022, Proc 409348/2022-8), and SISBIO gave permission to carry out the research in the protected area (Proc. 24557), issued by ICMBio, Brazilian Environment Agency.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J.; et al. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: A better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 2001, 51, 633–641. [Google Scholar] [CrossRef]
- Brown, C.J.; Harborne, A.R.; Paris, C.B.; Mumby, P.J. Uniting paradigms of connectivity in marine ecology. Ecology 2016, 97, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Gillanders, B.M.; Able, K.W.; Brown, J.A.; Eggleston, D.B.; Sheridan, P.F. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: An important component of nurseries. Mar. Ecol. Prog. Ser. 2003, 247, 281–295. [Google Scholar] [CrossRef]
- Johnsen, S.; Widder, E.A. Ultraviolet absorption in transparent zooplankton and its implications for depth distribution and visual predation. Mar. Biol. 2001, 138, 717–730. [Google Scholar] [CrossRef]
- Sheaves, M. Consequences of ecological connectivity: The coastal ecosystem mosaic. Mar. Ecol. Prog. Ser. 2009, 391, 107–115. [Google Scholar] [CrossRef]
- Kimirei, I.A.; Nagelkerken, I.; Griffioen, B.; Wagner, C.; Mgaya, Y.D. Ontogenetic habitat use by mangrove/seagrass-associated coral reef fishes shows flexibility in time and space. Estuar. Coast. Shelf. Sci. 2011, 92, 47–58. [Google Scholar] [CrossRef]
- Whitfield, A.K. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Rev. Fish. Biol. Fish 2017, 27, 75–110. [Google Scholar] [CrossRef]
- Sales, N.S.; Dias, T.L.P.; Baeta, A.; Pessanha, A.L.M. Dependence of juvenile reef fishes on semi-arid hypersaline estuary microhabitats as nurseries. J. Fish. Biol. 2016, 89, 661–679. [Google Scholar] [CrossRef]
- Sales, N.S.; Baeta, A.S.B.V.; de Lima, L.G.; Pessanha, A.L.M. Do the shallow-water habitats of a hypersaline tropical estuary act as nursery grounds for fishes? Mar. Ecol. 2018, 39, e12473. [Google Scholar] [CrossRef]
- Silva, R.S.; Baeta, A.S.; Pessanha, A.L. Are vegetated areas more attractive for juvenile fishin estuaries? A comparison in a tropical estuary. Environ. Biol. Fishes 2018, 101, 1427–1442. [Google Scholar] [CrossRef]
- Laegdsgaard, P.; Johnson, C. Why do juvenile fish utilise mangrove habitats? J. Exp. Mar. Biol. Ecol. 2001, 257, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Sheaves, M. Nature and consequences of biological connectivity in mangrove systems. Mar. Ecol. Prog. Ser. 2005, 302, 293–305. [Google Scholar] [CrossRef]
- Campos, D.M.; Silva, A.F.; Sales, N.S.; Oliveira, R.E.; Pessanha, A.L. Trophic relationships among fish assemblages in a mudflat within Brazilian marine protected area. Braz. J. Oceanogr. 2015, 63, 135–146. [Google Scholar] [CrossRef]
- Lugendo, B.R.; Nagelkerken, I.; Velde, G.V.; Mgaya, Y.D. The importance of mangroves, mud and sand flats, and seagrass beds as feeding areas for juvenile fishes in Chwaka Bay, Zanzibar: Gut content and stable isotope analyses. J. Fish. Biol. 2006, 69, 1639–1661. [Google Scholar] [CrossRef]
- Favero, J.M.; Dias, J.F. Juvenile fish use of the shallow zone of beaches of the Cananéia-Iguape coastal system, southeastern Brazil. Braz. J. Oceanogr. 2015, 63, 103–114. [Google Scholar] [CrossRef]
- Oliveira, R.E.; Pessanha, A.L. Fish assemblages along a morphodynamic continuum on three tropical beaches. Neotrop. Ichthyol. 2014, 12, 165–175. [Google Scholar] [CrossRef]
- Sheaves, M.; Baker, R.; Nagelkerken, I.; Connolly, R.M. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuar. Coast 2015, 38, 401–414. [Google Scholar] [CrossRef]
- Becker, A.; Coppinger, C.; Whitfield, A.K. Influence of tides on assemblages and behaviour of fishes associated with shallow seagrass edges and bare sand. Mar. Ecol. Prog. Ser. 2012, 456, 187–199. [Google Scholar] [CrossRef]
