Biological and Physico-Chemical Properties of Lobosphaera sp. Packed in Metallized Polyethylene Terephthalate/Polyethylene (PETmet/PE)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga Biomass
2.2. Storage Studies at Different Conditions of Light and Relative Humidity
2.3. Packaging Studies
2.3.1. Packaging Materials
2.3.2. Packaging of Biomass and Quality Analysis During Storage
2.4. Physico-Chemical Characterization of Lobosphaera sp. Biomass
2.4.1. Color Analysis
2.4.2. Moisture Content Determination
2.4.3. Carotenoid and Chlorophyll Content Determination
2.5. Extraction of Bioactive Compounds from Lobosphaera sp.
2.6. Characterization of the Bioactivity of Lobosphaera sp. Extracts
2.6.1. Determination of Total Phenolic Content (TPC)
2.6.2. Antioxidant Activity Assays
2.6.3. Antimicrobial Activity Assays
2.7. Statistical Analysis
3. Results and Discussion
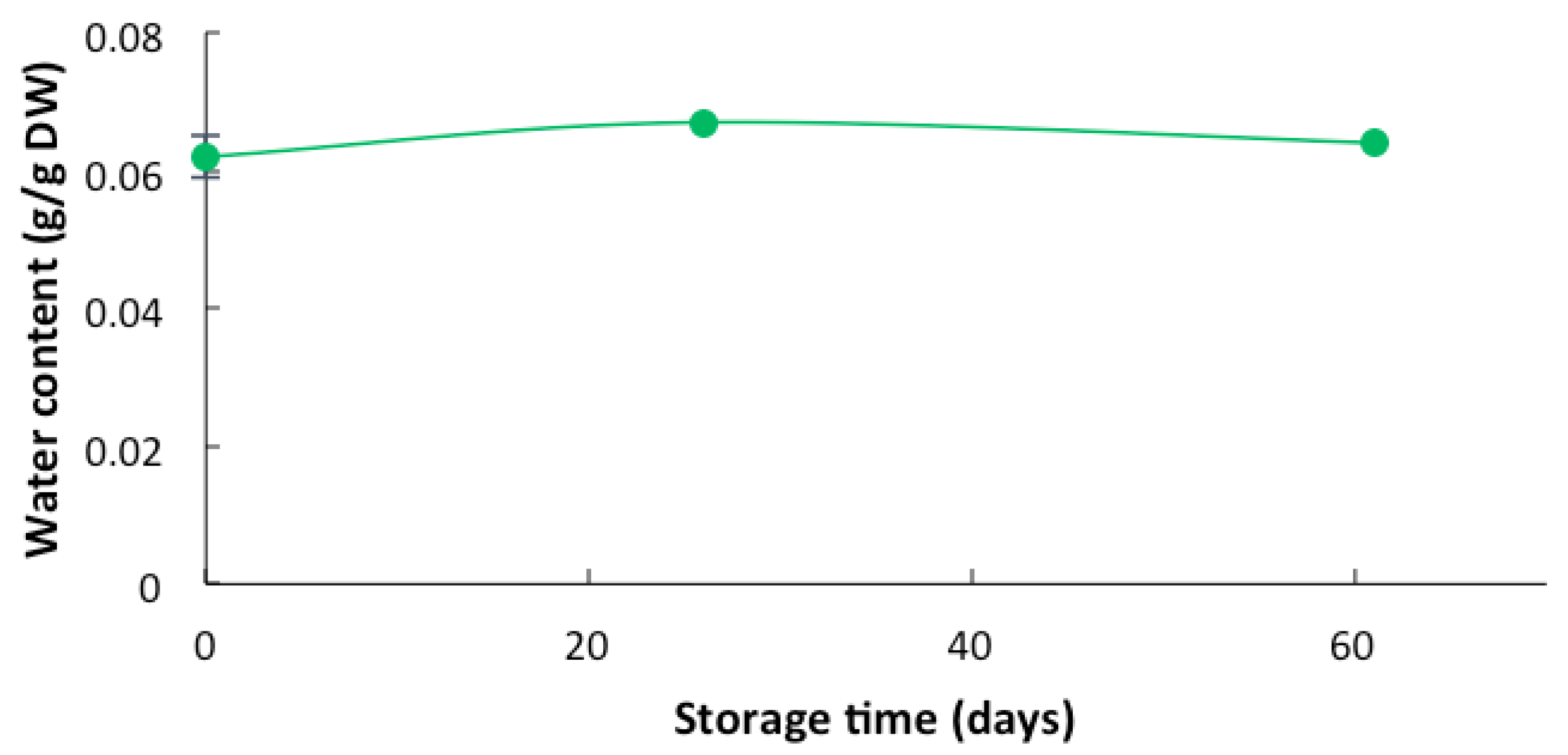
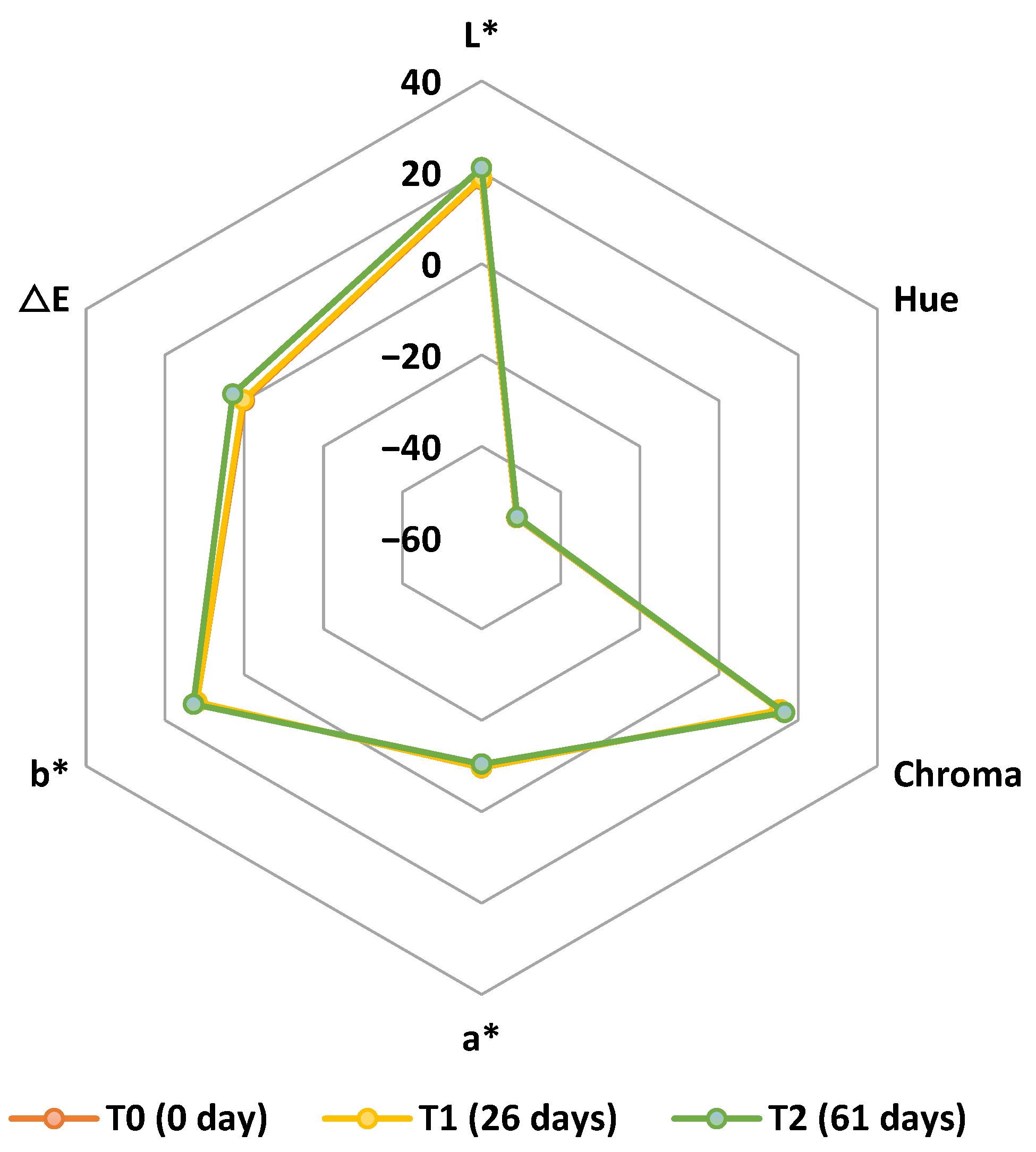
3.1. Effects of the Storage of Lobosphaera sp. Biomass Under Different Conditions of Light and Relative Humidity
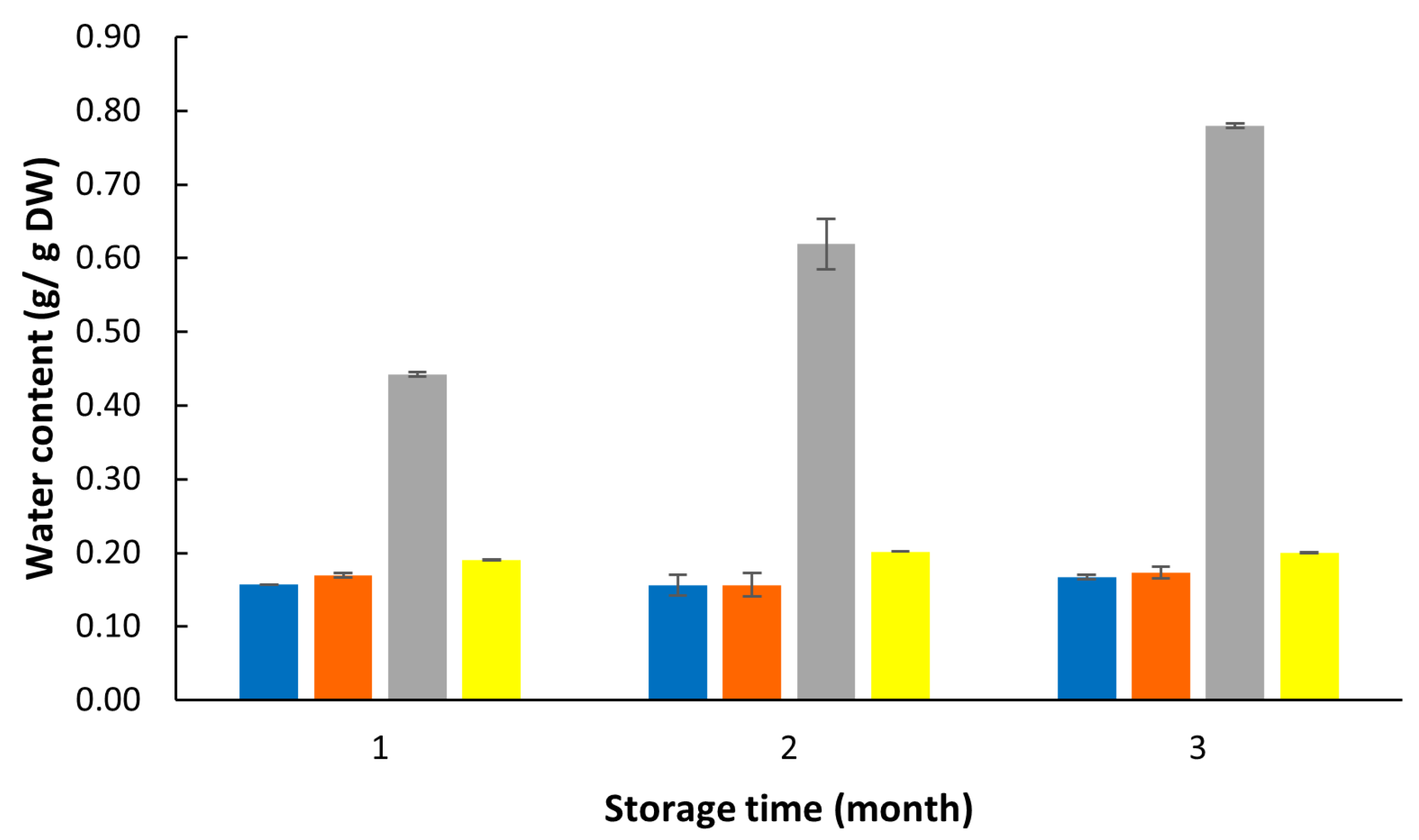
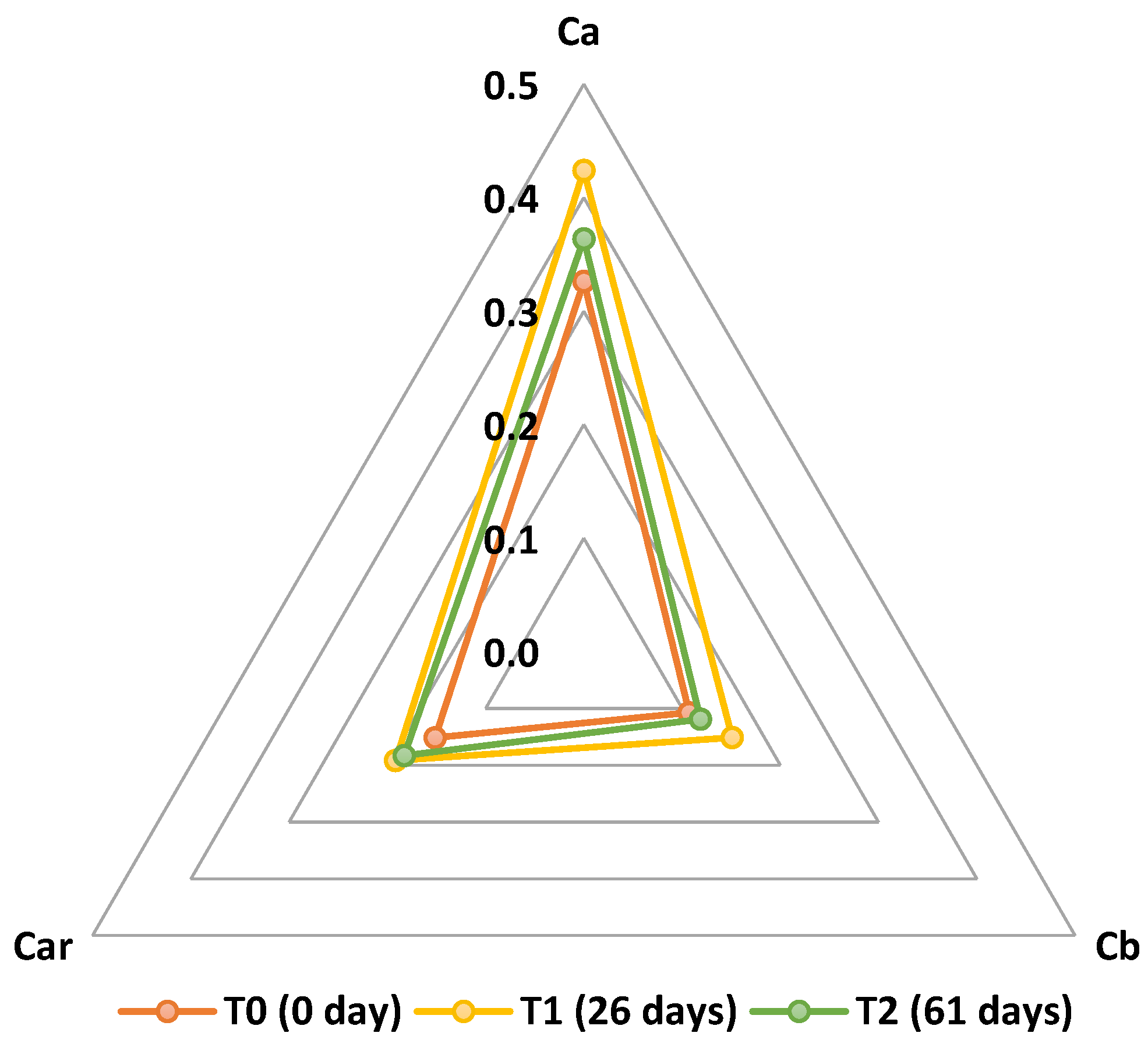
3.2. Effects of the PETmet/PE Packaging on Biomass Quality
3.3. Yield of Extraction of Bioactive Compounds from Lobosphaera sp.
3.4. Effects of PETmet/PE Packaging on the Bioactivity of Lobosphaera sp. Bioactive-Rich Extracts
3.4.1. Total Phenolic Content (TPC) and Antioxidant Activity (ABTS)
