Macroalgae Biorefinery for the Cosmetic Industry: Basic Concept, Green Technology, and Safety Guidelines
Abstract
:1. Introduction
2. Overview of Marine Macroalgae Chemical Composition and Bioactivities
| Putative Efficacies/Activities | Polysaccharides | PUFAs | Mycosporine-like Amino Acids | Phenolic Compounds | Pigments | Sterols |
|---|---|---|---|---|---|---|
| Anti-acne | [12] | |||||
| Anti-inflammatory | [13,14,15,16] | [15,17] | [15,17,18] | [15,17,19] | [17,18] | [20] |
| Anti-photoaging | [17,18,19,21] | [18] | [17] | [18,22] | [20] | |
| Anti-skin aging | [16,23] | [23] | [24] | |||
| Anti-wrinkling | [16,23,25] | [26] | ||||
| Antiapoptotic | [21,27] | [28] | ||||
| Antioxidant | [19,25,27,29] | [18] | [17,19,23,29] | [17,18,19] | [17] | |
| Antipollution | [28] | |||||
| Depigmenting, bleaching, and lightening | [16,19,23,29] | [17,26,29] | [17] | |||
| Hair growth | [29] | |||||
| Hydration | [29] | [29] | ||||
| Photoprotection | [29] | [17,18] | [17,29] | [17,18] | ||
| Skin barrier | [19] | [18] | ||||
| Wound healing | [30,31] |
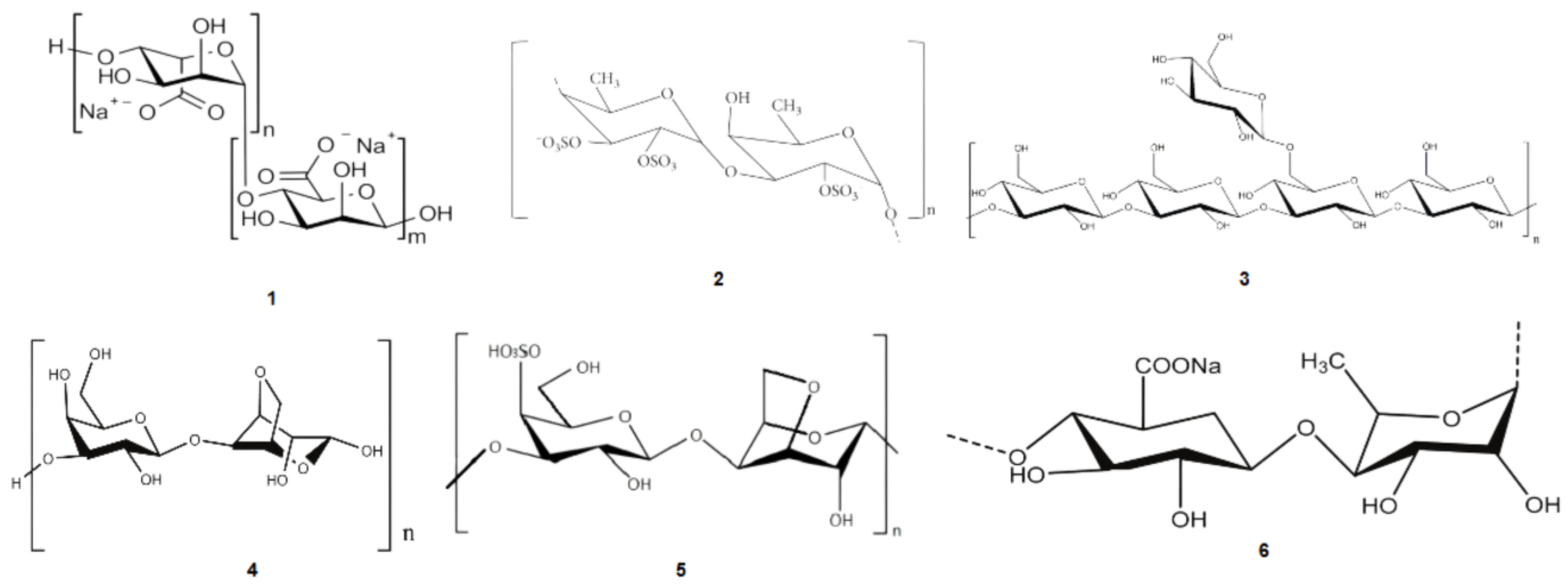
2.1. Polysaccharides
2.2. Polyunsaturated Fatty Acids (PUFAs)
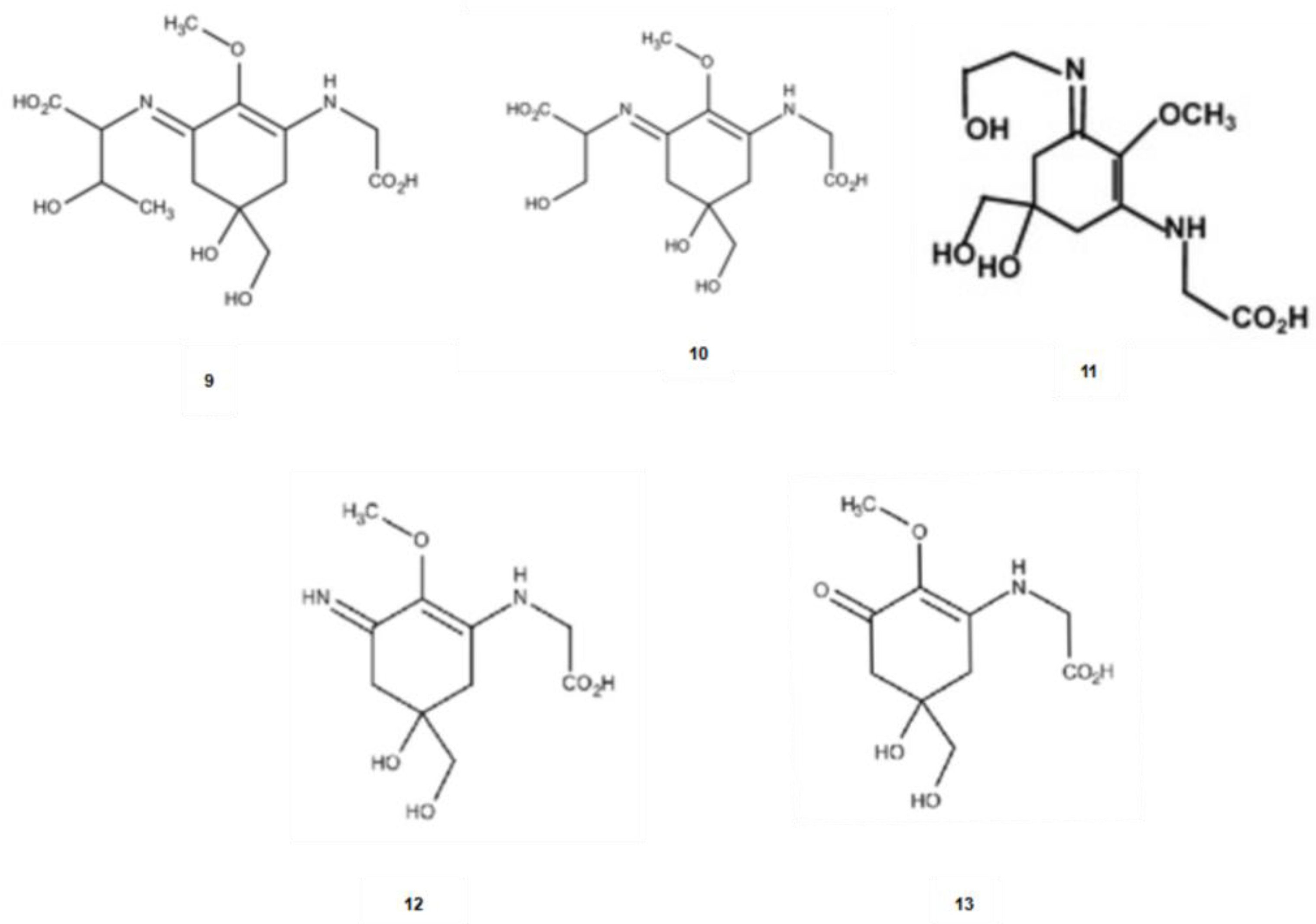
2.3. Mycosporine-like Amino Acids (MAAs)
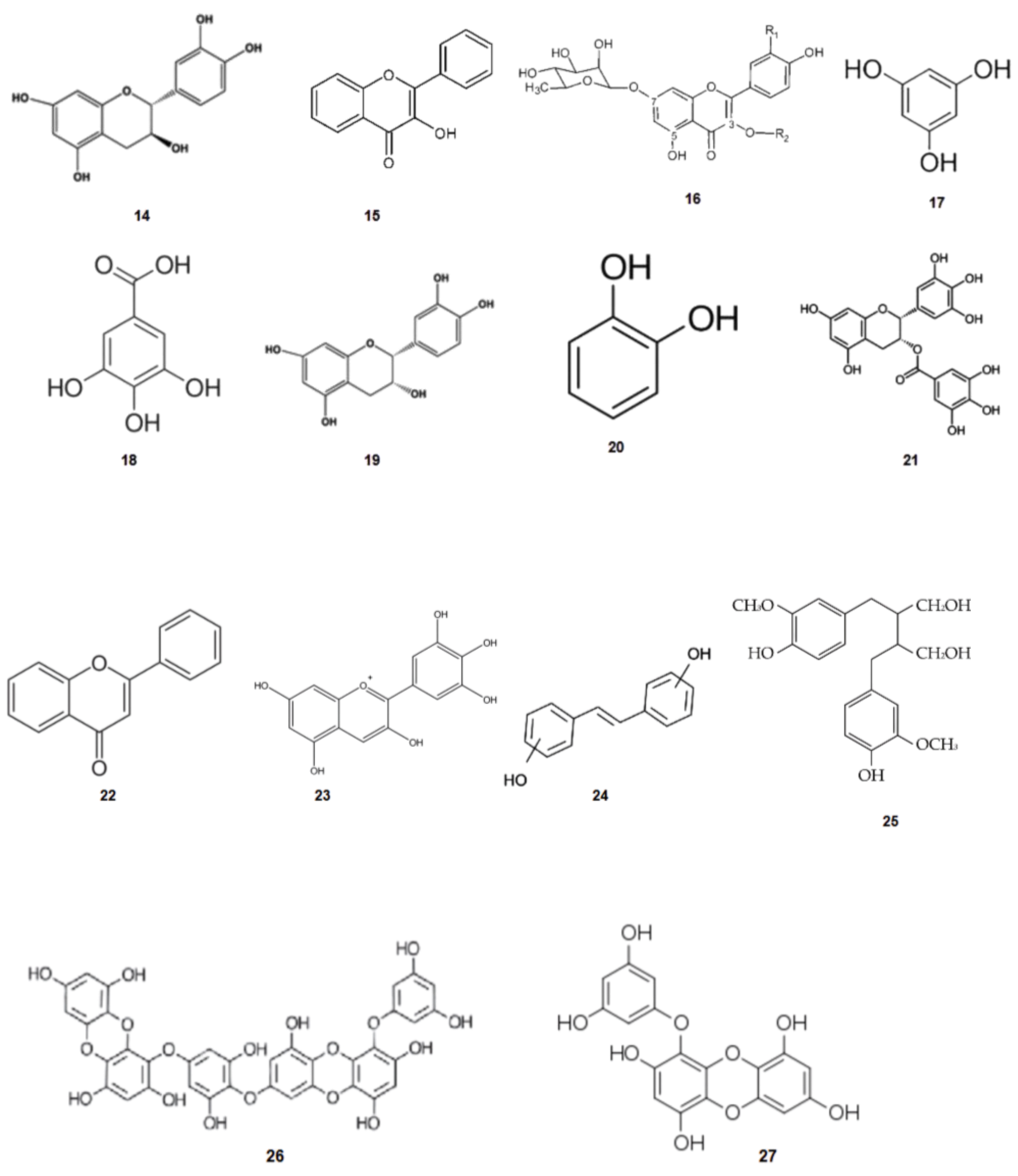
2.4. Phenolic Compounds
2.5. Pigments
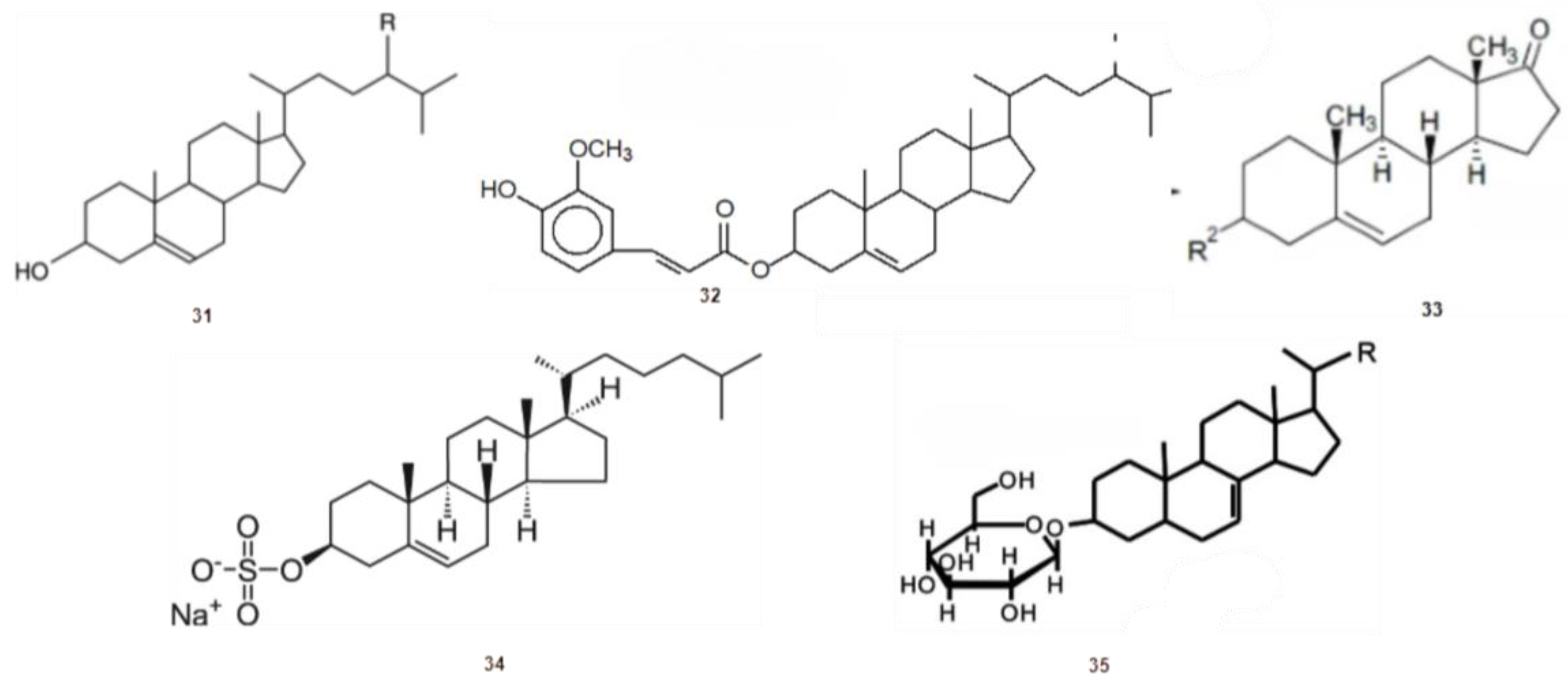
2.6. Sterols
3. The Application of Algae Extracts in Cosmetics
4. Production of Macroalgae Ingredients with Green Technology
4.1. Supercritical CO2 Extraction (CO2-SFE)
4.2. Pressurized Liquid Extracion (PLE)
4.3. Subcritical Water Extraction
4.4. Microwave-Assisted Extracition (MAE)
5. Safety of Algae in Cosmetics
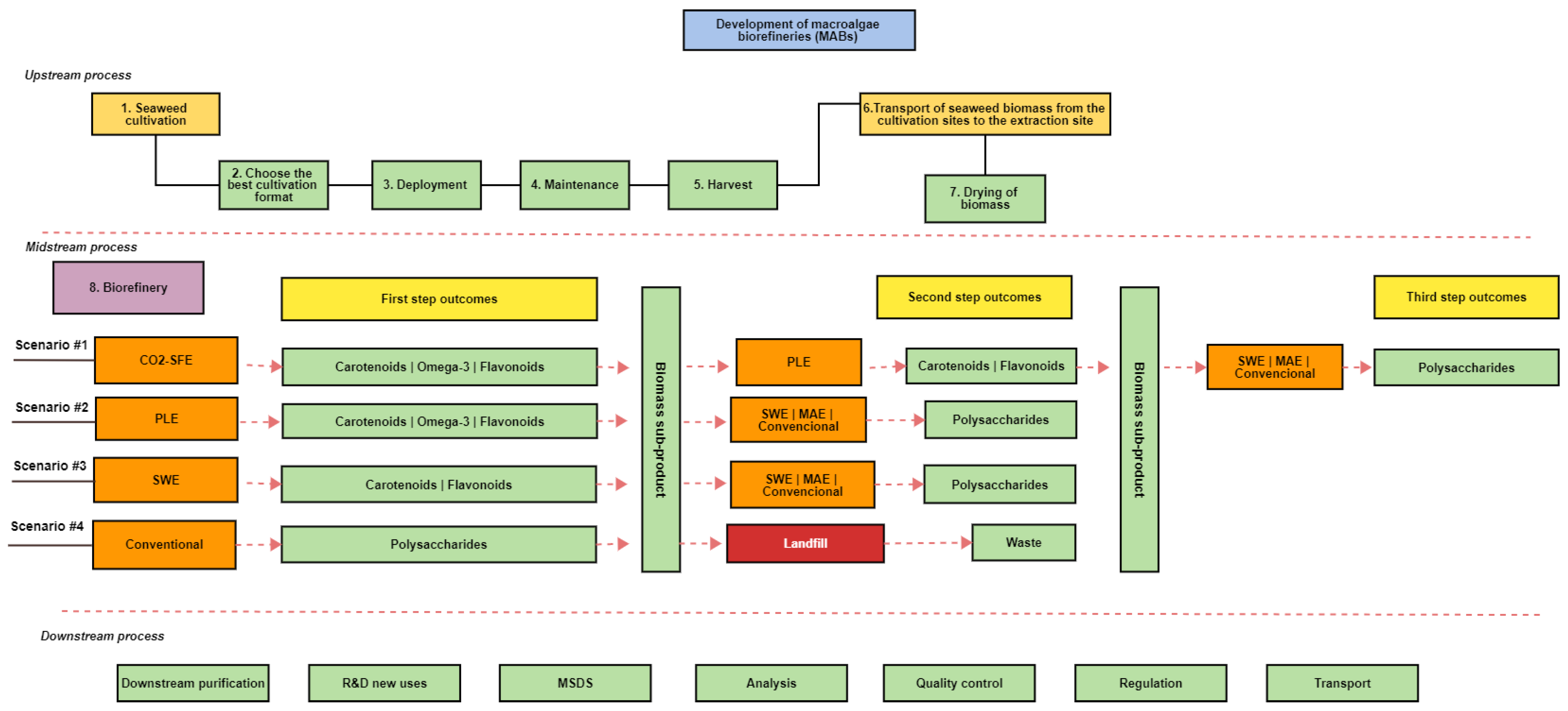
6. Industrial Perspectives in a Macroalgae Biorefinery Concept and Its Social and Environmental Impact
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Directorate-General for Research and Innovation (European Commission). A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment: Updated Bioeconomy Strategy; Publications Office Belgium: Brussels, Belgium, 2018. [Google Scholar]
- Executive Agency for Small and Medium-sized Enterprises. Blue Bioeconomy Forum: Highlights: Synthesis of the Roadmap and a Selection of Viable and Innovative Projects; Publications Office Luxembourg: Luxembourg, 2020. [Google Scholar]
- ISO 16128-2:2017; Cosmetics—Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients—Part 2: Criteria for Ingredients and Products. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/65197.html (accessed on 11 July 2022).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development (A/RES/70/1). 2015. Available online: https://sdgs.un.org/2030agenda (accessed on 15 July 2022).
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E. Sensory Testing Methods; Wolf, M.B., Ed.; ASTM: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Milstein, S.R.; Halper, A.R.; Katz, L.M. Regulatory Requirements for the Marketing of Cosmetics in the United States. In Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2006; pp. 862–889. [Google Scholar]
- Couteau, C.; Coiffard, L. Seaweed application in cosmetics. In Seaweed in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2016; pp. 423–441. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Available online: https://www.algaebase.org/ (accessed on 10 November 2022).
