The Significant Role of Physical Activity and Exercise in Health and Metabolic Diseases
Abstract
1. Introduction
2. Physiology of Insulin Effects on Target Tissues
3. The Importance of Insulin Sensitivity in Metabolic Regulation
4. Gender Differences in Insulin Sensitivity
5. The Adverse Effects of Physical Inactivity on the Development of Insulin Resistance and Metabolic Dysregulation
5.1. Short-Term Physical Inactivity
5.2. Long-Term Physical Inactivity
5.3. Clinical Relevance
6. Types of Exercise
6.1. Aerobic (Endurance) Training
6.2. Anaerobic (Resistance) Training
6.3. High Intensity Interval Training (HIIT)
6.4. Comparison of the Effects of Exercise Modalities in T2D and Obesity
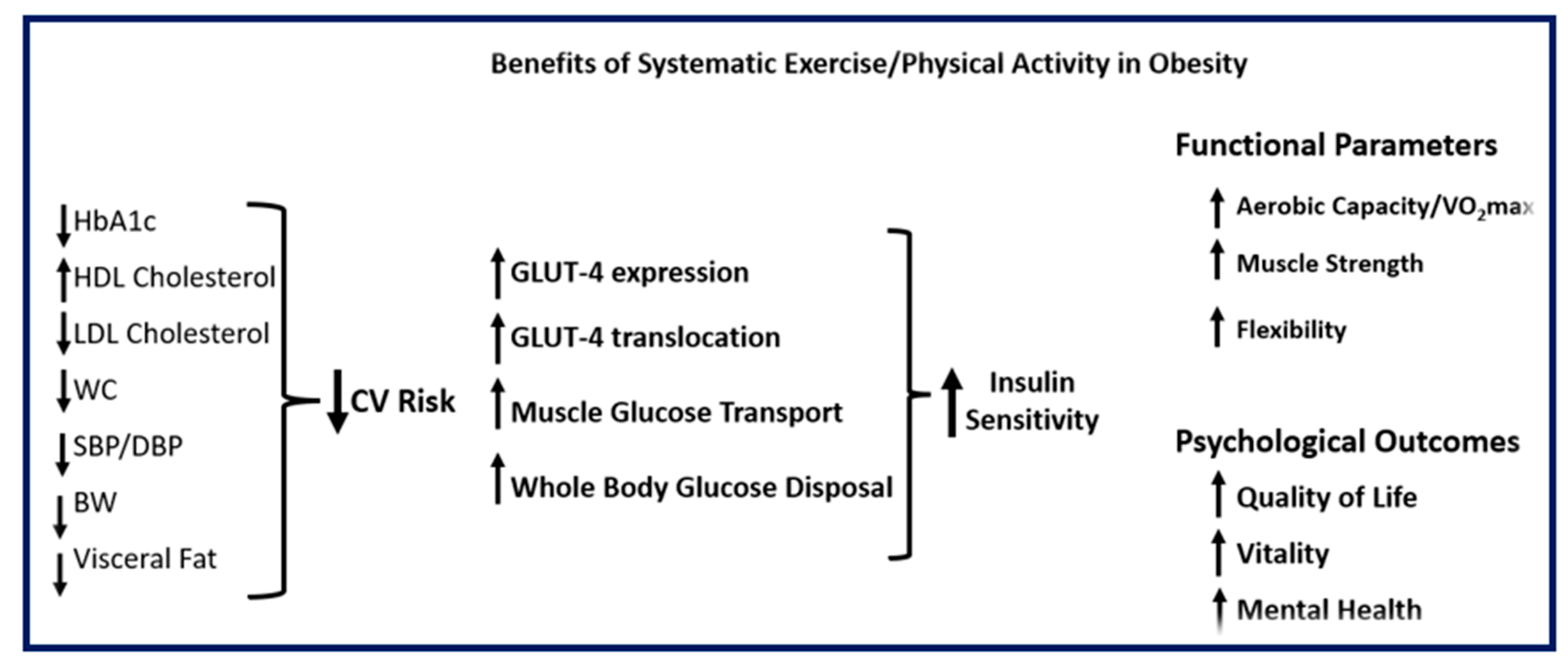
7. The Significant Role of Regular Physical Activity and Exercise in Metabolic Diseases
- ▪
- Review treatment schedules, especially when using insulin and sulfonylureas, to prevent hypoglycemia during and after exercise, as increased insulin sensitivity and muscle glucose uptake can last more than 24 h post-exercise. The interaction of exercise with anti-diabetic medications like metformin, GLP-1 receptor agonists (GLP-1RAs), and SGLT2 inhibitors (SGLT2i) should also be considered (Section 8).
- ▪
- Evaluate the presence of chronic diabetic complications, such as a history of stroke, retinopathy, autonomic neuropathy, coronary artery disease, hypertension, chronic kidney disease, peripheral neuropathy, peripheral arterial disease, and diabetic foot syndrome, when designing a training program.
- ▪
- Consider response heterogeneity, especially in people with long-standing T2D, as metabolic flexibility in utilizing glucose or NEFA in muscle during exercise can be reduced [7].
- ▪
- Consider factors such as sleep quality, exercise timing, and specific dietary issues related to carbohydrate/protein intake. Choose the appropriate type, amount, duration, and intensity of exercise to achieve the individual’s goals for optimal results. Preferences regarding exercise type and related concerns are essential for reducing dropout rates [141].
8. Exercise-Drug Interactions in People with T2D or Obesity
8.1. Metformin
8.2. GLP1RAs
8.3. SGLT2i
9. Exercise Timing
10. Effects of Physical Activity and Exercise on Immunological Regulation and Autonomic Function
11. The Role of Physical Activity and Exercise in Well-Being and Quality of Life (QoL)
12. Risks and Adverse Events of Physical Activity and Exercise
13. Future Perspectives
- The primary limitation in comparing how different workouts affect biochemical and clinical parameters is the considerable variability, which significantly hinders direct comparison. Future research should assess all exercise modalities within the same population, while accounting for factors that could influence the results, including exercise intensity and duration (Section 6.4).
- Since there may be differences between men and women (pre- or postmenopausal) in insulin sensitivity (Section 4), studies should also account for this factor when designing workout programs.
- An issue requiring further investigation is clarifying how meal timing and time of day influence the effects of physical activity and exercise on metabolic regulation and clinical benefits, while accounting for sleep quality and quantity. Long-term studies are required to clarify the effects of exercise timing on metabolic control.
- Personalized exercise dosing based on genetics or phenotype is a challenging issue for achieving optimal benefits.
- Studies are required to investigate molecular mechanisms underlying exercise modality-specific benefits.