3.4.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, V.F.R.; Lopes, A.I.; Machado, M.; Costa, E.M.; Ribeiro, T.B.; Poças, F.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicrobial Activities. Foods 2025, 14, 1327. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T. Eicosapentanoic and docosahexaenoicacids as inflammation-modulating and lipid homeostasis influencing nutraceuticals: A review. J. Funct. Foods 2012, 4, 25–38. [Google Scholar] [CrossRef]
- Lazado, C.C.; Nayak, S.; Khozin-Goldberg, I.; Zilberg, D. The Gut Mucosal Barrier of Zebrafish (Danio rerio) Responds to the Time-Restricted Delivery of Lobosphaera incisa-Enriched Diets. Fish Shellfish Immunol. 2019, 89, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Maté, J.I.; Saltveit, M.E.; Krochta, J.M. Peanut and Walnut Rancidity: Effects of Oxygen Concentration and Relative Humidity. J. Food Sci. 1996, 61, 465–469. [Google Scholar] [CrossRef]
- Wang, A.; Catherine, H.D.; Rachel, M.H.; Sean, F.O.; J’Nai, B.P.; Kemia, A.A.; Susan, E.D. Efficacy of light-protective additive packaging in protecting milk freshness in a retail dairy case with LED lighting at different light intensities. Food Res. Int. 2018, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dakhili, S.; Hashami, Z.; Rostami, N.; Moradi, S.; Moslehishad, M.; Shojaee-Aliabadi, S. Innovative applications of microalgae in biodegradable food packaging: A review. J. Agric. Food Res. 2025, 19, 101723. [Google Scholar] [CrossRef]
- Morais, A.M.M.B.; Martins, V.F.; Alves, A.J.; Poças, F.; Morais, R.M.S.C. Packaging and storage of Porphyridium cruentum: Metallised polyethylene terephthalate/polyethylene (PETmet/PE) versus polyethylene (PE). Acta Aliment. 2024, 53, 419–431. [Google Scholar] [CrossRef]
- Benedicte, L.; Philippe, R. Poly (ethylene terephthalate). In Handbook of Engineering and Speciality Thermoplastics, Polyethers and Polyesters; Thomas, S., Visakh, P.M., Eds.; Scrivener LLC: Beverly, MA, USA, 2011; Volume 3, p. 97. [Google Scholar]
- Garnier, G.; Yrieix, B.; Brechet, Y.; Flandin, L. Influence of structural feature of aluminum coatings on mechanical and water barrier properties of metallized PET films. J. App. Polym. Sci. 2010, 115, 3110–3119. [Google Scholar] [CrossRef]
- Anonymous. Metallized PET Film: What Can PET Met be Used for? AlFiPa. Available online: https://alfipa.com/applications/metallized-pet-met-foil/?utm_source=chatgpt.com (accessed on 10 July 2025).