- Naga, S.; Neha, M.; Vitika, V. Seaweed Protein Market by Source (Red, Brown, Green), by Application (Food, Animal Feed and Additives, Cosmetics and Personal Care, Others): Global Opportunity Analysis and Industry Forecast, 2021–2030; Allied Market Research: Portland, OR, USA, 2022; p. 293. Available online: https://www.alliedmarketresearch.com/seaweed-protein-market-A16894 (accessed on 23 November 2022).
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Kok, J.M.L.; Jee, J.M.; Chew, L.Y.; Wong, C.L. The potential of the brown seaweed Sargassum polycystum against acne vulgaris. J. Appl. Phycol. 2016, 28, 3127–3133. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.J.; Ryu, B. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme: Short communication. Int. J. Biol. Macromol. 2020, 142, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Januário, A.P.; Félix, R.; Félix, C.; Reboleira, J.; Valentão, P.; Lemos, M.F.L. Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care. Pharmaceutics 2021, 13, 1930. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.-H. Seaweed-Based Molecules and Their Potential Biological Activities: An Eco-Sustainable Cosmetics. Molecules 2021, 26, 5313. [Google Scholar]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Pangestuti, R.; Shin, K.H.; Kim, S.K. Anti-Photoaging and Potential Skin Health Benefits of Seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef]
- Lee, M.K.; Ryu, H.; Lee, J.Y.; Jeong, H.H.; Baek, J.; Van, J.Y.; Kim, M.J.; Jung, W.K.; Lee, B. Potential Beneficial Effects of Sargassum spp. in Skin Aging. Mar. Drugs 2022, 20, 540. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. Int. J. Biol. Macromol. 2020, 163, 26–35. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A Treasure from the Sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Goncalves, L.; Petrovski, Z.; et al. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Sohag, A.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.B.; Zheng, C.Q.; Cheong, K.L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef]
- Gam, D.; Park, J.H.; Hong, J.W.; Jeon, S.J.; Kim, J.H.; Kim, J.W. Effects of Sargassum thunbergii Extract on Skin Whitening and Anti-Wrinkling through Inhibition of TRP-1 and MMPs. Molecules 2021, 26, 7381. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Isolated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef] [Green Version]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesumani, V.; Du, H.; Pei, P.B.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.; Iqbal, H.M.N. Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Mar. Drugs 2020, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.X.; Qin, L.; Guo, M.; Geng, J.J.; Dong, S.T.; Wang, K.; Xu, H.; Qu, C.F.; Miao, J.L.; Liu, M. A novel alginate from Sargassum seaweed promotes diabetic wound healing by regulating oxidative stress and angiogenesis. Carbohydr. Polym. 2022, 289, 119437. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Zelinsky, N.D. Chemical structures of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing Series in Food Science, Technology and Nutrition: Amsterdam, The Netherlands, 2013; pp. 23–86. [Google Scholar]
- Torabi, P.; Hamdami, N.; Keramat, J. Microwave-assisted extraction of sodium alginate from brown macroalgae Nizimuddinia zanardini, optimization and physicochemical properties. Sep. Sci. Technol. 2022, 57, 872–885. [Google Scholar] [CrossRef]