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckel, R.H.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Kon Koh, K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Konsgen, N.; Neuhaus, A.L.; Semwai, M. Exercise/Physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef] [PubMed]
- Dasso, N.A. How is exercise different from physical activity? A concept analysis. Nurs. Forum 2019, 54, 45–52. [Google Scholar] [CrossRef]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Haring, H.U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef]
- Baron, A.; Steinberg, H.; Brechtel, G.; Johnson, A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am. J. Physiol. 1994, 266, E248–E253. [Google Scholar] [CrossRef]
- Coggins, M.; Lindner, J.; Rattigan, S.; Jahn, L.; Fasy, E.; Kaul, S.; Barrett, E. Physiological hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001, 50, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Karpe, F. Regulation of human subcutaneous adipose tissue blood flow. Int. J. Obes. 2014, 38, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Fielding, B.; Ilic, V.; Macdonald, I.A.; Summers, L.; Frayn, K.N. Adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes 2002, 51, 2467–2473. [Google Scholar] [CrossRef]
- Wahren, J.; Felig, P.; Ahlborg, G.; Jorfeldt, L. Glucose metabolism during leg exercise in man. J. Clin. Investig. 1971, 50, 2715–2725. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diab. Res. Clin. Pract. 2011, 93 (Suppl. 1), 52–59. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Lambadiari, V.; Mitrou, P.; Maratou, E.; Boutati, E.; Panagiotakos, D.; Economopoulos, T.; Raptis, S.A. Impaired postprandial blood flow in adipose tissue may be an early marker of insulin resistance in type 2 diabetes. Diabetes Care 2007, 30, 3128–3130. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Mitrou, P.; Maratou, E.; Raptis, A.; Raptis, S.A.; Dimitriadis, G. Increases in muscle blood flow after mixed meals are decreased at all stages of type 2 diabetes. Clin. Endocrinol. 2012, 76, 825–830. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Gooke, N.; Keaney JFJr Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef]
- Kahn, S.; Prigeon, R.L.; McCulloch, D.; Boyko, E.J.; Bergman, R.N.; Schwartz, M.W.; Neifing, J.L.; Ward, W.K.; Beard, J.C.; Palmer, J.P. Quantification of the relationship between insulin sensitivity and cell function in human subjects. Diabetes 1993, 42, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B.; Cohen, R.A. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am. J. Physiol. Endocrinol. Meta 1992, 263, H321–H326. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes and cardiovascular disease? The common soil hypothesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Lemkes, B.A.; Hermanides, J.; Devries, J.H.; Holleman, F.; Meijers, J.C.M.; Hoekstra, J.B.L. Hyperglycemia: A prothrombotic factor? J. Thromb. Haemost. 2010, 8, 1663–1669. [Google Scholar] [CrossRef]
- Stout, R.W. Insulin-stimulated lipogenesis in arterial tissue in relation to diabetes and atheroma. Lancet 1968, 2, 283–290. [Google Scholar] [CrossRef]
- Rizza, R.A.; Mandarino, L.J.; Genest, J.; Baker, B.A.; Gerich, J.E. Production of insulin resistance by hyperinsulinemia in man. Diabetologia 1985, 28, 70–75. [Google Scholar] [CrossRef]
- Ferrannini, E.; Galvan, A.Q.; Gastaldelli, A.; Camastra, S.; Sironi, A.M.; Toschi, E.; Baldi, S.; Frascerra, S.; Monzani, F.; Antonelli, A.; et al. Insulin: New roles for an ancient hormone. Eur. J. Clin. Investig. 1999, 29, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C. Glycemic variability. Diabetes Care 2008, 31, S150–S154. [Google Scholar] [CrossRef] [PubMed]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31, S262–S268. [Google Scholar] [CrossRef]
- Dandona, P.; Chaudhuri, A.; Dhindsa, S. Proinflammatory and prothrombotic effects of hypoglycemia. Diabetes Care 2010, 33, 1686–1687. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.M.; Chibrikov, E.; Twells, L.K.; Midodzi, W.K.; Young, S.W.; MacDonald, D.; Majumdar, S.R. Association of insulin dosage with mortality or major adverse cardiovascular events: A retrospective cohort study. Lancet Diabetes Endocrinol. 2016, 5, 43–52. [Google Scholar] [CrossRef]
- Christakis, M.K.; Hasan, H.; De Souza, L.R.; Shirreff, L. The effect of menopause on metabolic syndrome: Cross-sectional results from the Canadian Longitudinal Study on aging. Menopause 2020, 27, 999–1009. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender differences in insulin resistance: New knowledge and perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef]
- White, U.A.; Tchoukalova, Y.D. Sex dimorphism and depot differences in adipose tissue function. Biochim. Biophys. Acta 2014, 1842, 377–392. [Google Scholar] [CrossRef]
- Chen, X.; McClusky, R.; Itoh, Y.; Reue, K.; Arnold, A.P. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 2013, 154, 1092–1104. [Google Scholar] [CrossRef]
- Kerr, N.R.; Booth, F.W. Contributors of physical inactivity and sedentary behavior to metabolic and endocrine diseases. Trends Endocrinol. Metab. 2022, 33, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; Davis, J.C.; Liu-Ambrose, T. What is the association between sedentary behavior and cognitive function? A systematic review. Br. J. Sports Med. 2017, 5, 800–811. [Google Scholar] [CrossRef]
- Yang, Y.; Shin, J.C.; Li, D.; An, R. Sedentary Behavior and Sleep Problems: A Systematic Review and Meta-Analysis. Int. J. Behav. Med. 2017, 24, 481–492. [Google Scholar] [CrossRef]
- Stuart, C.A.; Shangraw, R.E.; Prince, M.J.; Peters, E.J.; Wolfe, R.R. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism 1988, 37, 802–806. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Arciero, P.J.; Kohrt, W.M.; Recette, S.B.; Hansen, P.A.; Holloszy, J.O. Changes in insulin action and GLUT-4 within 6 days of inactivity in endurance runners. J. Appl. Physiol. 1996, 80, 240–244. [Google Scholar] [PubMed]
- Bergouignan, A.; Rudwill, F.; Simon, C.; Blanc, S. Physical inactivity is the culprit of metabolic inflexibility: Evidence from bed-rest studies. J. Appl. Physiol. 2011, 111, 1201–1210. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; van de Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goosens, G.H.; van Loon, L.J.C. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef] [PubMed]
- Eggelbusch, M.; Charlton, B.T.; Bosutti, A.; Gansa, B.; Giakoumaki, I.; Grootemaat, A.E.; Hendrickse, P.W.; Jaspers, Y.; Kemp, S.; Kerkhoff, T.J.; et al. The impact of bed rest on human skeletal muscle metabolism. Cell Rep. Med. 2024, 5, 101372. [Google Scholar] [CrossRef]
- Alibegovic, A.C.; Sonne, M.P.; Hojbjerre, L.; Bork-Jensen, J.; Jacobsen, S.; Nilsson, E.; Faerch, K.; Hiscock, N.; Mortensen, B.; Friedrichsen, M.; et al. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E752–E763. [Google Scholar] [CrossRef]
- Handy, R.M.; Holloway, G.P. Insights into the development of insulin resistance: Unraveling the interaction of physical inactivity, lipid metabolism, and mitochondrial biology. Front. Physiol. 2023, 14, 1151389. [Google Scholar] [CrossRef]
- Bergouignan, A.; Schoeller, D.A.; Normand, S.; Gauquelin-Koch, G.; Laville, M.; Shriver, T.; Desage, M.; Le Maho, Y.; Oshima, H.; Gharib, C.; et al. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary fatty acids: Results of a randomized trial. PLoS Clin. Trials 2006, 1, e27. [Google Scholar] [CrossRef] [PubMed]
- Bilet, L.; Phielix, E.; van de Weijer, T.; Gemmink, A.; Bosma, M.; Moonen-Kornips, E.; Jorgensen, J.A.; Schaart, G.; Zhang, D.; Meijer, K.; et al. One-leg inactivity induces a reduction in mitochondrial oxidative capacity, intramyocellular lipid accumulation, and reduced insulin signaling upon lipid infusion: A human study with unilateral limb suspension. Diabetologia 2020, 63, 1211–1222. [Google Scholar] [CrossRef]