- Aboeinga, M.M.; Kalyaanamoorthy, S. QM/MM investigation to identify the Hallmarks of superior PET biodegradation activity of PETases over cutinase. ACS Sustain. Chem. Eng. 2022, 10, 15618–16069. [Google Scholar] [CrossRef]
- Gao, R.; Pan, H.; Lian, J. Recent advances in the discovery, characterization, and engineering of poly(ethylene terephthalate) (PET) hydrolases. Enzym. Microb. Technol. 2021, 150, 109868. [Google Scholar] [CrossRef] [PubMed]
- Commission Internationale de L’Eclairage (C.I.E.). Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms. Supplement No. 2 to C.I.E. Publication No. 15, (E.-1.3. 1) 1971; C.I.E.: Paris, France, 1978; Volume TC1. [Google Scholar]
- A.O.A.C. Method, A.O.A.C. 935.29. In Official Methods of Analysis, 18th ed.; Association of the Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Lichtenthaler, H.K.; Abuslima, E.; Nick, P. Strong increase of photosynthetic pigments and leaf size in a pruned Ginkgo biloba tree. Photosynthetica 2023, 61, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.F.R.; Ribeiro, T.B.; Lopes, A.I.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules 2024, 29, 1935. [Google Scholar] [CrossRef]
- Mostafa, H.S.; Hashem, M.M. Microalgae as a source of carotenoids in foods, obstacles and solutions. Phytochem. Rev. 2024, 1–43. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Edible Films with Protein and Bioactive Compounds from Arthrospira sp. Biol. Life Sci. Forum 2024, 40, 6. [Google Scholar]
- Monteiro, M.; Santos, R.A.; Iglesias, P.; Couto, A.; Serra, C.R.; Gouvinhas, I.; Barros, A.; Oliva-Teles, A.; Enes, P.; Díaz-Rosales, P. Effect of Extraction Method and Solvent System on the Phenolic Content and Antioxidant Activity of Selected Macro- and Microalgae Extracts. J. Appl. Phycol. 2020, 32, 349–362. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Coimbra, R.S.T.; Caetano, N.S. Exploring the Antioxidant Potential of Lobosphaera Sp. and Odontella Sp. Biomasses under Different Extraction and Preservation Conditions. In Proceedings of the Seagriculture EU 2023 12th International Seaweed Conference, Trondheim, Norway, 21–22 June 2023. [Google Scholar]
- Torres, P.; Osaki, S.; Silveira, E.; dos Santos, D.Y.A.C.; Chow, F. Comprehensive evaluation of Folin-Ciocalteu Assay for Total Phenolic Quantification in Algae (Chlorophyta, Phaeophyceae and Rhodophyta). Algal Res. 2024, 80, 103503. [Google Scholar] [CrossRef]