- Harwood, J.L. Algae: Critical Sources of Very Long-Chain Polyunsaturated Fatty Acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The Red Seaweed Gracilaria gracilis as a Multi Products Source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; Garcia-Risco, M.R. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Pathak, J.; Pandey, A.; Singh, V.; Ahmed, H.; Rajneesh; Kumar, D.; Sinha, R.P. Ultraviolet-screening compound mycosporine-like amino acids in cyanobacteria: Biosynthesis, functions, and applications. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 219–233. [Google Scholar]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Bedoux, G.; Pliego-Cortes, H.; Dufau, C.; Hardouin, K.; Boulho, R.; Freile-Pelegrin, Y.; Robledo, D.; Bourgougnon, N. Production and properties of mycosporine-like amino acids isolated from seaweeds. Seaweeds Around World State Art Perspect. 2020, 95, 213–245. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Sinha, R.P.; Singh, S.P.; Hader, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Rangel, K.C.; Villela, L.Z.; Pereira, K.D.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Assessment of the photoprotective potential and toxicity of Antarctic red macroalgae extracts from Curdiea racovitzae and Iridaea cordata for cosmetic use. Algal Res. Biomass Biofuels Bioprod. 2020, 50, 101984. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenco-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Polo, L.K.; Chow, F. Variation of antioxidant capacity and antiviral activity of the brown seaweed Sargassum filipendula (Fucales, Ochrophyta) under UV radiation treatments. Appl. Phycol. 2022, 3, 260–273. [Google Scholar]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Saul, R.C.; Saraí, C.H.; Karen, L.H.; Luis, A.C.C.; María, I.E.A.; Laura, E.G.; José de Jesús, O.P.; Marco, A.L.M. Flavonoids: Important Biocompounds in Food. In Flavonoids—From Biosynthesis to Human Health; INTECH: Eagle, Creek, 2017; pp. 353–369. [Google Scholar]
- Castejon, N.; Thorarinsdottir, K.A.; Einarsdottir, R.; Kristbergsson, K.; Marteinsdottir, G. Exploring the Potential of Icelandic Seaweeds Extracts Produced by Aqueous Pulsed Electric Fields-Assisted Extraction for Cosmetic Applications. Mar. Drugs 2021, 19, 662. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M.; et al. Antioxidant and Antiproliferative Activities of Heterofucans from the Seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [Green Version]
- Caferri, R.; Guardini, Z.; Bassi, R.; Dall’Osto, L. Assessing photoprotective functions of carotenoids in photosynthetic systems of plants and green algae. Methods Enzym. 2022, 674, 53–84. [Google Scholar] [CrossRef]
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2017, 56, 410–422. [Google Scholar] [CrossRef]
- Benveniste, P. Biosynthesis and accumulation of sterols. Annu. Rev. Plant Biol. 2004, 55, 429–457. [Google Scholar] [CrossRef]
- Brufau, G.; Canela, M.A.; Rafecas, M. Phytosterols: Physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 2008, 28, 217–225. [Google Scholar] [CrossRef]
- RIBEIRO, C. Cosmetologia Aplicada a Dermoestética 2a Edição; Pharmabooks: Sao Paulo, Brazil, 2010. [Google Scholar]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [PubMed]
- Suphasomboon, T.; Vassanadumrongdee, S. Toward sustainable consumption of green cosmetics and personal care products: The role of perceived value and ethical concern. Sustain. Prod. Consum. 2022, 33, 230–243. [Google Scholar] [CrossRef]
- Brooking, F. What Is the Clean Beauty Movement? Available online: https://pebblemag.com/magazine/living/clean-beauty-movement (accessed on 22 June 2022).