- Kelley, D.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef]
- Hawley, J.A. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab. Res. Rev. 2004, 20, 383–393. [Google Scholar] [CrossRef]
- Hamer, M.; Sabia, S.; Batty, G.D.; Shipley, M.J.; Tabak, A.G.; Singh-Manoux, A.; Kivimaki, M. Physical activity and inflammatory markers over 10 years. Circulation 2012, 126, 928–933. [Google Scholar] [CrossRef]
- Phillips, C.M.; Dillon, C.B.; Perry, I.J. Does replacing sedentary behavior with light or moderate to vigorous physical activity modulate inflammatory status in adults? Int. J. Behav. Nutr. Phys. Act. 2017, 14, 138. [Google Scholar] [CrossRef]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: Metabolic and musculoskeletal implications. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819888824. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pathophysiology of physical inactivity-dependent insulin resistance: A theoretical mechanistic review emphasizing clinical evidence. J. Diabetes Res. 2021, 2021, 7796727. [Google Scholar] [CrossRef] [PubMed]
- Bowden Davies, K.A.; Norman, J.A.; Thompson, A.; Mitchell, K.L.; Harrold, J.A.; Halford, J.C.; Wilding, J.P.H.; Kemp, G.J.; Cuthbertson, D.J.; Sprung, V.S. Short-term physical inactivity induces endothelial dysfunction. Front. Physiol. 2021, 12, 659834. [Google Scholar] [CrossRef]
- Kullmann, S.; Goj, T.; Veit, R.; Fritsche, L.; Wagner, L.; Schneeweiss, P.; Hoene, M.; Hoffmann, C.; Machann, J.; Niess, A.; et al. Exercise restores brain insulin sensitivity in sedentary adults who are overweight and obese. JCI Insight 2022, 7, e161498. [Google Scholar] [CrossRef]
- Sonne, M.P.; Alibegovic, A.C.; Hojbjerre, L.; Vaag, A.; Stallknecht, B.; Dela, F. Effect of 10 days of bed rest on metabolic and vascular insulin action: A study in individuals at risk for type 2 diabetes. J. Appl. Physiol. 2010, 108, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Alibegovic, A.C.; Hojbjerre, L.; Sonne, M.P.; van Hall, G.; Alsted, T.J.; Kiens, B.; Stallknecht, B.; Dela, F.; Vaag, A. Increased rates of whole body lipolysis before and after 9 days of bed rest in healthy young men born with low birth weight. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E555–E564. [Google Scholar] [CrossRef] [PubMed]
- Alibegovic, A.C.; Hojbjerre, L.; Sonne, M.P.; van Hall, G.; Stallknecht, B.; Dela, F.; Vaag, A. Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes 2009, 58, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Bowden Davies, K.A.; Sprung, V.S.; Norman, J.A.; Thompson, A.; Mitchell, K.L.; Halford, J.C.G.; Harrold, J.A.; Wilding, J.P.H.; Kemp, G.J.; Cuthbertson, D.J. Short-term decreased physical activity with increased sedentary behavior causes metabolic derangements and altered body composition: Effects in individuals with and without first-degree relatives with type 2 diabetes. Diabetologia 2018, 61, 1282–1294. [Google Scholar] [CrossRef]
- Smorawinski, J.; Kaciuba-Uscilco, H.; Nazar, K.; Kubala, P.; Kaminska, E.; Ziemba, A.W.; Adrian, J.; Greenleaf, J.E. Effects of three-day bed rest on metabolic, hormonal and circulatory responses to an oral glucose load in endurance or strength trained athletes and untrained subjects. J. Physiol. Pharmacom. 2000, 51, 279–289. [Google Scholar]
- Kroger, K.; Kucharczik, A.; Hirche, H.; Rudofsky, G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 1999, 50, 649–654. [Google Scholar] [CrossRef]
- Aboyans, V.; McClelland, R.L.; Allison, M.A.; McDermott, M.M.; Blumenthal, R.S.; Macura, K.; Criqui, M.H. Lower extremity peripheral artery disease in the absence of traditional risk factors. The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2011, 214, 169–173. [Google Scholar] [CrossRef]
- Restaino, R.M.; Walsh, L.K.; Morishima, T.; Vranish, J.R.; Martinez-Lemus, L.A.; Fadel, P.J.; Padilla, J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H648–H653. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, Metabolic Syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Barr, E.L.M.; Healy, G.N.; Salmon, J.; Shaw, J.E.; Balkau, B.; Magliano, D.J.; Cameron, A.J.; Zimmet, P.Z.; Owen, N. Television viewing, and mortality. The Australasian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation 2010, 121, 384–391. [Google Scholar] [CrossRef]
- Wennberg, P.; Gustafsson, P.E.; Dunstan, D.W.; Wennberg, M.; Hammarstrom, A. Television viewing and low-leisure time physical activity in adolescence independently predict the Metabolic Syndrome in early adulthood. Diabetes Care 2013, 36, 2090–2097. [Google Scholar] [CrossRef]
- Bressler, P.; Bailey, S.R.; Matsuda, M.; DeFronzo, R.A. Insulin resistance and coronary artery disease. Diabetologia 1996, 39, 1345–1350. [Google Scholar] [CrossRef]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.S.; Smit, J.W.A.; Dekkers, O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS ONE 2012, 7, e52036. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 4, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Lambadiari, V.; Roden, M.; Dimitriadis, G.D. Insulin resistance in type 1 diabetes: Pathophysiological, clinical, and therapeutic relevance. Endocr. Rev. 2025, 46, 317–348. [Google Scholar] [CrossRef]
- Stout, R.W. Insulin and atheroma: A 20-year perspective. Diabetes Care 1990, 13, 631–654. [Google Scholar] [CrossRef]
- Meigs, J.B.; Mittleman, M.A.; Nathan, D.M.; Tofler, G.H.; Singer, D.E.; Murphy-Sheehy, P.M.; Lipinska, I.; D’Agostino, R.B.; Wilson, P.W.F. Hyperinsulinemia, hyperglycemia, and impaired hemostasis. JAMA 2000, 283, 221–228. [Google Scholar] [CrossRef]
- Tall, A.R. Exercise to reduce cardiovascular risk—How much is enough? N. Engl. J. Med. 2002, 347, 1522–1524. [Google Scholar] [CrossRef]
- Rabol, R.; Petersen, K.F.; Dufour, S.; Flannery, C.; Shulman, G.I. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin-resistant individuals. Proc. Natl. Acad. Sci. USA 2011, 108, 13705–13709. [Google Scholar] [CrossRef]
- Van Baak, M.A.; Pramono, A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraca, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of different types of regular exercise on physical fitness in adults with overweight or obesity: Systematic review and meta-analyses. Obes. Rev. 2021, 22, e13239. [Google Scholar] [CrossRef]
- Hadjispyrou, S.; Dinas, P.C.; Delitheos, S.M.; Koumprentziotis, I.-A.; Chryssanthopoulos, C.; Philippou, A. The Effect of High-Intensity Interval Training on Mitochondrial-Associated Indices in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. Front. Biosci. (Landmark Ed.) 2023, 28, 281. [Google Scholar] [CrossRef]
- Batacan RBJr Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef]
- Philippou, A.; Chryssanthopoulos, C.; Maridaki, M.; Dimitriadis, G.; Koutsilieris, M. Exercise metabolism in health and disease. In Cardiorespiratory Fitness in Cardiometabolic Diseases; Kokkinos, P., Narayan, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2019, pp. 57–96. [Google Scholar]
- Miles, J.M.; Wooldridge, D.; Grellner, W.J.; Windsor, S.; Isley, W.L.; Klein, S.; Harris, W.S. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: Effects of insulin sensitization therapy. Diabetes 2003, 52, 675–676. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed]
- Clutter, W.E.; Bier, D.M.; Shah, S.D.; Cryer, P.E. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J. Clin. Investig. 1980, 66, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Dona, S.; Negri, C. Metabolic Effects of Exercise. Front. Horm. Res. 2016, 47, 44–57. [Google Scholar]