- Cuong, D.M.; Yang, S.H.; Kim, J.S.; Moon, J.Y.; Choi, J.; Go, G.M.; Cho, S.K. Evaluation of Antioxidant and Anti-Inflammatory Activity and Identification of Bioactive Compound from the Marine Diatom, Odontella aurita Extract. Appl. Biol. Chem. 2024, 67, 46. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of Exhausted Olive Pomace by an Ecofriendly Solvent Extraction Process of Natural Antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.F.R.; Lopes, A.I.; Gomes, D.; Parreira, C.; Badenes, S.M.; Costa, L.; Pintado, M.; Morais, A.M.M.B.; Morais, R.M.S.C. Unravelling the Potential of Seven Microalgae Species: Nutritional, Antioxidant, and Antimicrobial Properties and Application. Appl. Sci. 2025, 15, 6691. [Google Scholar] [CrossRef]
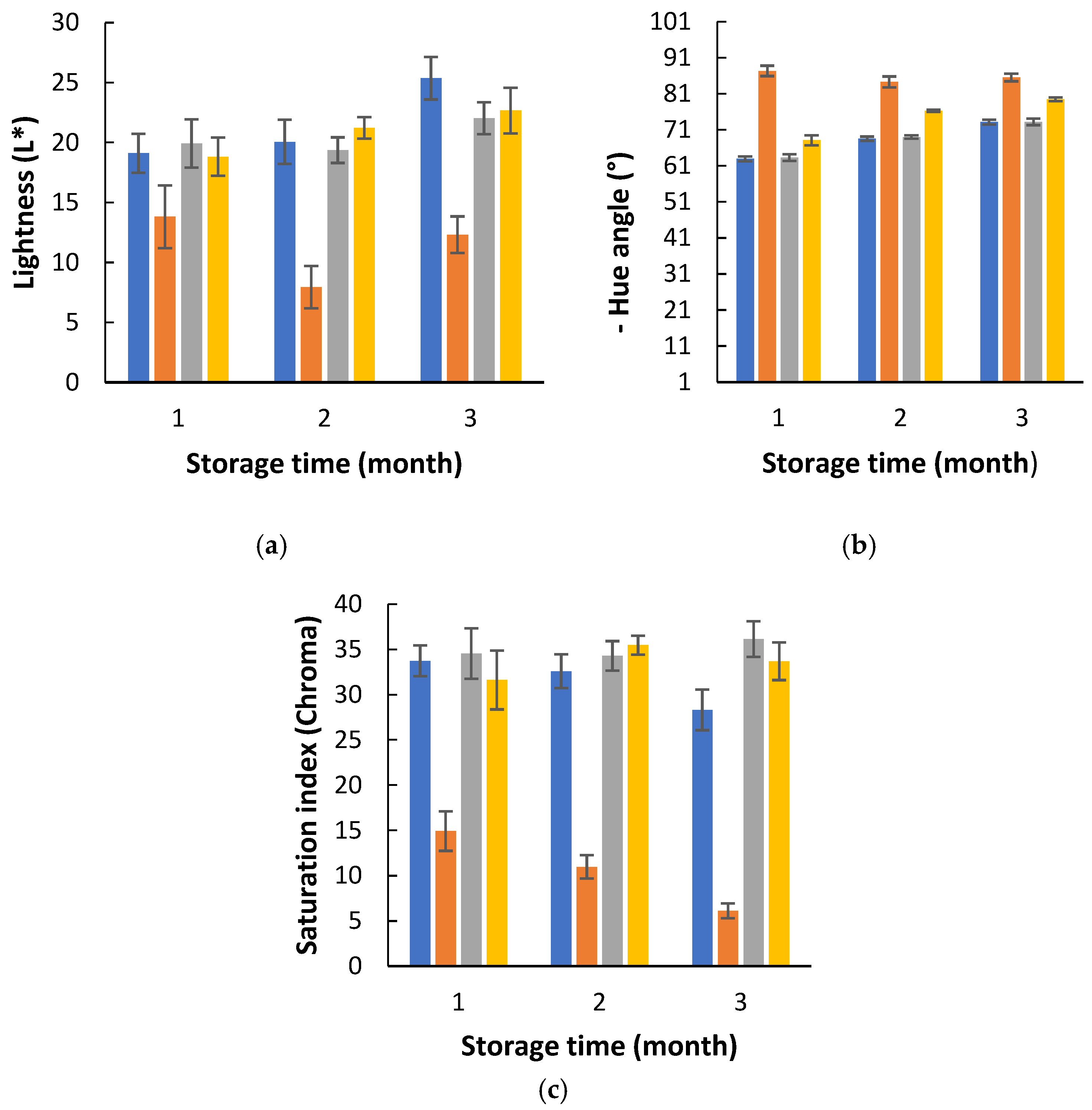
| Packaging Material | Storage Time (Month) | TPC (mg GAE/100 mg Sample DW) | ABTS (µmol of TE/100 mg Sample DW) |
|---|---|---|---|
| - | 0 | 2.83 ± 0.09 | 3.61 ± 0.39 |
| PETmet/PE | 1 | 2.53 ± 0.33 | 3.65 ± 0.32 |
| LDPE | 2.52 ± 0.08 | 3.45 ± 0.47 | |
| PETmet/PE | 2 | 2.45 ± 0.14 | 3.47 ± 0.26 |
| LDPE | 2.19 ± 0.16 | 3.28 ± 0.46 |
| Packaging Material | Storage Time (Month) | Bacteria | Inhibition (%) | ||
|---|---|---|---|---|---|
| 1% Extract | 2% Extract | 3% Extract | |||
| - | 0 | Escherichia coli | 34.18 ± 4.05 | 39.02 ± 4.49 | 56.44 ± 2.85 |
| PETmet/PE | 1 | 24.56 ± 5.99 | 30.03 ± 7.01 | 52.60 ± 4.45 | |
| LDPE | 1 | 6.06 ± 1.50 | 29.69 ± 5.58 | 48.93 ± 2.27 | |
| PETmet/PE | 2 | 14.86 ± 5.25 | 13.80 ± 2.74 | 31.73 ± 2.91 | |
| LDPE | 2 | 0.28 ± 1.11 | 0.72 ± 2.26 | 5.66 ± 1.82 | |
| - | 0 | Yersinia enterocolitica | 41.25 ± 1.06 | 55.13 ± 1.26 | 71.15 ± 2.51 |
| PETmet/PE | 1 | 33.32 ± 1.06 | 37.43 ± 1.63 | 58.54 ± 1.96 | |
| LDPE | 1 | 10.13 ± 3.17 | 19.14 ± 1.61 | 52.08 ± 2.02 | |
| PETmet/PE | 2 | 14.69 ± 2.12 | 28.16 ± 1.92 | 52.45 ± 1.18 | |
| LDPE | 2 | 1.28 ±0.72 | 4.16 ± 1.93 | 12.57 ± 1.39 | |
| - | 0 | Salmonella enterica serovar Enteritidis | 41.37 ± 1.17 | 59.57 ± 1.04 | 69.35 ± 2.84 |
| PETmet/PE | 1 | 30.16 ± 1.99 | 39.65 ± 1.27 | 61.61 ± 1.61 | |
| LDPE | 1 | 21.23 ± 2.39 | 24.41 ± 1.38 | 56.73 ± 1.36 | |
| PETmet/PE | 2 | 27.33 ± 1.57 | 36.80 ± 1.53 | 46.78 ± 1.43 | |