- Kolanjinathan, K.; Ganesh, P.; Saranraj, P. Pharmacological importance of seaweeds: A review. World J. Fish Mar. Sci. 2014, 6, 1–15. [Google Scholar]
- Charlier, R.H.; Chaineux, M.-C.P. The healing sea: A sustainable coastal ocean resource: Thalassotherapy. J. Coast. Res. 2009, 25, 838–856. [Google Scholar] [CrossRef]
- Fabrowska, J.; Kapuscinska, A.; Leska, B.; Feliksik-Skrobich, K.; Nowak, I. In vivo studies and stability study of Cladophora glomerata extract as a cosmetic active ingredient. Acta Pol. Pharm. 2017, 74, 633–641. [Google Scholar]
- Samarakoon, K.; Jeon, Y.J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 7, 212530. [Google Scholar] [CrossRef] [Green Version]
- Brummer, R.; Godersky, S. Rheological studies to objectify sensations occurring when cosmetic emulsions are applied to the skin. J. Coast. Res. 1999, 152, 89–94. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Resende, D.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine Ingredients for Sensitive Skin: Market Overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef]
- Groniger, A.; Sinha, R.P.; Klisch, M.; Hader, D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Photobiol. B-Biol. 2000, 58, 115–122. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Tyskiewicz, K.; Tyskiewicz, R.; Konkol, M.; Roj, E.; Jaroszuk-Scisel, J.; Skalicka-Wozniak, K. Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract Against Fusarium culmorum and Fusarium oxysporum. Molecules 2019, 24, 3518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, C.; Paiga, P.; Marques, M.; Neto, T.; Carvalho, A.P.; Paiva, A.; Simoes, P.; Costa, L.; Bernardo, A.; Fernandez, N.; et al. Multi-Step Subcritical Water Extracts of Fucus vesiculosus L. and Codium tomentosum Stackhouse: Composition, Health-Benefits and Safety. Processes 2021, 9, 893. [Google Scholar] [CrossRef]
- Falkenberg, M.; Nakano, E.; Zambotti-Villela, L.; Zatelli, G.A.; Philippus, A.C.; Imamura, K.B.; Velasquez, A.M.A.; Freitas, R.P.; Tallarico, L.D.; Colepicolo, P.; et al. Bioactive compounds against neglected diseases isolated from macroalgae: A review. J. Appl. Phycol. 2019, 31, 797–823. [Google Scholar] [CrossRef] [Green Version]
- Prado, J.M.; Veggi, P.C.; Meireles, M.A.A. Extraction Methods for Obtaining Carotenoids from Vegetables—Review. Curr. Anal. Chem. 2014, 10, 29–66. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Ktari, L.; Mdallel, C.; Aoun, B.; Ajjabi, L.C.; Sadok, S. Fucoxanthin and Phenolic Contents of Six Dictyotales From the Tunisian Coasts With an Emphasis for a Green Extraction Using a Supercritical CO2 Method. Front. Mar. Sci. 2021, 8, 647159. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical Carbon Dioxide Extraction of Fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef] [PubMed]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1,. Extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Rozo, G.; Rozo, C.; Puyana, M.; Ramos, F.A.; Almonacid, C.; Castro, H. Two compounds of the Colombian algae Hypnea musciformis prevent oxidative damage in human low density lipoproteins LDLs. J. Funct. Foods 2019, 60, 103399. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachewac, A.T.; Cho, Y.J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Fitzpatrick, L.J.; Zuloaga, O.; Etxebarria, Ν.; Dean, J.R. Environmental Applications of Pressurised Fluid Extraction. Rev. Anal. Chem. 2000, 19, 75–122. [Google Scholar] [CrossRef]
- Silva, E.K.; Martelli-Tosi, M.; Vardanega, R.; Nogueira, G.C.; Zabot, G.L.; Meireles, M.A.A. Technological characterization of biomass obtained from the turmeric and annatto processing by using green technologies. J. Clean. Prod. 2018, 189, 231–239. [Google Scholar] [CrossRef]
- Alcazar-Alay, S.C.; Osorio-Tobon, J.F.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining bixin from semi-defatted annatto seeds by a mechanical method and solvent extraction: Process integration and economic evaluation. Food Res. Int. 2017, 99, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Fayad, S.; Nehme, R.; Tannoury, M.; Lesellier, E.; Pichon, C.; Morin, P. Macroalga Padina pavonica water extracts obtained by pressurized liquid extraction and microwave-assisted extraction inhibit hyaluronidase activity as shown by capillary electrophoresis. J. Chromatogr. A 2017, 1497, 19–27. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid-liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Otero, P.; Lopez-Martinez, M.I.; Garcia-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef]
- Haraldsson, G. Separation of saturated/unsaturated fatty acids. J. Am. Oil Chem. Soc. 1984, 61, 219–222. [Google Scholar] [CrossRef]
- Dobrincic, A.; Pedisic, S.; Zoric, Z.; Jurin, M.; Roje, M.; Coz-Rakovac, R.; Dragovic-Uzelac, V. Microwave Assisted Extraction and Pressurized Liquid Extraction of Sulfated Polysaccharides from Fucus virsoides and Cystoseira barbata. Foods 2021, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Diop, C.I.K.; Beltran, S.; Jaime, I.; Sanz, M.T. Adjustable Gel Texture of Recovered Crude Agar Induced by Pressurized Hot Water Treatment of Gelidium sesquipedale Industry Waste Stream: An RSM Analysis. Foods 2022, 11, 2081. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.; Mariatti, F.; Cravotto, G. Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 128, 244–253. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Phycol. 2019, 31, 2517–2528. [Google Scholar] [CrossRef]
- Florez-Fernandez, N.; Lopez-Garcia, M.; Gonzalez-Munoz, M.J.; Vilarino, J.M.L.; Dominguez, H. Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- Zhang, X.; Thomsen, M. Techno-economic and environmental assessment of novel biorefinery designs for sequential extraction of high-value biomolecules from brown macroalgae Laminaria digitata, Fucus vesiculosus; Saccharina latissima. Algal Res. 2021, 60, 102499. [Google Scholar] [CrossRef]
- Beoletto, V.G.; De Las Mercedes Oliva, M.; Marioli, J.M.; Carezzano, M.E.; Demo, M.S. Antimicrobial Natural Products against Bacterial Biofilms. In Antibiotic Resistance; Elsevier: Amsterdam, The Netherlands, 2016; Volume 14, pp. 291–307. [Google Scholar]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Robledo, D.; Freile-Pelegrín, Y. Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta). J. Appl. Phycol. 2014, 26, 901–907. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Park, J.S.; Han, J.M.; Surendhiran, D.; Chun, B.S. Physicochemical and biofunctional properties of Sargassum thunbergii extracts obtained from subcritical water extraction and conventional solvent extraction. J. Supercrit. Fluids 2022, 182. [Google Scholar] [CrossRef]
- Jang, J.H.; So, B.R.; Yeo, H.J.; Kang, H.J.; Kim, M.J.; Lee, J.J.; Jung, S.K.; Jung, Y.H. Preparation of cellulose microfibril (CMF) from Gelidium amansii and feasibility of CMF as a cosmetic ingredient. Carbohydr. Polym. 2021, 257, 117569. [Google Scholar] [CrossRef]
- Toan, T.Q.; Phong, T.D.; Tien, D.D.; Linh, N.M.; Anh, N.T.M.; Minh, P.T.H.; Duy, L.X.; Nghi, D.H.; Thi, H.H.P.; Nhut, P.T.; et al. Optimization of Microwave-Assisted Extraction of Phlorotannin from Sargassum swartzii (Turn.) C. Ag. with Ethanol/Water. Nat. Prod. Commun. 2021, 16, 1934578x21996184. [Google Scholar] [CrossRef]
- Grillo, G.; Tabasso, S.; Solarino, R.; Cravotto, G.; Toson, C.; Ghedini, E.; Menegazzo, F.; Signoretto, M. From Seaweeds to Cosmeceutics: A Multidisciplinar Approach. Sustainability 2021, 13, 13443. [Google Scholar] [CrossRef]
- Monde, L. Les enfants du talc Morhange Les Victimes D’intoxication à L’hexachlorophène ont Souffert, Selon une Étude de l’OMS, de Retards Dans Leur Développement Intellectuel. Available online: https://www.lemonde.fr/archives/article/1991/02/27/les-enfants-du-talc-morhange-les-victimes-d-intoxication-a-l-hexachlorophene-ont-souffert-selon-une-etude-de-l-oms-de-retards-dans-leur-developpement-intellectuel_4159753_1819218.html (accessed on 13 July 2022).