- Coderre, L.; Kandror, K.V.; Vallega, G.; Pilch, P.F. Identification and characterization of an exercise-sensitive pool of glucose transporters in skeletal muscle. J. Biol. Chem. 1995, 270, 27584–27588. [Google Scholar] [CrossRef] [PubMed]
- Ploug, T.; Galbo, H.; Richter, E.A. Increased muscle glucose uptake during contractions: No need for insulin. Am. J. Physiol. 1984, 247, E726–E731. [Google Scholar] [CrossRef]
- Richter, E.A. Is GLUT4 translocation the answer to exercise-stimulated muscle glucose uptake? Am. J. Physiol. Endocrinol. Metab. 2021, 320, E240–E243. [Google Scholar] [CrossRef]
- O’Doherry, R.M.; Bracy, D.P.; Osawa, H.; Wasserman, D.H.; Granner, D.K. Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. Am. J. Physiol. 1994, 266, E171–E178. [Google Scholar]
- Perseghin, G.; Price, T.B.; Petersen, K.F.; Roden, M.; Cline, G.W.; Gerov, K.; Rothman, D.L.; Shulman, G.I. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N. Engl. J. Med. 1996, 335, 1357–1362. [Google Scholar] [CrossRef]
- Jensen, T.E.; Richter, E.A. Regulation of glucose and glycogen metabolism during and after exercise. J. Physiol. 2012, 590, 1069–1076. [Google Scholar] [CrossRef]
- Gejl, K.D.; Ortenblad, N.; Andersson, E.; Plomgaard, P.; Holmberg, H.C.; Nielsen, J. Local depletion of glycogen with supramaximal exercise in human skeletal muscle fibers. J. Physiol. 2017, 595, 2809–2821. [Google Scholar] [CrossRef]
- Lewis, G.F. Fatty acid regulation of very low density lipoprotein production. Curr. Opin. Lipidol. 1997, 8, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Leighton, B.; Blomstrand, E.; Challiss, R.A.; Lozeman, F.J.; Parry-Billings, M.; Dimitriadis, G.D.; Newsholme, E.A. Acute and chronic effects of strenuous exercise on glucose metabolism in isolated, incubated soleus muscle of exercise-trained rats. Acta Physiol. Scand. 1989, 136, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bogardus, C.; Thuillez, P.; Ravussin, E.; Vasquez, B.; Narimiga, M.; Azhar, S. Effect of muscle glycogen depletion on in vivo insulin action in man. J. Clin. Investig. 1983, 72, 1605–16100. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Exercise-induced increase in muscle insulin sensitivity. J. Appl. Physiol. 2005, 99, 338–343. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Marzolini, S.; Oh, P.I.; Brooks, D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: A meta-analysis. Eur. J. Prev. Cardiol. 2012, 19, 81–94. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Newsholme, E.A. Integration of Biochemical and Physiologic Effects of Insulin on the Control of Blood Glucose Concentrations. In Diabetes Mellitus a Fundamental and Clinical Text, 3rd ed.; LeRoith, D., Taylor, S., Olefsky, J., Eds.; Lippincott Williams &Wilkins: Philadelphia, PA, USA, 2003; Chapter 12; pp. 183–197. [Google Scholar]
- Newsholme, E.A. A possible metabolic basis for the control of body weight. N. Engl. J. Med. 1980, 302, 400–405. [Google Scholar] [CrossRef]
- Codella, R.; Ialacqua, M.; Terruzzi, I.; Luzi, L. May the force be with you: Why resistance training is essential for subjects with type 2 diabetes mellitus without complications. Endocrine 2018, 62, 14–25. [Google Scholar] [CrossRef]
- Philippou, A.; Halapas, A.; Maridaki, M.; Koutsilieris, M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J. Musculoskelet. Neuronal Interact. 2007, 7, 208–218. [Google Scholar]
- Kido, K.; Ato, S.; Yokokawa, T.; Makanae, Y.; Sato, K.; Fujita, S. Acute resistance exercise-induced IGF1 expression and subsequent GLUT4 translocation. Physiol. Rep. 2016, 4, e12907. [Google Scholar] [CrossRef]
- Mangine, G.T.; Hoffman, J.R.; Gonzalez, A.M.; Townsend, J.R.; Wells, A.J.; Jajtner, A.R.; Beyer, K.S.; Boone, C.H.; Miramont, A.A.; Wang, R.; et al. The effect of training volume and intensity on improvements in muscular strength and size in resistance-trained men. Physiol. Rep. 2015, 3, e12472. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Parry-Billings, M.; Bevan, S.; Dunger, D.; Piva, T.; Krause, U.; Wegener, G.; Newsholme, E.A. Effects of insulin-like growth factor I on the rates of glucose transport and utilization in rat skeletal muscle in vitro. Biochem. J. 1992, 285, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Parry-Billings, M.; Dunger, D.; Bevan, S.; Colquhoun, A.; Taylor, A.; Calder, P.; Krause, U.; Wegener, G.; Newsholme, E.A. Effects of in-vivo administration of insulin-like growth factor-I on the rate of glucose utilization in the soleus muscle of the rat. J. Endocrinol. 1992, 133, 37–43. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Dvorak, R.V.; DeNino, W.F.; Brochu, M.; Ades, P.A. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: A controlled randomized trial. J. Clin. Endocrinol. Metab. 2000, 85, 2463–2468. [Google Scholar] [CrossRef]
- Paquin, J.; Lagace, J.C.; Brochu, M.; Dionne, I.J. Exercising for insulin sensitivity. Is there a mechanistic relationship with quantitative changes in skeletal muscle mass? Front. Physiol. 2021, 12, 656909. [Google Scholar] [CrossRef]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, R.S.; Babraj, J.A.; Fawkner, S.G.; Vollaard, N.B.J. Towards the minimal amount of exercise for improving metabolic health: Beneficial effects of reduced-exertion high-sensitivity interval training. Eur. J. Appl. Physiol. 2012, 112, 2767–2775. [Google Scholar] [CrossRef]
- Gallardo-Gomez, D.; Salazar-Martinez, E.; Alfonso-Rosa, R.M.; Ramos-Munell, J.; del Pozzo-Cruz, J.; del Pozo-Cruz, B.; Alvarez-Barbosa, F. Optimal dose and type of physical activity to improve glycemic control in people diagnosed with type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2024, 47, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.N.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Pan, B.; Ge, L.; Xun, Y.-Q.; Chen, Y.-J.; Gao, C.-Y.; Han, X.; Zuo, L.-Q.; Shan, H.-Q.; Yang, K.-H.; Ding, G.-W.; et al. Exercise training modalities in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Int. J. Behav. Nut Phys. Act. 2018, 15, 72. [Google Scholar] [CrossRef]
- Hou, L.; Wang, Q.; Pan, B.; Li, R.; Li, Y.; He, J.; Qin, T.; Cao, L.; Zhang, N.; Cao, C.; et al. Exercise modalities for type 2 diabetes: A systematic review and network meta-analysis of randomized trials. Diabetes Metab. Res. Rev. 2023, 39, e3591. [Google Scholar] [CrossRef]
- Liang, M.; Pan, Y.; Zhong, T.; Zeng, Y.; Cheng, A.S.K. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: A systematic review and network meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1523–1533. [Google Scholar] [CrossRef]
- Sparks, L.M.; Johannsen, N.M.; Church, T.S. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J. Clin. Endocrinol. Metab. 2013, 98, 1694–1702. [Google Scholar] [CrossRef]
- Acosta-Manzano, P.; Rodriguez-Ayllon, M.; Acosta, F.M.; Niederseer, D.; Niebauer, J. Beyond general resistance training. Hypertrophy versus muscular endurance training as therapeutic interventions in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13007. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Blair, S.N.; Cocreham, S.; Johannsen, N.; Johnson, W.; Kramer, K.; Mikus, C.R.; Myers, V.; Nauta, M.; Rodarte, R.Q.; et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes. JAMA 2010, 304, 2253–2262. [Google Scholar] [CrossRef]
- Snowling, N.J.; Hopkins, W.G. Effects of different models of exercise training on glucose control and risk factors for complications in type 2 diabetic patients. Diabetes Care 2006, 29, 2518–2527. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; Dias, S.; König, J.; Hoffmann, G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetologia 2014, 57, 1789–1797. [Google Scholar] [CrossRef]