| LDPE | 2 | 8.03 ± 4.74 | 18.10 ± 0.98 | 23.99 ± 1.04 | |
| - | 0 | Staphylococus aureus | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| PETmet/PE | 1 | 85.92 ± 2.74 | 91.94 ± 1.92 | 98.33 ± 2.23 | |
| LDPE | 1 | 18.13 ± 2.76 | 23.66 ± 2.72 | 51.29 ± 3.98 | |
| PETmet/PE | 2 | 25.14 ± 1.97 | 31.45 ± 1.51 | 44.94 ± 1.37 | |
| LDPE | 2 | 1.68 ± 1.05 | 2.04 ± 1.57 | 16.94 ± 1.60 | |
| - | 0 | Bacillus cereus | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| PETmet/PE | 1 | 91.04 ± 1.70 | 94.65 ± 1.44 | 100.00 ± 0.00 | |
| LDPE | 1 | 16.85 ± 2.82 | 19.68 ± 2.72 | 42.33 ± 2.89 | |
| PETmet/PE | 2 | 36.42 ± 1.66 | 40.81 ± 1.50 | 50.37 ± 1.02 | |
| LDPE | 2 | 9.60 ± 0.98 | 13.41 ± 2.69 | 16.43 ± 1.55 | |
| - | 0 | Listeria monocytogenes | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| PETmet/PE | 1 | 96.44 ± 3.09 | 99.62 ± 2.43 | 100.00 ± 0.00 | |
| LDPE | 1 | 5.95 ± 2.95 | 8.00 ± 1.89 | 53.99 ± 1.31 | |
| PETmet/PE | 2 | 7.52 ± 1.72 | 12.84 ± 3.02 | 33.76 ± 1.83 | |
| LDPE | 2 | 1.59 ± 3.80 | 3.32 ± 1.30 | 9.81 ± 1.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, V.F.R.; Alves, A.J.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Biological and Physico-Chemical Properties of Lobosphaera sp. Packed in Metallized Polyethylene Terephthalate/Polyethylene (PETmet/PE). Phycology 2025, 5, 35. https://doi.org/10.3390/phycology5030035
Martins VFR, Alves AJ, Poças F, Pintado M, Morais RMSC, Morais AMMB. Biological and Physico-Chemical Properties of Lobosphaera sp. Packed in Metallized Polyethylene Terephthalate/Polyethylene (PETmet/PE). Phycology. 2025; 5(3):35. https://doi.org/10.3390/phycology5030035
Chicago/Turabian StyleMartins, Valter F. R., Ana J. Alves, Fátima Poças, Manuela Pintado, Rui M. S. C. Morais, and Alcina M. M. B. Morais. 2025. "Biological and Physico-Chemical Properties of Lobosphaera sp. Packed in Metallized Polyethylene Terephthalate/Polyethylene (PETmet/PE)" Phycology 5, no. 3: 35. https://doi.org/10.3390/phycology5030035
APA StyleMartins, V. F. R., Alves, A. J., Poças, F., Pintado, M., Morais, R. M. S. C., & Morais, A. M. M. B. (2025). Biological and Physico-Chemical Properties of Lobosphaera sp. Packed in Metallized Polyethylene Terephthalate/Polyethylene (PETmet/PE). Phycology, 5(3), 35. https://doi.org/10.3390/phycology5030035