- Marley, G.S.; Deacon, A.E.; Phillip, D.A.; Lawrence, A.J. Mangrove or mudflat: Prioritising fish habitat for conservation in a turbid tropical estuary. Estuar. Coast. Shelf. Sci. 2020, 240, 106788. [Google Scholar] [CrossRef]
- Pessanha, A.L.M.; Araújo, F.G. Recrutamento do peixe-rei, Atherinella brasiliensis (Quoy & Gaimard) (Atheriniformes, Atherinopsidae), na margem continental da Baía de Sepetiba, Rio de Janeiro, Brasil. Rev. Bras. Zool. 2001, 18, 1265–1274. [Google Scholar] [CrossRef]
- Motta, P.J.; Clifton, K.B.; Hernandez, P.; Eggold, B.T. Ecomorphological correlates in ten species of subtropical seagrass fishes: Diet and microhabitat utilization. Environ. Biol. Fishes 1995, 44, 37–60. [Google Scholar] [CrossRef]
- Norton, S.F.; Luczkovich, J.J.; Motta, P.J. The role of ecomorphological studies in the comparative biology of fishes. Environ. Biol. Fishes 1995, 44, 287–304. [Google Scholar] [CrossRef]
- Shuai, F.; Yu, S.; Lek, S.; Li, X. Habitat effects on intra-species variation in functional morphology: Evidence from freshwater fish. Ecol. Evol. 2018, 8, 10902–10913. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Early environment influences later performance in fishes. J. Fish. Biol. 2014, 85, 151–188. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.D.G.F.V.; Lima, D.E.P.C.; Santos, N.S.; Terra, B.F.; Pessanha, A.L.M. Trade-offs between ontogenetic changes and food consumption in Brazilian silverside Atherinella brasiliensis from two tropical estuaries. J. Fish. Biol. 2020, 98, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Baldasso, M.C.; Wolff, L.L.; Neves, M.P.; Delariva, R.L. Ecomorphological variations and food supply drive trophic relationships in the fish fauna of a pristine neotropical stream. Environ. Biol. Fishes 2019, 102, 783–800. [Google Scholar] [CrossRef]
- Delariva, R.L.; Neves, M.P. Morphological traits correlated with resource partitioning among small characin fish species coexisting in a Neotropical river. Ecol. Freshw. Fish 2020, 29, 640–653. [Google Scholar] [CrossRef]
- Alves, V.E.N.; Patrício, J.; Dolbeth, M.; Pessanha, A.; Palma, A.R.T.; Dantas, E.W.; Vendel, A.L. Do different degrees of human activity affect the diet of Brazilian silverside Atherinella brasiliensis? J. Fish. Biol. 2016, 89, 1239–1257. [Google Scholar] [CrossRef]
- Neves, L.M.; Pereira, H.H.; Costa, M.R.; Araújo, F.G. Uso do manguezal de Guaratiba, Baía de Sepetiba, Rio de Janeiro, pelo peixe-rei Atherinella brasiliensis (Quoy & Gaimard) (Atheriniformes, Atherinopsidae). Rev. Bras. Zool. 2006, 23, 421–428. [Google Scholar] [CrossRef]
- Andreata, J.V.; Manzano, F.V.; Baptista, M.G.S.; Teixeira, D.E.; de Oliveira, L.O.V.; Longo, M.M.; Freret, N.V.; Valois, A.S. Assembléia de peixes da lagoa Rodrigo de Freitas, Rio de Janeiro. Bioikos 2002, 16, 19–28. [Google Scholar]
- Neves, L.M.; Teixeira, T.P.; Franco, T.P.; Pereira, H.H.; Araújo, F.G. Fish composition and assemblage structure in the estuarine mixing zone of a tropical estuary: Comparisons between the main channel and an adjacent lagoon. Mar. Biol. Res. 2013, 9, 661–675. [Google Scholar] [CrossRef]
- Pessanha, A.L.M.; Araújo, F.G.; Oliveira, R.E.M.; Silva, A.F.D.; Sales, N.S. Ecomorphology and resource use by dominant species of tropical estuarine juvenile fishes. Neotrop. Ichthyol. 2015, 13, 401–412. [Google Scholar] [CrossRef]
- de Carvalho, B.M.; Spach, H.L. Habitat use by Atherinella brasiliensis (Quoy & Gaimard, 1825) in intertidal zones of a subtropical estuary, Brazil. Acta. Sci. Biol. Sci. 2015, 37, 177–184. [Google Scholar] [CrossRef]