- Eschner, K. Three Horrifying Pre-FDA Cosmetics. Available online: https://www.smithsonianmag.com/smart-news/three-horrifying-pre-fda-cosmetics-180963775/ (accessed on 2 February 2022).
- OECD. OECD Test Guidelines for Chemicals—OECD. Available online: https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788#:~:text=The%20OECD%20Guidelines%20for%20the,characterise%20potential%20hazards%20of%20chemicals. (accessed on 13 July 2022).
- Resende, D.I.S.P.; Ferreira, M.; Magalhaes, C.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. Biomass Biofuels Bioprod. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Dejoye Tanzi, C.; Abert Vian, M.; Ginies, C.; Elmaataoui, M.; Chemat, F. Terpenes as green solvents for extraction of oil from microalgae. Molecules 2012, 17, 8196–8205. [Google Scholar] [CrossRef] [Green Version]
- Tavares, R.S.N.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schafer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfuhler, S.; Pirow, R.; Downs, T.R.; Haase, A.; Hewitt, N.; Luch, A.; Merkel, M.; Petrick, C.; Said, A.; Schäfer-Korting, M.; et al. Validation of the 3D reconstructed human skin Comet assay, an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis 2021, 36, 19–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, M.A.D.; Resende, J.F.D.; Oliveira, S.R.; Fernandes, F.D.; Borburema, H.D.D.; Barbosa-Silva, M.S.; Ferreira, A.B.G.; Marinho-Soriano, E. Performance of the agarophyte Gracilariopsis tenuifrons in a multi-trophic aquaculture system with Litopenaeus vannamei using water recirculation. J. Appl. Phycol. 2021, 33, 481–490. [Google Scholar] [CrossRef]
- Kumar, B.R.; Mathimani, T.; Sudhakar, M.P.; Rajendran, K.; Nizami, A.S.; Brindhadevi, K.; Pugazhendhi, A. A state of the art review on the cultivation of algae for energy and other valuable products: Application, challenges, and opportunities. Renew. Sustain. Energy Rev. 2021, 138, 110649. [Google Scholar] [CrossRef]
- Seghetta, M.; Marchi, M.; Thomsena, M.; Bjerre, A.B.; Bastianoni, S. Modelling biogenic carbon flow in a macroalgal biorefinery system. Algal Res. Biomass Biofuels Bioprod. 2016, 18, 144–155. [Google Scholar] [CrossRef]
- Saral, J.S.; Ajmal, R.S.; Ranganathan, P. Bioeconomy of hydrocarbon biorefinery processes. In Hydrocarbon Biorefinery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 355–385. [Google Scholar]


| International Nomenclature of Cosmetic Ingredients (INCI) Name | Type (Color) | Ingredient Name | INCI Functions |
|---|---|---|---|
| Hypnea musciformis Extract | Rhodophyta | Algae hypnea contains alginic acid, mannitol, carrageenan, galactosides, amino acids, mineral salts, oligoelements, vitamins, and pigments | skin protection |
| Gelidiella acerosa Extract | Rhodophyta | Hypnea Musciformis Extract (and) Gelidiella Acerosa Extract (and) Sargassum Filipendula Extract (and) Sorbitol | skin protection |
| Sargassum filipendula Extract | Phaeophyceae | Hypnea Musciformis Extract (and) Gelidiella Acerosa Extract (and) Sargassum Filipendula Extract (and) Cellulose | skin protection |
| Gymnogongrus durvillei (formerly Ahnfeltiopsis concinna) Extract | Rhodophyta | Ahnfeltia Concinna Extract | viscosity control |
| Botryocladia occidentalis Extract | Rhodophyta | Water (and) Botryocladia Occidentalis Extract (and) Hypnea Musciformis Extract (and) Sargassum Vulgare Extract | skin conditioning |
| Chondrus crispus Extract | Rhodophyta | Chondrus Crispus Extract | film-forming, skin-smoothing, and moisturizing properties, viscosity control |
| Ascophyllum nodosum Extract | Phaeophyceae | Ascophyllum nodosum Extract | skin conditioning, may help protect against UVB ray |
| Laminaria ochroleuca Extract | Phaeophyceae | Caprylic/Capric Triglyceride (and) Laminaria ochroleuca Extract | skin conditioning |
| Kappaphycus alvarezii Extract (Telosomyl) | Rhodophyta | Water (and) Kappaphycus alvarezii Extract (and) Laminaria saccharina Extract (and) Hydrolyzed Rice Protein | skin conditioning |
| Laminaria digitata Extract (Horsetail Kelp Extract) | Phaeophyceae | Aqua (and) Propylene Glycol (and) Laminaria digitata Extract | skin protection |
| Neopyropia yezoensis (formerly Porphyra yezoensis) Extract | Rhodophyta | Water (and) Butylene Glycol (and) Porphyra yezoensis Extract | skin conditioning |
| Eisenia bicyclis Extract | Phaeophyceae | Aqua, Aloe Barbadensis, Cocos Nucifera Milk, Melaleuca Oil, Cucumis Sativus, Anthemis Nobilis, Hyaluronic Acid, Camellia Sinensis, Xanthan Gum, Arame algae Extract, Vitis Vinifera, Matricaria Chamomilla, C, Betaine, Lavender Extract, Citrus Sinensis, Camellia Sinensis, Mangifera Indica, Leuconostoc Radish Root Ferment Filtrate, Tetrasodium Glutamate Diacetate | skin conditioning, skin protection, anti-inflammatory effects, soothing effect |
| Ecklonia cava Extract | Phaeophyceae | Ecklonia cava Extract | skin conditioning agent |
| Eisenia arborea Extract | Phaeophyceae | Water, Butylene Glycol, Eisenia arborea Extract | skin conditioning agent |
| Gracilariopsis longissimaf (ormerly Gracilaria verrucosa) Extract | Rhodophyta | Gracilaria verrucosa Extract | humectant, skin protection, production of agar, potential antiseptic function for human skin |
| Porphyra umbilicalis Extract | Rhodophyta | Aqua (and) Lecithin (and) Alcohol (and) Sodium Lactate (and) Porphyra umbilicalis Extract (and) Phenoxyethanol | skin conditioning agent |
| Codium tomentosum Extract | Chlorophyta | Propylene Glycol (and) Aqua (and) Codium tomentosum Extract | skin protection |
| Fucus vesiculosus Extract (Bladderwrack) | Phaeophyceae | Fucus vesiculosus Extract | emollient, skin conditioning, smoothing, soothing |
| Pelvetia canaliculata Extract | Phaeophyceae | Aqua (and) Pelvetia canaliculata Extract | skin protection |
| Caulerpa racemosa Extract | Chlorophyta | Caulerpa racemosa Extract | skin conditioning |
| Furcellaria lumbricalis Extract | Rhodophyta | Furcellaria lumbricalis Extract and Perna canaliculus Extract | skin conditioning |
| Ulva lactuca Extract | Chlorophyta | Glycerin (and) Aqua (and) Hydrolyzed Ulva lactuca Extract | skin conditioning, skin protection |
| Saccharina japonica Extract | Phaeophyceae | Saccharina japonica Extract | skin conditioning |
| Cladosiphon okamuranus Extract | Phaeophyceae | Cladosiphon okamuranus Extract | skin conditioning |
| Sargassum vulgare Extract | Phaeophyceae | Water (and) Botryocladia occidentalis Extract (and) Hypnea musciformis Extract (and) Sargassum vulgare Extract | skin conditioning |
| Polysaccharide (Phycocolloid) | Patent Number | Priority Date | Brief Description of Polysaccharide Preparation Methods |
|---|---|---|---|
| Non-specific | WO2021133148 | 27 December 2019 | The present invention provides a method for separating polysaccharides from seaweed. The method for obtaining polysaccharides comprises a pre-treatment step that ages seaweed at 20–50 °C before extraction with a solvent to remove salts and color materials; an extraction step with hot water that removes the seaweed from which salts and color materials have been removed; a separation step where seaweed is categorized according to size; and a purification step at 40–60 °C. |
| Agar and Agarose | WO2010109289 | 24 March 2009 | The present invention relates to a more convenient and energy-efficient process for the preparation of agarose from Gracilaria and Gelidiella spp. (Rhodophyta), more particularly Gracilaria dura and Gelidella acerosa (Rhodophyta) from Indian waters. Said process comprises steps where the dry seaweed is pre-treated with alkali and then rinsed until a pH ranging between 7 and 9 is shown, with water then added before subsequent autoclaving to obtain the extract, which is then centrifuged before being treated with surface-active chemicals to induce the precipitation of agarose. This is followed by centrifugation of the mass to remove the adhering liquid, which is then rinsed with water to remove the surface-active chemical. A hot sol of the agarose mass is then prepared in a minimum quantity of water before the agarose is then re-precipitated with iso-propanol to achieve a gel product with dispersibility. The described gel showed performance equal to that of gel obtained through a more conventional process of agarose preparation in DNA gel electrophoresis studies involving the same seaweed extractives, as well as performance akin to that of gel prepared from a commercial benchmark. |