- Hussey, S.E.; McGee, S.L.; Garnham, A.; Wentworth, J.M.; Jeukendrup, A.E.; Hargreaves, M. Exercise training increases adipose tissue GLUT4 expression in patients with type 2 diabetes. Diabetes Obes. Metab. 2011, 13, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.C.; Franke, W.D.; Sharp, R.L.; Lee, D. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease factors: A randomized controlled trial. PLoS ONE 2019, 14, e0210292. [Google Scholar] [CrossRef]
- Amare, F.; Alemu, Y.; Enichalew, M.; Demilie, Y.; Adamu, S. Effects of aerobic, resistance, and combined exercise training on body fat and glucolipid metabolism in inactive middle-aged adults with overweight or obesity: A randomized trial. BMC Sports Sci. Med. Rehabil. 2024, 16, 189. [Google Scholar] [CrossRef]
- Lafontant, K.; Rukstela, A.; Hanson, A.; Chan, J.; Alsayed, Y.; Ayers-Creech, W.A.; Bale, C.; Ohigashi, Y.; Solis, J.; Shelton, G.; et al. Comparison of concurrent, resistance, or aerobic training on body fat loss: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2025, 22, 2507949. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, F.M.; Lake, Y.A.; Enchalew, M.E.; Miherete, Y.D.; Zewdie, S.A. Impact of aerobic, resistance, and combined training on cardiometabolic health-related indicators in inactive middle-aged men with excess body weight and obesity. Front. Physiol. 2025, 16, 1519180. [Google Scholar] [CrossRef]
- Durstine, J.L.; Grandjean, P.W.; Davis, P.G.; Ferguson, M.A.; Alderson, N.L.; DuBose, K. Blood lipid and lipoprotein adaptations to exercise. Sports Med. 2001, 31, 1033–1062. [Google Scholar] [CrossRef]
- Batrakoulis, A.; Jamurtas, A.Z.; Metsios, G.S.; Perivoliotis, K.; Liguori, G.; Feito, Y.; Riebe, D.; Thompson, W.R.; Angelopoulos, T.J.; Krustrup, P.; et al. Comparative efficacy of 5 exercise types on cardiometabolic health in overweight and obese adults: A systematic review and network meta-analysis of 81 randomized controlled trials. Circulation 2022, 15, e008243. [Google Scholar] [CrossRef]
- Seip, R.L.; Angelopoulos, T.J.; Semenkovich, C.F. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am. J. Physiol. Endocrinol. Metab. 1995, 268, E229–E236. [Google Scholar] [CrossRef]
- Watt, M.J.; Spriet, L.L. Regulation and role of hormone-sensitive lipase activity in human skeletal muscle. Proc. Nutr. Soc. 2004, 63, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Alsted, T.J.; Ploug, T.; Prats, C.; Serup, A.K.; Høeg, L.; Schjerling, P.; Holm, C.; Zimmermann, R.; Fledelius, C.; Galbo, H.; et al. Contraction-induced lipolysis is not impaired by inhibition of hormone-sensitive lipase in skeletal muscle. J. Physiol. 2013, 591, 5141–5155. [Google Scholar] [CrossRef] [PubMed]
- Kiens, B.; Lithell, H. Lipoprotein metabolism influenced by training-induced changes in human skeletal muscle. J. Clin. Investig. 1989, 83, 558–564. [Google Scholar] [CrossRef]
- Smart, N.A.; Downes, D.; van der Touw, T.; Hada, S.; Dieberg, G.; Pearson, M.J.; Wolden, M.; King, N.; Goodman, S.P.J. The effect of exercise training on blood lipids: A systematic review and meta-analysis. Sports Med. 2025, 55, 67–78. [Google Scholar] [CrossRef]
- Kelley, D. Skeletal muscle fat oxidation: Timing and flexibility are everything. J. Clin. Investig. 2005, 115, 1699–1702. [Google Scholar] [CrossRef]
- Yun, H.; Su, W.; Zhao, H.; Li, H.; Wang, Z.; Cui, X.; Xi, C.; Gao, R.; Sun, Y.; Liu, C. Effects of different exercise modalities on lipid profile in the elderly population. Medicine 2023, 102, e33854. [Google Scholar] [CrossRef]
- Serrablo-Torrejon, I.; Lopez-Valenciano, A.; Ayuso, M.; Horton, E.; Mayo, X.; Medina-Gomez, G.; Liguori, G.; Jimenez, A. A meta-analysis of high-intensity training exercise-induced physiological changes and their potential influence on metabolic syndrome clinical biomarkers. BMC Endocr. Disord. 2020, 20, 167. [Google Scholar] [CrossRef]
- Sanca-Valeriano, S.; Espinola-Sanchez, M.; Caballero-Alvarado, J.; Canelo-Aybar, C. Effect of high-intensity interval training compared to moderate-intensity continuous training on body composition and insulin sensitivity in overweight and obese adults: A systematic review and meta-analysis. Heliyon 2023, 9, e20402. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, A.T.; Tolves, T.; Lenzi, T.L.; Signori, L.U.; da Silva, A.M.V. High-intensity interval training versus continuous training on physiological and metabolic variables in prediabetes and type 2 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2017, 137, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mansberg, P.; Frandsen, C.; Carl, C.S.; Espersen, E.; Leineweber, T.; Larsen, E.L.; Storgaard, H.; Schlawitz, K.; Petersen, T.H.D.; Poulsen, J.N.; et al. High-intensity interval training improves insulin sensitivity in individuals with prediabetes. Eur. J. Endocrinol. 2025, 192, 456–465. [Google Scholar] [CrossRef]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-intensity interval training for patients with cardiovascular disease. Is it safe? A systematic review. J. Amer. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef]
- Ko, J.M.; So, W.Y.; Park, S.E. Narrative review of high-intensity interval training: Positive impacts on cardiovascular health and disease prevention. J. Cardiovasc. Dev. Dis. 2025, 12, 158. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, K.; Sundquist, J.; Winkleby, M.A. Exercise is medicine: Primary care counseling on aerobic fitness and muscle strengthening. J. Am. Board. Fam. Med. 2019, 32, 103–107. [Google Scholar] [CrossRef]
- Voudouris, D.; Horianopoulou, M.; Apostolopoulou, Z.; Chryssanthopoulos, C.; Bardopoulou, M.; Maridaki, M.; Vassilakopoulos, T.; Koutsilieris, M.; Philippou, A. The effects of a short-term combined exercise program on liver steatosis indices and the lipemic and glycemic profile in NAFLD individuals: A pilot study. Metabolites 2023, 13, 1074. [Google Scholar] [CrossRef]
- Papaioannou, F.; Karatzanos, E.; Chatziandreou, I.; Philippou, A.; Nanas, S.; Dimopoulos, S. Epigenetic effects following acute and chronic exercise in cardiovascular disease: A systematic review. Int. J. Cardiol. 2021, 341, 88–95. [Google Scholar] [CrossRef]
- Steinacker, J.M.; van Mechelen, W.; Bloch, W.; Börjesson, M.; Casasco, M.; Wolfarth, B.; Knoke, C.; Papadopoulou, T.; Wendt, J.; Al Tunaiji, H.; et al. Global alliance for the promotion of physical activity: The Hamburg declaration. BMJ Open Sport Exerc. Med. 2023, 9, e001626. [Google Scholar] [CrossRef]
- Brinkman, C. Roadmap for personalized exercise medicine in T2DM. Trends Endocrinol. Metab. 2023, 34, 789–798. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Care. Diabetes Care 2025, 48 (Suppl. 1), S100–S104. [Google Scholar]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical activity in individuals with type 2 diabetes: A consensus statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Apovian, C.M.; Barr-Anderson, D.J.; Courcoulas, A.P.; Donnelly, J.E.; Ekkekakis, P.; Hopkins, M.; Lampert, E.V.; Napolitano, M.A.; Volpe, S.L. Physical activity and excess body weight and adiposity for adults. American College of Sports Medicine Consensus Statement. Med. Sci. Sports Exerc. 2024, 56, 2076–2091. [Google Scholar] [CrossRef]
- Blackwell, D.L.; Clarke, T.C. State Variation in Meeting the 2008 Federal Guidelines for Both Aerobic and Muscle-strengthening Activities Through Leisure-time Physical Activity Among Adults Aged 18–64: United States, 2010–2015. Natl. Health Stat. Rep. 2018, 112, 1–21. [Google Scholar]