- Dolbeth, M.; Vendel, A.L.; Pessanha, A.; Patrício, J. Functional diversity of fish communities in two tropical estuaries subjected to anthropogenic disturbance. Mar. Pollut. Bull. 2016, 112, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.P.M.D.; Xavier, J.H.D.A.; Rosa, I.M.D.L. Diet and trophic organization of the fish assemblage from the Mamanguape River Estuary, Brazil. Lat. Am. J. Aquat. Res. 2017, 45, 879–890. [Google Scholar] [CrossRef]
- Claudino, M.C.; Pessanha, A.L.M.; Araújo, F.G.; Garcia, A.M. Trophic connectivity and basal food sources sustaining tropical aquatic consumers along a mangrove to ocean gradient. Estuar. Coast. Shelf. Sci. 2015, 167, 45–55. [Google Scholar] [CrossRef]
- Contente, R.F.; Stefanoni, M.F.; Spach, H. Feeding ecology of the Brazilian silverside Atherinella brasiliensis (Atherinopsidae) in a sub-tropical estuarine ecosystem. J. Mar. Biolog. Assoc. UK 2011, 91, 1197. [Google Scholar] [CrossRef]
- Prado, A.V.; Goulart, E.; Pagotto, J.P. Ecomorphology and use of food resources: Inter- and intraspecific relationships of fish fauna associated with macrophyte stands. Neotrop. Ichthyol. 2016, 14, e150140. [Google Scholar] [CrossRef]
- Soares, B.E.; Ruffeil, T.O.; Montag, L.F. Ecomorphological patterns of the fishes inhabiting the tide pools of the Amazonian Coastal Zone, Brazil. Neotrop. Ichthyol. 2013, 11, 845–858. [Google Scholar] [CrossRef]
- Figueiredo, G.G.A.A.; Pessanha, A.L.M. Comparative study of trophic organization of juvenile fish assemblages of three tidal creeks in a tropical semi-arid estuary. J. Fish. Biol. 2016, 89, 680–695. [Google Scholar] [CrossRef]
- Manna, L.R.; Rezende, C.F.; Mazzoni, R. Effect of body size on microhabitat preferences in stream-dwelling fishes. J. Appl. Ichthyol. 2017, 33, 193–202. [Google Scholar] [CrossRef]
- Manna, L.R.; Villéger, S.; Rezende, C.F.; Mazzoni, R. High intraspecific variability in morphology and diet in tropical stream fish communities. Ecol. Freshw. Fish 2019, 28, 41–52. [Google Scholar] [CrossRef]
- Brito, G.J.; Lima, L.G.D.; Oliveira, R.E.; Pessanha, A. Intraspecific food resource partitioning in Brazilian silverside Atherinella brasiliensis (Atheriniformes: Atherinopsidae) in a tropical estuary, Brazil. Neotrop. Ichthyol. 2019, 17, e180108. [Google Scholar] [CrossRef]
- Medeiros, I.D.S.; de Assis, H.Y.; Dantas, M.D.S.; Clemente, T.S.; Almeida, N.V. Environmental Vulnerability of the Environmental Protection Area of the Mamanguape River Bar-PB. In Proceedings of the XIX GEOINFO, Campina Grande, Brazil, 5–7 December 2018; pp. 92–102. [Google Scholar]
- Rocha, M.D.S.P.; Mourão, J.D.S.; Souto, W.D.M.S.; Barboza, R.R.D.; Alves, R.R.D.N. O uso dos recursos pesqueiros no estuário do rio mamanguape, estado da Paraíba, Brasil. Interciencia 2008, 33, 903–910. [Google Scholar]
- Mourão, J.; Nordi, N. Etnoictiologia de pescadores artesanais do estuário do rio Mamanguape, Paraíba, Brasil. Bol. Inst. Pesca 2003, 29, 9–17. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef]
- Magalhães, K.M.; Borges, J.C.; Pitanga, M.E. Halophila baillonis Ascherson: First population dynamics data for the Southern Hemisphere. An. Acad. Bras. Ciênc. 2015, 87, 861–865. [Google Scholar] [CrossRef]
- Xavier, J.H.; Cordeiro, C.A.; Tenório, G.D.; Diniz, A.D.; Júnior, E.P.; Rosa, R.S.; Rosa, I.L. Fish assemblage of the Mamanguape Environmental Protection Area, NE Brazil: Abundance, composition and microhabitat availability along the mangrove-reef gradient. Neotrop. Ichthyol. 2012, 10, 109–122. [Google Scholar] [CrossRef]
- Sibbing, F.A.; Nagelkerke, L.A. Resource partitioning by Lake Tana barbs predicted from fish morphometrics and prey characteristics. Rev. Fish. Biol. Fish 2001, 10, 393–437. [Google Scholar] [CrossRef]
- Schaefer, K.M. An evaluation of geographic and annual variation in morphometric characters and gill-raker counts of yellowfin tuna, Thunnus albacares, from the Pacific Ocean. Inter-Am. Trop. Tuna Comm. Bull. 1992, 20, 133–163. [Google Scholar]