| Agar and Carrageenan | WO2015102021 | 30 December 2013 | The present invention provides an integrated process for the recovery of a spectrum of commercially valuable products (including agar, cellulose, lipids, pigments, and liquid rich in minerals of agricultural importance) directly from fresh seaweed without employing any catalyst-driven in situ chemical conversions. Additionally, the solvents used during lipid extraction were capable of being used for three cycles without the yield and quality of successive products being affected. Furthermore, this new process is highly efficient and utilizes the total amount of raw seaweed material without any biomass leftover as solid waste. |
| Carragenan | WO2012123422 | 11 March 2011 | The present invention relates to a method for processing fresh seaweed. This method relates to the use of the processed seaweed components in the food sector, in the pharmaceutical sector, as food supplements, in cosmetics, and also in animal husbandry as feedstuff, as well as to the products made using such a method. |
| Alginates | WO2022139115 | 22 December 2020 | The present invention is designed to increase production efficiency and decrease the production costs of alginic acid and fucoidan. The method comprises the following steps: separating a mixture including ground sea algae, water, and an organic acid into a primary solid and liquid extraction; adding calcium chloride to the separate liquid to aggregate a secondary solid; separating the liquid containing the secondary solid aggregated therein into a secondary solid and residual liquid; extracting fucoidan from the residual liquid; and extracting alginic acid from the primary and secondary solid. |
| WO2021090023 | 7 November 2019 | The present invention relates to a method of processing macroalgae in which superheated solvent (water or alcohol [methanol, ethanol, or propanol] or water/alcohol mixtures) is used in an initial pre-treatment step (temperature range: 101 °C to 150 °C; pressure range: 105 kPa to 500 kPa). Polysaccharide extraction is carried out for up to 24 h in an alkali solution (sodium hydroxide, potassium hydroxide, calcium hydroxide, magnesium hydroxide, or sodium carbonate). To precipitate alginate from the previous solution, the alginate composition can be contacted with acid, calcium salt, or an anti-solvent. |
| Technology | Summary of the Technique | Ref |
|---|---|---|
| Supercritical CO2 Extraction (CO2-SFE) |
| [72] |
| Pressurized Liquid Extraction (PLE) |
| [73] |
| Subcritical Water Extraction (SWE) |
| [74] |
| Microwave-Assisted Extraction (MAE) |
| [75,76,77] |
| Technology | Species of Seaweed, Color | Highlights | Reference |
|---|---|---|---|
| Supercritical CO2 Extraction (CO2-SFE) | Dictyopteris polypodioides, brown | The extraction with CO2 at 60 °C resulted in higher global extraction yield and fucoxanthin content than lower temperatures but was not affected by the tested pressures. Total phenolic content and DPPH antiradical scavenging capacity results showed the same behavior. | [78] |
| Gracilaria mammillaris, red | The extraction operated with CO2 + ethanol (as a co-solvent). The co-solvent concentration and pressure positively correlated with global extraction yield and the concentration of total phenolics extracted, while pressure was the most relevant factor for total carotenoid (TC) content. The extracts reduced the lipid oxidation of the tested edible oil, although they were not as effective as the reference antioxidants. | [80] | |
| Hypnea musciformis, red | Different conditions of extraction with CO2 resulted in extracts with antioxidant activities, and the proportion of ethanol (co-solvent) directly correlated with the increase in antioxidant activity (ABTS) and protection against lipid peroxidation. Phloretin and (-)-epicatechin were the phenolics found in the study. | [81] | |
| Saccharina japonica, brown | The use of CO2 + sunflower oil (as co-solvent) favored the extraction of total carotenoids and fucoxanthin, the yield of fatty acids, high antioxidant activity, and high oil stability; CO2 + water (as co-solvent) was more efficient for the extraction of phlorotannins. | [82] | |
| Undaria pinnatifida, brown | The use of CO2 extraction recovered fucoxanthin from seaweeds discharged into the environment due to non-adherence to food quality standards. In subcritical conditions (400 bar, 25 °C), the extraction was more selective, while supercritical conditions favored high fucoxanthin recovery. | [79] | |
| Pressurized Liquid Extraction (PLE) | Codium fragile, green | The extraction with ethanol:water (80:20) at 100 °C and 68.9 bar was more selective in the recovery of TP than other solvents tested with PLE, despite having lower extraction yield than the protocol with water at 100 °C and 103 bar. Additionally, the extracts had higher antioxidant activity. However, most PLE protocols had lower performance than solid–liquid extraction (SLE). | [88] |
| Gongolaria barbata, brown | Pre-treatment of the raw material included seaweed maceration with (1st step) acetone (18 h, at room temperature) and (2nd step) ethanol (4 h, at 70 °C). Distilled water and H2SO4 were the solvents tested at a constant pressure under different temperatures to extract polysaccharides (PS). The optimized protocol reduced the extraction time to 30 min (3 h for conventional extraction) and resulted in similar PS extraction yield. Furthermore, PS presented higher sulfate group proportions and lower uronic acid content than PS from conventional extraction, although with lower antioxidant activity. | [91] | |
| Fucus virsoides, brown | Pre-treatment of the raw material included seaweed maceration with (1st step) acetone (18 h, at room temperature) and (2nd step) ethanol (4 h, at 70 °C). Distilled water and H2SO4 were the solvents tested at a constant pressure under different temperatures to extract PS. The optimized protocol reduced the extraction time to 30 min (3 h for conventional extraction) with a higher PS extraction yield. Likewise, the PS had higher sulfate group proportions and fucose content alongside lower uronic acid content and antioxidant activity than PS from conventional extraction. | [91] | |
| Gelidium corneum, red | In an upcycling approach (algae from a waste stream of a primary industrial phycocolloid extraction), agar extraction was possible with water at 100 °C and 10.13 bar. It showed that the different temperatures, pressures, algae-to-water ratios, and extraction times tested affected gel strength, hysteresis, hardness, cohesiveness, gumminess, adhesiveness, and springiness. | [92] | |
| Gracilaria gracilis, red | Extraction with water at 120 °C and 103 bar was the protocol with the highest extraction yield. Statistically, there was no difference among the protocols tested for PLE regarding TP content, DPPH scavenging activity, and antioxidant activity. The extraction yield of PLE with water was similar to most SLE protocols, though the extract presented lower TP recovery, DPPH scavenging activity, and antioxidant activity. | [88] | |
| Laminaria ochroleuca, brown | The use of ethanol at 120 °C and 100 bar was the optimized protocol that favored the extraction of unsaturated fatty acids (USFAs), while ethanol:water (2:1) favored the extraction of saturated fatty acids (SFAs). However, the absolute content of USFAs was ~2.26-fold superior with ethanol:water because of the higher global extraction yield (~3-fold) than ethanol. In addition, ethanol:water was more efficient at recovering TP content. | [89] | |
| Padina pavonica, brown | The optimum extraction condition was two extraction cycles with water at 60 °C and 150 bar, in which the extract inhibited 100% of hyaluronidase activity. | [86] | |
| Subcritical Water Extraction (SWE) | Caulerpa racemosa, green | Processes tested at different temperatures (110 to 230 °C with 40 °C increments) and 50 to 70 bar yielded up to ~60% (dw basis) acidic hydrolysate. Regarding global yield, 190 °C was the optimal temperature. At 230 °C, the extract had stronger ultraviolet B (UVB) absorption, higher total protein content, higher TP content, higher total flavonoid (TF) content, higher total saponin (TS) content, and greater antioxidant activity. | [18] |