- Dos Santos, M.; Ferrari, G.; Lee, D.H.; Rey-Lopez, J.P.; Aune, D.; Liao, B.; Huang, W.; Nie, J.; Wang, Y.; Giovannucci, E.; et al. Association of the “weekend warrior” and other leisure-time physical activity patterns with all-cause and cause-specific mortality. JAMA Intern. Med. 2022, 182, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Mn Clare, P.J.; Katzmarzyk, P.T.; del Pozo Cruz, B.; Lee, I.M.; Stamatakis, E. Vigorous physical activity, incident heart disease, and cancer: How little is enough? Eur. Heart J. 2022, 43, 4801–4814. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all-cause mortality: Systematic review and harmonized meta-analysis. Brit. Med. J. 2019, 366, l4570. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Kamada, M.; Bassett, D.R.; Matthews, C.E.; Buring, J.E. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern. Med. 2019, 179, 1105–1112. [Google Scholar] [CrossRef]
- Ding, D.; Nguyen, B.; Nav, T.; Luo, M.; del Polo Cruz, B.; Dempsey, P.C.; Munn, Z.; Jefferis, B.J.; Sherrington, C.; Calleja, E.A.; et al. Daily steps and health outcomes in adults: A systematic review and dose-response meta-analysis. Lancet Public Health 2025, 10, e668-81. [Google Scholar] [CrossRef]
- Doewes, R.I.; Gharibian, G.; Zadeh, F.A.; Zanan, B.A.; Vahdat, S.; Akhavan-Sigari, R. An Updated Systematic Review on the Effects of Aerobic Exercise on Human Blood Lipid Profile. Curr. Probl. Cardiol. 2023, 48, 101108. [Google Scholar] [CrossRef]
- Kannan, U.; Vasudevan, K.; Balasubramaniam, K.; Yerrabelli, D.; Shanmugavel, K.; John, N.A. Effect of exercise intensity on lipid profile in sedentary obese adults. J. Clin. Diagn. Res. 2014, 8, BC08–BC10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qun Zhai, Q.; Ge Li, G.; Weihang Peng, W. Effects of Different Aerobic Exercises on Blood Lipid Levels in Middle-Aged and Elderly People: A Systematic Review and Bayesian Network Meta-Analysis Based on Randomized Controlled Trials. Healthcare 2024, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, R.; Liu, X.; Wang, J.; Wang, L.; Lv, Y.; Yu, L. Effects of Aerobic Exercise on Blood Lipids in People with Overweight or Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Life 2025, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.B.; Hivert, M.F.; Jerome, G.J.; Kraus, W.E.; Rosenkranz, S.K.; Schorr, E.N.; Spartano, N.L.; Lobelo, F. Physical Activity as a Critical Component of First-Line Treatment for Elevated Blood Pressure or Cholesterol: Who, What, and How? A Scientific Statement From the American Heart Association. Hypertension 2021, 78, e26–e37. [Google Scholar] [CrossRef]
- Philippou, A.; Chryssanthopoulos, C.; Maridaki, M.; Koutsilieris, M. The role of exercise in obesity. Diabetes Metab. Syndr. 2019, 13, 2861–2862. [Google Scholar] [CrossRef]
- Carraça, E.V.; Encantado, J.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; van Baak, M.; Dicker, D.; Ermolao, A.; Farpour-Lambert, N.; et al. Effect of exercise training on psychological outcomes in adults with overweight or obesity: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13261. [Google Scholar] [CrossRef]
- Garthwaite, T.; Sjoros, T.; Laine, S.; Vaha-Ypya, H.; Loyttyniemi, E.; Sievanen, H.; Houttu, N.; Laitinen, K.; Kalliokoski, K.; Vasankari, T.; et al. Effects of reduced sedentary time on cardiometabolic health in adults with metabolic syndrome: A three-month randomized controlled trial. J. Sci. Med. Sport. 2022, 25, 579–585. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Larsen, R.N.; Sethi, P.; Sacre, J.W.; Straznicky, N.E.; Cohen, N.D.; Cerin, E.; Lambert, G.W.; Owen, N.; Kingwell, B.A.; et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care 2016, 39, 964–972. [Google Scholar] [CrossRef]
- Hespel, P.; Vergauwen, L.; Vandenberghe, K.; Richter, E.A. The important role of insulin and blood flow in stimulating glucose uptake in contracting skeletal muscle. Diabetes 1995, 44, 210–215. [Google Scholar] [CrossRef]
- Inyard, A.C.; Chong, D.G.; Klibanov, A.L.; Barrett, E.J. Muscle contraction but not insulin increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes 2009, 58, 2457–2463. [Google Scholar] [CrossRef]
- Santos, J.M.; Ribeiro, S.B.; Gaya, A.R.; Appell, H.J.; Duarte, J.A. Skeletal muscle pathways of contraction-enhanced glucose uptake. Int. J. Sports Med. 2008, 29, 785–794. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef]
- Mulya, A.; Haus, J.M.; Solomon, T.P.; Kelly, K.R.; Malin, S.K.; Rocco, M.; Barkoukis, H.; Kirwan, J.P. Exercise training-induced improvement in skeletal muscle PGC-1alpha-mediated fat metabolism is independent of dietary glycemic index. Obesity 2017, 25, 721–729. [Google Scholar] [CrossRef]
- McAuley, P.A.; Artero, E.G.; Sui, X.; Lavie, C.J.; Almeida, M.J.; Blair, S.N. Fitness, fatness, and survival in adults with prediabetes. Diabetes Care 2014, 37, 529–536. [Google Scholar] [CrossRef]
- Rao, S.; Pandey, A.; Garg, S.; Park, B.; Mayo, H.; Despres, J.P.; Kumbhani, D.; de Lemos, J.A.; Neeland, I.J. Effect of exercise and pharmacological interventions on visceral adiposity: A systematic review and meta-analysis of long-term randomized controlled trials. Mayo Clin. Proc. 2019, 94, 211–224. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Despres, J.P. Physical activity for type 2 diabetes prevention: Some is better than none, more is better, and earlier is best. Diabetes Care 2023, 46, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Sharoff, C.G.; Hagobian, T.A.; Malin, S.K.; Chipkin, S.R.; Yu, H.; Hirshman, M.F.; Goodyear, L.J.; Braun, B. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E815–E823. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Nightingale, J.; Choi, S.E.; Chipkin, S.R.; Braun, B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose-tolerant adults. Obesity 2013, 21, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Clarson, C.L.; Mahmud, F.H.; Baker, J.E.; Clark, H.E.; McKay, W.M.; Schauteet, V.D.; Hill, D.J. Metformin, in combination with a structured lifestyle intervention, improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine 2009, 36, 141–146. [Google Scholar] [CrossRef]
- Boule, N.G.; Robert, C.; Bell, G.J.; Johnson, S.T.; Bell, R.C.; Lewanczuk, R.Z.; Gabr, R.Q.; Brocks, D.R. Metformin and exercise in type 2 diabetes. Diabetes Care 2011, 34, 1469–1474. [Google Scholar] [CrossRef]
- Boule, N.G.; Kenny, G.P.; Larose, J.L.; Khandwala, F.; Kuzik, N.; Sigal, R.J. Does metformin modify the effect on glycemic control of aerobic exercise, resistance exercise, or both? Diabetologia 2013, 56, 2378–2382. [Google Scholar] [CrossRef]
- Carillo, P.B.J.; Cope, E.; Gurel, S.; Traslosheros, A.; Kenny, A.; Michot-Duval, O.; Mody, N.; Delibegovic, M.; Philip, S.; Thies, F.; et al. Morning exercise and pre-breakfast metformin interact to reduce glycemia in people with type 2 diabetes: A randomized crossover trial. J. Physiol. 2024, 602, 6491–6506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yang, Q.; Feuerbacher, J.F.; Yu, B.; Brinkmann, C.; Cheng, S.; Bloch, W.; Schumann, M. Effects of exercise, metformin and their combination on glucose metabolism in individuals with abnormal glycaemic control: A systematic review and network meta-analysis. Br. J. Sports Med. 2024, 58, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Sandsdal, R.M.; Juhl, C.R.; Jensen, S.B.K.; Lundgren, J.R.; Janus, C.; Blond, M.B.; Rosenkilde, M.; Bogh, A.F.; Glieman, L.; Jensen, J.E.B.; et al. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: A randomized controlled trial. Cardiovasc. Diabetol. 2023, 22, 41. [Google Scholar] [CrossRef]
- Jensen, S.B.K.; Blond, M.B.; Sandsdal, R.M.; Olsen, L.M.; Juhl, C.R.; Lundgren, J.R.; Janus, C.; Stallknecht, B.M.; Holst, J.J.; Madsbad, S.; et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined, followed by one year without treatment: A post-treatment analysis of a randomized placebo-controlled trial. eClinicalMedicine 2024, 69, 102475. [Google Scholar] [CrossRef]
- Mehrtash, F.; Dushay, J.; Manson, J.E. Integrating diet and physical activity when prescribing GLP-1s-Lifestyle factors remain crucial. JAMA Intern. Med. 2025, 185, 1151–1152. [Google Scholar] [CrossRef]