- Gatz, A.J. Community organization in fishes as indicate. Ecology 1979, 60, 711–718. [Google Scholar] [CrossRef]
- Keast, A.; Webb, D. Mouth and body form relative to feeding ecology in the fish fauna of a small lake, Lake Opinicon, Ontario. J. Fish. Res. Board. Can. 1966, 23, 1845–1874. [Google Scholar] [CrossRef]
- Watson, D.J.; Balon, E.K. Ecomorphological analysis of fish taxocenes in rainforest streams of northern Borneo. J. Fish. Biol. 1984, 25, 371–384. [Google Scholar] [CrossRef]
- Freire, K.M.; Nascimento, F.P.; Rosário, L.M.; Rocha, G.R.; Alves, G.A.; Lins-Oliveira, J.E. Characterization of some biological aspects of Atherinella brasiliensis caught during sport fishing tournaments: A case study from northeastern Brazil. Bol. Inst. Pesca 2012, 38, 171–180. [Google Scholar]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish. Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Bemvenuti, M.D.A. Hábitos alimentares de peixes-rei (Atherinidae) na região estuarina da Lagoa dos Patos, RS, Brasil. Atlântica 1990, 12, 79–102. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall International, Inc.: Hoboken, NJ, USA, 2009; p. 960. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing, Version 4.2.2; R Development Core Team: Vienna, Austria, 2022.
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.; Clarke, K.P. PRIMER: Guide to Software and Statistical Methods; Primer-e: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer; PRIMER-e: Plymouth, UK, 2006. [Google Scholar]
- Ruehl, C.B.; Shervette, V.; Dewitt, T.J. Replicated shape variation between simple and complex habitats in two estuarine fishes. Biol. J. Linn. Soc. 2011, 103, 147–158. [Google Scholar] [CrossRef]
- Becker, A.; Cowley, P.D.; Whitfield, A.K. Use of remote underwater video to record littoral habitat use by fish within a temporarily closed South African estuary. J. Exp. Mar. Biol. Ecol. 2010, 391, 161–168. [Google Scholar] [CrossRef]
- Fluker, B.L.; Pezold, F.; Minton, R.L. Molecular and morphological divergence in the inland silverside (Menidia beryllina) along a freshwater-estuarine interface. Environ. Biol. Fishes 2011, 91, 311. [Google Scholar] [CrossRef]
- Harding, H.R.; Gordon, T.A.; Eastcott, E.; Simpson, S.D.; Radford, A.N. Causes and consequences of intraspecific variation in animal responses to anthropogenic noise. Behav. Ecol. 2019, 30, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Wainwright, P.C. Ecomorphology of the eyes and skull in zooplanktivorous labrid fishes. Coral. Reefs 2011, 30, 415–428. [Google Scholar] [CrossRef]
- Caves, E.M.; Sutton, T.T.; Johnsen, S. Visual acuity in ray-finned fishes correlates with eye size and habitat. J. Exp. Biol. 2017, 220, 1586–1596. [Google Scholar] [CrossRef]
- Hartman, E.J.; Abrahams, M.V. Sensory compensation and the detection of predators: The interaction between chemical and visual information. Proc. R. Soc. Biol. Sci. 2000, 267, 571–575. [Google Scholar] [CrossRef]
- Pangle, K.L.; Malinich, T.D.; Bunnell, D.B.; DeVries, D.R.; Ludsin, S.A. Context-dependent planktivory: Interacting effects of turbidity and predation risk on adaptive foraging. Ecosphere 2012, 3, 1–18. [Google Scholar] [CrossRef]
- Lowe, M.L.; Morrison, M.A.; Taylor, R.B. Harmful effects of sediment-induced turbidity on juvenile fish in estuaries. Mar. Ecol. Prog. Ser. 2015, 539, 241–254. [Google Scholar] [CrossRef]
- Nilsson, D.E.; Warrant, E.; Johnsen, S. Computational visual ecology in the pelagic realm. Philos. Trans. R. Soc. Biol. Sci. 2014, 369, 20130038. [Google Scholar] [CrossRef]
- Ortega, J.C.; Figueiredo, B.R.; da Graça, W.J.; Agostinho, A.A.; Bini, L.M. Negative effect of turbidity on prey capture for both visual and non-visual aquatic predators. J. Anim. Ecol. 2020, 89, 2427–2439. [Google Scholar] [CrossRef]