| Codium tomentosum, green | The multi-step isobaric process (100 bar, temperature range of 20 to 250 °C) yields up to 51.4% cumulative extract. The increase in temperature augmented TP and TF content, but phlorotannin content was higher at low temperatures. It is noteworthy that free amino groups positively correlate to higher temperatures and that reducing sugar content increased compared to the raw material, indicating the presence of hydrolysis. | [70] | |
| Hypnea musciformis, red | Hydrolysis efficiency ranged from 61.37 to 81.23% at the different solvent-to-feed (S/F) ratios (50:1, 100:1, and 150:1) and temperatures (120 to 270 °C with 30 °C increments) that yielded acidic extracts. Regarding global yield, the tested temperatures over 210 °C showed higher hydrolysis efficiencies and were not affected by the S/F ratio tested. The combination of 210 °C and an S/F ratio of 50:1 was the optimal condition for obtaining high total protein, high TP, and high TF content, which positively correlated with the evaluated antioxidant activity. | [95] | |
| Saccharina japonica, brown | The input raw material for the tests was a CO2 supercritical de-oiled seaweed, and SWE was an upstream step for alginate and fucoidan extraction. The procedures ran at different temperatures (100, 125, and 150 °C), pressures (10, 30, and 50 bar), deep eutectic solvent mixtures with water (30, 40, and 50%), and S/F ratios (30:1, 40:1, and 50:1). According to the statistical model, the optimal parameter combination was 150 °C, 19.85 bar, 30% choline chloride:glycerol (1:2) in water, and an S/F ratio of 36.81:1, which yielded 28.12% and 14.93% functional alginate and fucoidan, respectively. However, they presented lower antioxidant activity when compared to commercial standards. | [101] | |
| Sargassum thunbergii, brown | The processes tested at different temperatures (120 to 240 °C with 30 °C increments) at an isobaric pressure of 30 bar and with an S/F ratio of 20:1 reached up to ~80% extraction efficiency (EE). A temperature of 180 °C presented relevant EE (70.33%), and the maximum values observed for total phlorotannins positively correlated with the antioxidant activity tested. Additionally, some phenolic compound concentrations were evaluated and ranked as follows: pyrogallol > p-coumaric acid > chlorogenic acid = protocatechuic acid > gallic acid > syringic acid. | [102] | |
| Ulva lactuca, green | Processes tested at different temperatures (110 to 230 °C with 40 °C increments) and at 50 to 70 bar yielded up to ~45% (dw basis) acidic hydrolysate. Regarding global yield, 190 °C was the optimal temperature. At 230 °C, the extract had higher total protein, higher TP, higher TF, and higher TS content and greater antioxidant activity. UVB absorption was strong and similar for extracts obtained at 190 and 230 °C. | [18] | |
| Microwave-Assisted Extraction (MAE) | Gongolaria barbata, brown | Pre-treatment of the raw material included seaweed maceration with (1st step) acetone (18 h, at room temperature) and (2nd step) ethanol (4 h, at 70 °C). Distilled water, 0.1 M HCl, and 0.1 M H2SO4 were the solvents tested (S/F 30:1) at different temperatures (60, 80, and 100 °C) and with different extractions times (10, 20, and 30 min) for the extraction of polysaccharides (PS). The optimized protocol (0.1 M H2SO4 at 80 °C) reduced extraction time to 10 min [3 h for conventional extraction (CE)] and yielded ~15% PS. The PS had a higher sulfate group proportion and lower uronic acid content than PS extracted with CE. In addition, the extract had higher antioxidant capacity, though radical scavenging capacity was lower than that of CE. | [91] |
| Fucus virsoides, brown | Pre-treatment of the raw material included seaweed maceration with (1st step) acetone (18 h, at room temperature) and (2nd step) ethanol (4 h, at 70 °C). Distilled water, 0.1 M HCl, and 0.1 M H2SO4 were the solvents tested (S/F 30:1) at different temperatures (60, 80, and 100 °C) and with different extractions times (10, 20, and 30 min.) for the extraction of polysaccharides (PS). The optimized protocol (0.1 M H2SO4 at 80 °C) reduced extraction time to 10 min [3 h for conventional extraction (CE)] and yielded ~20% PS. The PS had a higher sulfate group proportion and lower uronic acid content than PS extracted with CE. In addition, the extract had higher antioxidant capacity and a similar radical scavenging capacity to CE. | [91] | |
| Gelidium amansii, red | MAE was the first step in producing cellulose microfibrils. Distilled water, 1% NaOH, and 1% H2SO4 were the solvents tested (S/F 10:1) at different temperatures (100 to 180 °C) in the exploration of conditions that favor cellulose extraction over agar. The optimized protocol was the use of distilled water for 10 min at 180 °C. The derived cellulose microfibrils exhibited anti-inflammatory activity. Additionally, this material might be employed as an active functional nanomaterial for cosmetics. | [103] | |
| Padina pavonica, brown | The optimum extraction condition was the use of water at 60 °C and with 1000 watts, in which the extract inhibited 100% of hyaluronidase activity. | [86] | |
| Sargassum swartzii, brown | The combination of different ethanol concentrations (20 to 96% with 20 °C increments), S/F ratios (15:1 to 40:1 with 5:1 increments), microwave power (80 to 720 W with 160 W increments), and extractions times (15 to 90 min with 15 min increments) yielded phlorotannin extracts with antioxidant activity. The optimized protocol determined by a statistical model had the following parameters: 65 min, ethanol 52%, microwave power 613 W, and S/F ratio 33:1. | ||
| Ulva lactuca, green | Extraction with 70% ethanol at 90 °C (1500 W) with an S/F ratio of 15:1 for 30 min resulted in a yield of ~22% of an extract with significant TP content and antioxidant activity. The dermo-cosmetic preparations (a gel and an emulsion) made with the extract were thermally and mechanically stable. Additionally, these formulations were efficient in the active skin permeation and cutaneous retention test. | [104] | |
| Ulva australis (formerly Ulva pertusa), green | Pre-treatment of the raw material consisted of seaweed maceration (S/F 4:1) with 80% ethanol (2 h, at 85 °C) for pigment removal, followed by centrifugation and drying. Distilled water was the solvent used for the tests with different extraction times (30, 45, and 60 min), power (500, 600, and 700 W), S/F ratios (40:1, 55:1, and 70:1), and pH levels (5, 6, and 7), which yielded up to ~41% of an ulvan extract that showed antioxidant activity. Additionally, it upregulated the expression and enhanced the activity of the antioxidant enzymes superoxide dismutase and catalase. The optimized protocol parameters were the following: distilled water, ~44 min, 600 W, S/F ratio of 55.45, and a pH of 6.57. | [105] |
| Definition of International Bodies of Standardization |
|---|
| About OECD “The Organization for Economic Co-operation and Development is an international organization that works to build better policy together with governments and citizens, thus setting evidence-based international standards and finding solutions to a range of social, economic, and environmental challenges.” |
| About ISO “International Organization for Standardization is an independent, non-governmental international organization with a membership of 167 national standards bodies. Through its members, it brings together experts to share knowledge and develop voluntary, consensus-based, market relevant International Standards that support innovation and provide solutions to global challenges.” |
| About SCCS “The Scientific Committee on Consumer Safetyprovides Opinions on health and safety risks (chemical, biological, mechanical and other physical risks) of non-food consumer products (e.g., cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products) and services (e.g., tattooing, artificial sun tanning).” |
| Guide | Briefing Description |
|---|---|
| ISO 10993-5 (Test for in vitro cytotoxicity) | It describes methods for assessing cytotoxicity in vitro and specifies the incubation of cultured cells in contact with a device and/or extracts from a device directly or by diffusion. These methods are designed to determine the biological response of mammalian cells in vitro using appropriate biological parameters. |