- Conte, C.; Hall, K.D.; Klein, S. Is weight loss-induced muscle mass loss clinically relevant? JAMA 2024, 332, 9–10. [Google Scholar] [CrossRef]
- Jensen, S.B.K.; Sorensen, V.; Sandsdal, R.M.; Lehman, E.W.; Lundgren, J.R.; Rimer, C.J.; Janus, C.; Ternhamar, T.; Stallknecht, B.M.; Holst, J.J.; et al. Bone health after exercise alone, GLP-1 Receptor Agonist treatment, or combination treatment. JAMA 2024, 7, e2416775. [Google Scholar] [CrossRef]
- Bouchi, R.; Sonoda, N.; Itoh, J.; Ono, Y.; Fukuda, T.; Takeuchi, T.; Kishimoto, J.; Yamada, T.; Ogawa, Y. Effects of intensive exercise combined with dapagliflozin on body composition in patients with type 2 diabetes: A randomized controlled trial. Endocr. J. 2021, 68, 329–343. [Google Scholar] [CrossRef]
- Newman, A.A.; Grimm, N.C.; Wolburn, J.R.; Schoenberg, H.M.; Trikha, S.R.J.; Luckasen, G.J.; Biela, L.M.; Melby, C.L.; Bell, C. Influence of Sodium Glucose Transporter 2 inhibition on physiological adaptations to endurance exercise training. J. Clin. Endocrinol. Metab. 2019, 104, 1953–1966. [Google Scholar] [CrossRef]
- Voorrips, S.N.; Saucedo-Orozco, H.; Sanchez-Aguilera, P.I.; De Boer, R.A.; Van der Meer, P.; Westenbrink, B.D. Could SGLT2 inhibitors improve exercise intolerance in chronic heart failure? Int. J. Mol. Sci. 2022, 23, 8631. [Google Scholar] [CrossRef]
- Peng, Y.; Qin, D.; Wang, Y.; Xue, L.; Qin, Y.X.; Xu, X. The effect of SGLT2 inhibitors on cardiorespiratory fitness capacity: A systematic review and meta-analysis. Front. Physiol. 2023, 13, 1081920. [Google Scholar] [CrossRef]
- Chaix, A.; Panda, S. Timing tweaks exercise. Nat. Rev. Endocrinol. 2019, 15, 440–441. [Google Scholar] [CrossRef]
- Gerhard-Hines, Z.; Lazar, M.A. Circadian metabolism in the light of evolution. Endocr. Rev. 2015, 36, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Esser, K.A. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med. Sci. Sports Exerc. 2012, 44, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- van Moorsel, D.; Hansen, J.; Havekes, B.; Scheer, F.A.J.L.; Jorgensen, J.A.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Duez, H.; Lefebvre, P.; Schaper, N.C.; et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab. 2016, 5, 635–645. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.N.J.; Bartlett, J.D. Is exercise a viable therapeutic intervention to mitigate mitochondrial dysfunction and insulin resistance induced by sleep loss? Sleep. Med. Rev. 2018, 37, 60–68. [Google Scholar]
- Gonzalez-Reytor, C.; Smancas-Racines, D.; Roman-Galeano, N.M.; Annunziata, G.; Galasso, M.; Zambrano-Villacres, R.; Verde, L.; Muscogiuri, G.; Frias-Toral, E.; Barrea, L. Chrononutrition and energy balance: How meal timing and circadian rhythms shape weight regulation and metabolic health. Nutrients 2025, 17, 2135. [Google Scholar] [CrossRef]
- Heden, T.D.; Kanaley, J.A. Syncing exercise with meals and circadian clocks. Exerc. Sport. Sci. Rev. 2019, 47, 22–28. [Google Scholar] [CrossRef]
- MacLeod, S.F.; Terada, T.; Chahal, B.S.; Boule, N.G. Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: A meta-analysis of studies using continuous glucose monitoring. Diabetes Metab. Res. Rev. 2013, 29, 593–603. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Mann, J.L.; Williams, S.; Venn, B.J. Advice to walk after meals is more effective for lowering postprandial glycemia in type 2 diabetes mellitus than advice that does not specify timing: A randomized crossover study. Diabetologia 2016, 59, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- Bolli, G.; Gerich, J. The “Dawn Phenomenon”—A Common Occurrence in Both Non-Insulin-Dependent and Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1984, 310, 746–750. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Vlachonikolis, I.G.; Hatziagellaki, E.; Linos, A.; Kordonouri, O.; Alexopoulos, E.; Raptis, S. The “dawn phenomenon” in patients with type II diabetes mellitus. Diab Nutr. Metab. 1988, 1, 37–41. [Google Scholar]
- Liu, P.Y. Rhythms in cortisol mediate sleep and circadian impacts on health. Sleep 2024, 47, zsae151. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Swirski, F.K. Circadian influence on metabolism and inflammation in atherosclerosis. Circ. Res. 2016, 119, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Porter, J.W.; Winn, N.C.; Lastra, G.; Chockalingam, A.; Pettit-Mee, R.J.; Petroski, G.F.; Cobelli, C.; Schiavon, M.; Parks, E.J. Temporal optimization of exercise to lower fasting glucose levels. J. Physiol. 2024, 602, 6447–6461. [Google Scholar] [CrossRef]
- Sabag, A.; Ahmadi, M.N.; Francois, M.E.; Postnova, S.; Cistulli, P.A.; Fontana, L.; Stamatakis, E. Timing of moderate to vigorous physical activity, mortality, cardiovascular disease, and microvascular disease in adults with obesity. Diabetes Care 2024, 47, 890–897. [Google Scholar] [CrossRef]
- Van der Velde, J.H.P.M.; Boone, S.C.; Winters-van Eekelen, E.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B.; Schrauwen, P.; Lamb, H.J.; Rossendaal, F.R.; de Mutsert, R. Timing of physical activity in relation to liver fat content and insulin resistance. Diabetologia 2022, 66, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.J.; Brady, A.J.; Smith, J.A.B.; Savikj, M.; MacGregor, K.; Jollet, M.; Oberg, S.B.; Nylen, C.; Bjornholm, M.; Rickenlund, A.; et al. Inflammatory markers and blood glucose are higher after morning vs afternoon exercise in type 2 diabetes. Diabetologia 2025, 68, 2023–2035. [Google Scholar] [CrossRef]
- Henson, J.; Rowlands, A.V.; Baldry, E.; Brady, E.M.; Davies, M.J.; Edwardson, C.L.; Yates, T.; Hall, A.P. Physical behaviors and chronotype in people with type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001375. [Google Scholar] [CrossRef]
- Tian, C.; Burki, C.; Westerman, K.E.; Patel, C.J. Association between timing and consistency of physical activity and type 2 diabetes: A cohort study on participants of the UK Biobank. Diabetologia 2023, 66, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Feng, Q.; Rutter, M.K.; Albalak, G.; Wang, H.; Noordam, R. Associations between the timing of 24 h physical activity and diabetes mellitus: Results from a nationally representative sample of the US population. Diabetologia 2025, 68, 1005–1015. [Google Scholar] [CrossRef]
- Campbell, M.D.; Alobaid, A.M.; Hopkins, M.; Dempsey, P.C.; Pearson, S.M.; Kietsiriroje, N.; Churm, R.; Ajjan, R.A. Interrupting prolonged sitting with frequent short bouts of light-intensity activity in people with type 1 diabetes improves glycemic control without increasing hypoglycemia: The SIT-LESS randomized controlled trial. Diabetes Obes. Metab. 2023, 25, 3589–3598. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the regulation of immune functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of exercise training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The interplay between oxidative stress, exercise, and pain in health and disease: Potential role of autonomic regulation and epigenetic mechanisms. Antioxidants 2020, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, J.-Y.; Zhang, H.-X.; Li, Q.; Zhang, S.-W. Exercise training attenuates sympathetic activation and oxidative stress in diet-induced obesity. Physiol. Res. 2015, 64, 355–367. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.-D.; Kumar, V.B. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Hautala, A.J.; Kiviniemi, A.M.; Tulppo, M.P. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009, 33, 107–115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Promoting Well-Being. Available online: https://www.who.int/activities/promoting-well-being (accessed on 3 April 2025).