- Buskey, E.J. Factors affecting feeding selectivity of visual predators on the copepod Acartia tonsa: Locomotion, visibility and escape responses. Hydrobiologia 1994, 292, 447–453. [Google Scholar] [CrossRef]
- Widder, E. Bioluminescence and the pelagic visual environment. Mar. Freshw. Behav. Phy. 2002, 35, 1–26. [Google Scholar] [CrossRef]
- Aksnes, D.L.; Nejstgaard, J.; Sædberg, E.; Sørnes, T. Optical control of fish and zooplankton populations. Limnol. Oceanogr. 2004, 49, 233–238. [Google Scholar] [CrossRef]
- Cochran-Biederman, J.L.; Winemiller, K.O. Relationships among habitat, ecomorphology and diets of cichlids in the Bladen River, Belize. Environ. Biol. Fishes 2010, 88, 143–152. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kohda, M. The effect of feeding habitats on dietary shifts during the growth in a benthophagous suction-feeding fish. Zool. Sci. 2002, 19, 709–714. [Google Scholar] [CrossRef]
- Svanbäck, R.; Eklöv, P. Morphology dependent foraging efficiency in perch: A trade-off for ecological specialization? Oikos 2003, 102, 273–284. [Google Scholar] [CrossRef]
- Wainwright, P.C.; Carroll, A.M.; Collar, D.C.; Day, S.W.; Higham, T.E.; Holzman, R.A. Suction feeding mechanics, performance, and diversity in fishes. Integr. Comp. Biol. 2007, 47, 96–106. [Google Scholar] [CrossRef]
- Wainwright, P.C.; Day, S.W. The forces exerted by aquatic suction feeders on their prey. J. R. Soc. Interface 2007, 4, 553–560. [Google Scholar] [CrossRef]
- Motta, P.J.; Norton, S.F.; Luczkovich, J.J. Perspectives on the ecomorphology of bony fishes. Environ. Biol. Fishes 1995, 44, 11–20. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Glover, T.; Weis, J.S. The Impact of Mangrove Prop-Root Epibionts on Juvenile Reef Fishes: A Field Experiment Using Artificial Roots and Epifauna. Estuar. Coasts 2008, 31, 981–993. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Weis, J.S. Fish community features correlate with prop root epibionts in Caribbean mangroves. J. Exp. Mar. Biol. Ecol. 2013, 441, 90–98. [Google Scholar] [CrossRef]
- Ferry, L.A.; Paig-Tran, E.M.; Gibb, A.C. Suction, ram, and biting: Deviations and limitations to the capture of aquatic prey. Integr. Comp. Biol. 2015, 55, 97–109. [Google Scholar] [CrossRef]
- Coughlin, D.J.; Strickler, J.R. Zooplankton capture by a coral reef fish: An adaptive response to evasive prey. Environ. Biol. Fishes 1990, 29, 35–42. [Google Scholar] [CrossRef]
- Norton, S.F. Capture success and diet of cottid fishes: The role of predator morphology and attack kinematics. Ecology 1991, 72, 1807–1819. [Google Scholar] [CrossRef]
- Burdon, F.J.; Harding, J.S. The linkage between riparian predators and aquatic insects across a stream-resource spectrum. Freshw. Biol. 2008, 53, 330–346. [Google Scholar] [CrossRef]
- Ramey, T.L.; Richardson, J.S. Terrestrial invertebrates in the riparian zone: Mechanisms underlying their unique diversity. BioScience 2017, 67, 808–819. [Google Scholar] [CrossRef]
- Nunn, A.D.; Tewson, L.H.; Cowx, I.G. The foraging ecology of larval and juvenile fishes. Rev. Fish. Biol. Fish 2012, 22, 377–408. [Google Scholar] [CrossRef]
- Pusey, B.J.; Arthington, A.H. Importance of the riparian zone to the conservation and management of freshwater fish: A review. Mar. Freshwater. Res. 2003, 54, 1–16. [Google Scholar] [CrossRef]
- Pease, A.A.; Justine, D.J.; Edwards, M.S.; Turner, T.F. Habitat and resource use by larval and juvenile fishes in an arid-land river (Rio Grande, New Mexico). Freshw. Biol. 2006, 51, 475–486. [Google Scholar] [CrossRef]
- Mise, F.T.; Fugi, R.; Pagotto, J.P.A.; Goulart, E. The coexistence of endemic species of Astyanax (Teleostei: Characidae) is propitiated by ecomorphological and trophic variations. Biota. Neotrop. 2013, 13, 21–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).