| OECD 432 (Test of photocytotoxicity) | It describes an in vitro method for the evaluation of photocytotoxicity via relative reductions in the viability of cells exposed to the chemical in the presence versus absence of light. |
| OECD 439 and ISO 10993-10 (Skin irritation test) | They describe in vitro procedures that can be used for hazard identification in irritating chemicals (substances and mixtures) following Category 2 of the UN Globally Harmonized System of Classification and Labeling of Chemicals (GHS). The methods are based on the reconstructed human epidermis (RhE), which in its overall design closely mimics the biochemical and physiological properties of the upper parts of human skin and offers key factors for the interpretation of results. |
| OECD 431 (Test of cutaneous corrosivity) | This method allows for the identification of corrosive chemical substances and mixtures, as well as the identification of non-corrosive substances and mixtures when supported by a weight of evidence determination using other existing information. The test protocol can also indicate the distinction between severe and less severe skin corrosives. This test guideline does not require the use of live animals or animal tissue for the assessment of skin corrosivity. |
| OECD 491 (Test of eye irritation/corrosivity) | It describes an in vitro cytotoxicity-based assay that is performed on a confluent monolayer of Statens Seruminstitut Rabbit Cornea (SIRC) cells cultured on a 96-well polycarbonate microplate. |
| OECD 428 (Test of skin permeation/absorption) | This method provides information on the absorption of a test substance (preferably radiolabelled) applied to the surface of a skin sample that separates the two chambers (a donor chamber and a recipient chamber) of a diffusion cell. Static and flow diffusion cells are both acceptable. Human or animal skin can be used. |
| OECD 129 (Test for prediction of oral toxicity) | This method is an in vitro alternative to animal testing that estimates starting doses for oral systemic toxicity tests. |
| OECD 442E (Test of skin sensitization) | The present key event-based test guideline (TG) addresses the human health hazard endpoint of skin sensitization following exposure to a test chemical. Skin sensitization refers to an allergic response following skin contact with the tested chemical, as defined by the United Nations Globally Harmonized System of Classification and Labelling of Chemicals (UN GHS). |
| OECD 487 (Test of genotoxicity) | The assay detects the activity of clastogenic and aneugenic test substances in cells that have undergone cell division during or after exposure to the test substance. It is an in vitro micronucleus test for the detection of micronuclei in the cytoplasm of interphase cells. |
| SDGs | MABs Contribution |
|---|---|
| SDG 1: No Poverty: “End poverty in all its forms everywhere.” | MABs contribution: Responsible harvesting and cultivation of macroalgae may contribute to incomes > USD 1.25 per capita per day. |
| SDG 2: Zero Hunger: “End hunger, achieve food security and improved nutrition and promote sustainable agriculture.” | MABs contribution: Indirect enhancement to productivity in macroalgae farming. An MAB transforms 100% of biomass into products, making less biomass necessary. Additionally, the price of raw materials increases in a Fairtrade business model, expanding farmers’ incomes. |
| SDG 3: Good Health and Well-Being: “Ensure healthy lives and promote well-being for all at all ages.” | MABs contribution: Macroalgae are a source of bioactive compounds against neglected tropical diseases. |
| SDG 4: Quality Education: “Ensure inclusive and equitable quality education and promote lifelong learning opportunities for all.” | MABs contribution: From macroalgae harvesting or cultivation until extraction with green technologies, MABs need qualified technical professionals. In a couple of years, women and men can develop the skills required for a new sustainable industry. |
| SDG 5: Gender Equality: “Achieve gender equality and empower all women and girls.” | MABs contribution: All businesses aware to stakeholder interests must implement corporate governance, in which a guideline addresses different day-to-day aspects in a company, including gender equality practices. |
| SDG 6: Clear Water and Sanitation: “Ensure availability and sustainable management of water and sanitation for all.” | MABs contribution: In an IMTA alongside macroalgae, the reduction in eutrophication is proven. |
| SDG 7: Afford Clean Energy: “Ensure access to affordable, reliable, sustainable and modern energy for all.” | Macroalgae contribution: Macroalgae can remediate polluted areas, e.g., areas contaminated with heavy metals. This biomass cannot be a raw material for an MAB but is useful for energy production. |
| SDG 8: Decent Work and Economic Growth: “Promote sustained, inclusive and sustainable economic growth, full and productive employment and decent work for all.” | MABs contribution: Green technologies have high productivity and add value to commodities locally. This favors the development of local technological industries and mitigates the carbon footprint resulting from overseas/road transportation of raw material commodities. |
| SDG 9: Industry, Innovation, and Infrastructure: “Build resilient infrastructure, promote inclusive and sustainable industrialization and foster innovation.” | MABs contribution: MABs can be highly technological, lean, and small-scale while also having the capability to increase the local gross domestic product. |
| SDG 10: Reduce Inequalities: “Reduce inequality within and among countries.” | MABs contribution: Increase in income per capita through a Fairtrade relationship. Local MABs may foster the replication of business models such as that of the Association of Seaweed Farming in northeast Brazil, in which women are the key players in the sustainable harvesting and cultivation of macroalgae and the sale of goods made of macroalgae. |
| SDG 11: Sustainable Cities and Communities: “Make cities and human settlements inclusive, safe, resilient and sustainable.” | MABs contribution: As a cultural and natural heritage, macroalgae harvesting traces its origins to ancient fishing traditions. Local MABs must allocate part of their profits to recover and preserve natural macroalgae beds as a long-term strategy for raw material supply. |
| SDG 12: Responsible Consumption and Production: “Ensure sustainable consumption and production patterns.” | MABs contribution: The supply chain is sustainable. When macroalgae origin is an IMTA, MABs promote lower water footprint and reduce eutrophication issues related to other commercial goods. |
| SDG 13: Climate Action: “Take urgent action to combat climate change and its impacts.” | MABs contribution: In a circular regenerative economy, MABs are pivotal in reducing the carbon footprint. |
| SDG 14: Life Below Water: “Conserve and sustainably use the oceans, seas and marine resources for sustainable development.” | MABs contribution: MABs reduce eutrophication and water and carbon footprints. With the implementation of a sustainable harvesting strategy, macroalgae provide shelter and food for ocean-living organisms. Aside from the MAB, macroalgae bioremediates polluted areas, and these biomasses can serve as raw material for energy production. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hempel, M.d.S.S.; Colepicolo, P.; Zambotti-Villela, L. Macroalgae Biorefinery for the Cosmetic Industry: Basic Concept, Green Technology, and Safety Guidelines. Phycology 2023, 3, 211-241. https://doi.org/10.3390/phycology3010014
Hempel MdSS, Colepicolo P, Zambotti-Villela L. Macroalgae Biorefinery for the Cosmetic Industry: Basic Concept, Green Technology, and Safety Guidelines. Phycology. 2023; 3(1):211-241. https://doi.org/10.3390/phycology3010014
Chicago/Turabian StyleHempel, Mariana de Sousa Santos, Pio Colepicolo, and Leonardo Zambotti-Villela. 2023. "Macroalgae Biorefinery for the Cosmetic Industry: Basic Concept, Green Technology, and Safety Guidelines" Phycology 3, no. 1: 211-241. https://doi.org/10.3390/phycology3010014
APA StyleHempel, M. d. S. S., Colepicolo, P., & Zambotti-Villela, L. (2023). Macroalgae Biorefinery for the Cosmetic Industry: Basic Concept, Green Technology, and Safety Guidelines. Phycology, 3(1), 211-241. https://doi.org/10.3390/phycology3010014