- World Health Organization. WHOQOL: Measuring Quality of Life. Available online: https://www.who.int/tools/whoqol (accessed on 3 April 2025).
- Marquez, D.X.; Aguiñaga, S.; Vásquez, P.M.; Conroy, D.E.; Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood Sheppard, B.; Petruzzello, S.J.; et al. A systematic review of physical activity and quality of life and well-being. Transl. Behav. Med. 2020, 10, 1098–1109. [Google Scholar] [CrossRef]
- Sabag, A.; Chang, C.R.; Francois, M.E.; Keating, S.E.; Coombes, J.S.; Johnson, N.A.; Pastor-Valero, M.; Lopez, J.P.R. The effect of exercise on quality of life in type 2 diabetes: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2023, 55, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Edwards, J.J.; McNamara, J.; Galbraith, A.; Bruce-Low, S.; O’Driscoll, J.M. The effects of high-intensity interval training on quality of life: A systematic review and meta-analysis. J. Public Health 2024, 33, 2175–2185. [Google Scholar] [CrossRef]
- Bispo, D.P.C.F.; Lins, C.C.S.A.; Hawkes, K.L.; Tripp, S.; Khoo, T.K. The positive effects of physical activity on quality of life in Parkinson’s Disease: A Systematic Review. Geriatrics 2024, 9, 94. [Google Scholar] [CrossRef]
- Dallas, K.; Dinas, P.C.; Chryssanthopoulos, C.; Dallas, G.; Maridaki, M.; Koutsilieris, M.; Philippou, A. The effects of exercise on VO2peak, quality of life and hospitalization in heart failure patients: A systematic review with meta-analyses. Eur. J. Sport. Sci. 2020, 21, 1337–1350. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Vincent, A.J.P.E. Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomized controlled trials. Br. J. Sports Med. 2015, 50, 796–803. [Google Scholar] [CrossRef]
- Reid, H.; Ridout, A.J.; Tomaz, S.A.; Kelly, P.; Jones, N.; on behalf of the Physical Activity Risk Consensus group. Benefits outweigh the risks: A consensus statement on the risks of physical activity for people living with long-term conditions. Br. J. Sports Med. 2022, 56, 427–438. [Google Scholar] [PubMed]
- Niemeijer, A.; Lund, H.; Stafne, S.N.; Ipsen, T.; Goldschmidt, C.L.; Jorgensen, C.T.; Juhl, C.B. Adverse events of exercise therapy in randomized controlled trials: A systematic review and meta-analysis. Br. J. Sports Med. 2020, 54, 1073–1080. [Google Scholar]
- Landolfi, E. Exercise addiction. Sports Med. 2013, 43, 111–119. [Google Scholar] [CrossRef]
- Colledge, F.; Cody, R.; Buchner, U.G.; Schmidt, A.; Pühse, U.; Gerber, M.; Wiesbeck, G.; Lang, U.E.; Walter, M. Excessive Exercise-A Meta-Review. Front. Psychiatry 2020, 11, 521572. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Best, S.; Meerstetter, F.; Isoard-Gautheur, S.; Gustafsson, H.; Bianchi, R.; Madigan, D.J.; Colledge, F.; Ludyga, S.; Holsboer-Trachsler, E.; et al. Cross-Sectional and Longitudinal Associations Between Athlete Burnout, Insomnia, and Polysomnographic Indices in Young Elite Athletes. J. Sport. Exerc. Psychol. 2018, 40, 312–324. [Google Scholar]
- Golshani, S.; Najafpour, A.; Hashemian, S.S.; Goudarzi, N.; Shahmari, F.; Golshani, S.; Babaei, M.; Firoozabadi, K.; Dürsteler, K.M.; Brühl, A.B.; et al. When Much Is Too Much-Compared to Light Exercisers, Heavy Exercisers Report More Mental Health Issues and Stress, but Less Sleep Complaints. Healthcare 2021, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Mittleman, M.A.; Chae, C.U.; Lee, I.M.; Hennekens, C.H.; Manson, J.E. Triggering of sudden death from cardiac causes by vigorous exertion. N. Engl. J. Med. 2000, 343, 1355–1361. [Google Scholar] [CrossRef]
- Mannakkara, N.N.; Finocchiaro, G. Exercise and the heart: Benefits, risks and adverse events of exercise training. Rev. Cardiovasc. Med. 2023, 24, 94. [Google Scholar] [CrossRef]
- Kim, J.H.; Rim, A.J.; Miller, J.T.; Jackson, M.; Patel, N.; Rajesh, S.; Ko, Y.A.; DiGregorio, H.; Chiampas, G.; McGillivray, D.; et al. Cardiac arrest during long-distance running races. JAMA 2025, 333, 1699–1707. [Google Scholar] [CrossRef]
- De Bosscher, R.; Dausin, C.; Claus, P.; Bogaert, J.; Dymarkowski, S.; Goetschalckx, K.; Ghekiere, O.; Van De Heyning, C.M.; Van Herck, P.; Paelinck, B.; et al. Lifelong endurance exercise and its relation with coronary atherosclerosis. Eur. Heart J. 2023, 44, 2388–2399. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Skouras, A.Z.; Antonakis-Karamintzas, D.; Tsantes, A.G.; Triantafyllou, A.; Papagiannis, G.; Tsolakis, C.; Koulouvaris, P. The acute and chronic effects of resistance and aerobic exercise in hemostatic balance: A brief review. Sports 2023, 11, 74. [Google Scholar] [CrossRef]
- Womack, C.J.; Nagelkirk, P.R.; Coughlin, A.M. Exercise-induced changes in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Med. 2003, 33, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.; Dudley, D.; Collins, N.J. Do the benefits of participation in sport and exercise outweigh the negatives? An academic review. Best Pract. Res. Clin. Rheumatol. 2019, 33, 172–187. [Google Scholar] [CrossRef]




| EXERCISE | Aerobic | Resistance | Combined |
|---|---|---|---|
| HbA1c (%) | −0.57 | −0.40 | −0.65 |
| Adipose tissue mass (%) | −0.93 | −1.03 | −1.34 |
| Lipids (mg/dL) Triglycerides LDL HDL | −17.9 −5.77 +10.9 | −9.24 −15.6 +8.61 | −20.6 −10.7 +4.32 |
| Increase in insulin sensitivity (%) | 28 | 12 | 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, G.D.; Chryssanthopoulos, C.; Philippou, A.; Koutsilieris, M. The Significant Role of Physical Activity and Exercise in Health and Metabolic Diseases. Physiologia 2025, 5, 57. https://doi.org/10.3390/physiologia5040057
Dimitriadis GD, Chryssanthopoulos C, Philippou A, Koutsilieris M. The Significant Role of Physical Activity and Exercise in Health and Metabolic Diseases. Physiologia. 2025; 5(4):57. https://doi.org/10.3390/physiologia5040057
Chicago/Turabian StyleDimitriadis, George D., Costas Chryssanthopoulos, Anastassios Philippou, and Michael Koutsilieris. 2025. "The Significant Role of Physical Activity and Exercise in Health and Metabolic Diseases" Physiologia 5, no. 4: 57. https://doi.org/10.3390/physiologia5040057
APA StyleDimitriadis, G. D., Chryssanthopoulos, C., Philippou, A., & Koutsilieris, M. (2025). The Significant Role of Physical Activity and Exercise in Health and Metabolic Diseases. Physiologia, 5(4), 57. https://doi.org/10.3390/physiologia5040057










