A Chemical Investigation of the Antioxidant Capacity of Extracts from Red Macroalga Gracilaria domingensis
Abstract
:1. Introduction
2. Material and Methods
2.1. Algal Samples
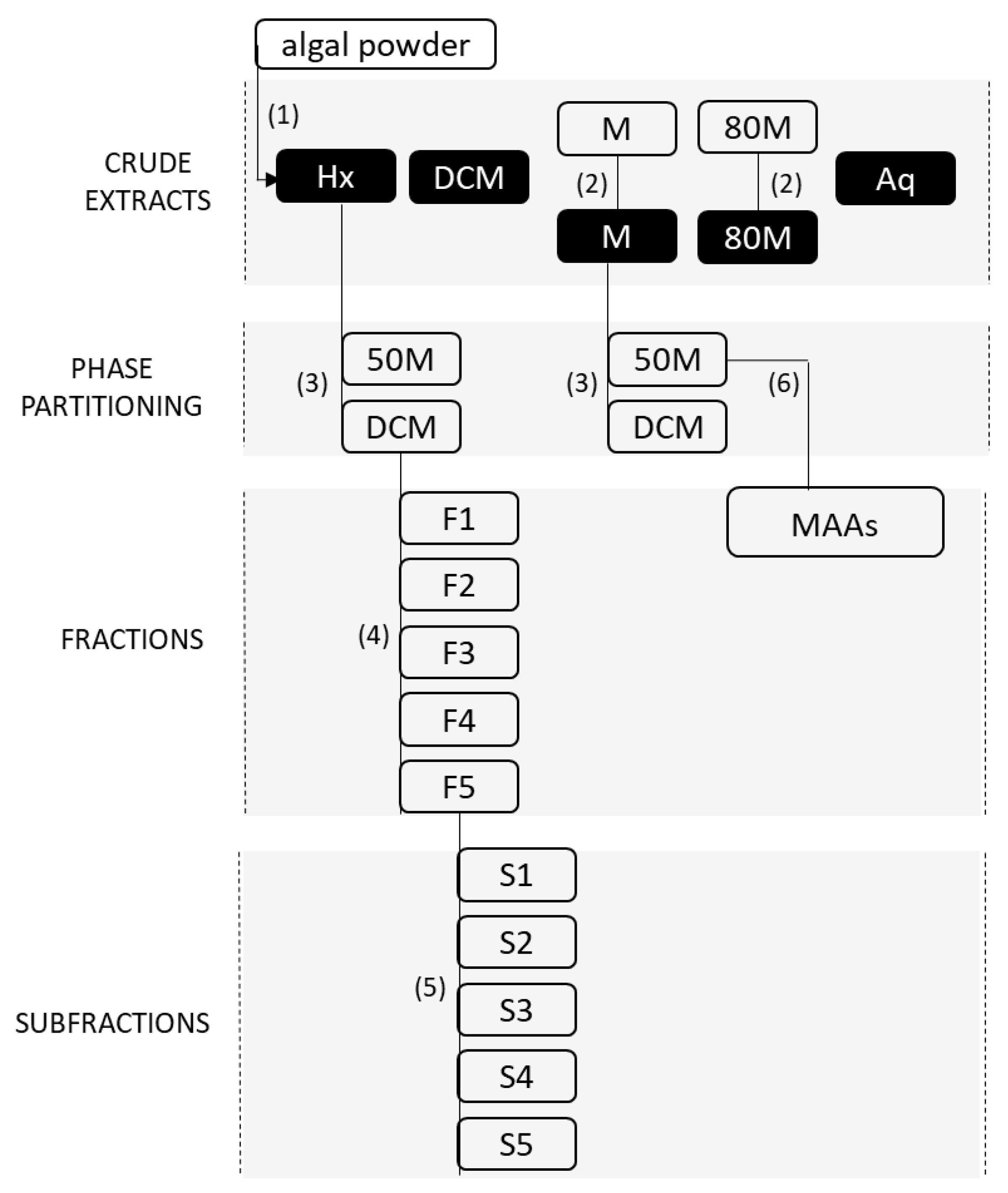
2.2. Preparation of the Crude Extracts and Fractionation of the Active Extracts
2.3. Determination of the Antioxidant Capacity
2.4. Ferric Reduction Antioxidant Power (FRAP) Assay
2.5. Metal Chelating Ability Based on the Measurement of Iron-Ferrozine Complex
2.6. Lipid Peroxidation Inhibition Using the β-Carotene-Linoleic Acid Assay
2.7. Folin–Ciocalteu (FC) Assay
2.8. Interaction Indexes
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, P.; Chaudhary, A.; Singh, R.D.; Gopichand; Prasad, R.; Singh, B. Spatial and temporal variation of secondary metabolite profiles in Ginkgo biloba leaves. Chem. Biodivers. 2012, 9, 409–417. [Google Scholar] [CrossRef]
- Urrea-Victoria, V.; Furlan, C.M.; dos Santos, D.Y.A.C.; Chow, F. Antioxidant potential of two Brazilian seaweeds in response to temperature: Pyropia spiralis (red alga) and Sargassum stenophyllum (brown alga). J. Exp. Mar. Biol. Ecol. 2022, 549, 151706. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlu, K.; çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlu, K.; çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 3, 152–178. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Dettweiler, M.; Marquez, L.; Bao, M.; Quave, C.L. Quantifying synergy in the bioassay-guided fractionation of natural product extracts. PLoS ONE 2020, 15, 0235723. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan peninsula, Mexico. J. Appl. Phycol. 2007, 19, 449–458. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Effects of UV radiation on photosynthesis, antioxidant capacity and the accumulation of bioactive compounds in Gracilariopsis longissima, Hydropuntia Cornea and Halopithys Incurva (Rhodophyta). J. Phycol. 2019, 55, 1258–1273. [Google Scholar] [CrossRef]
- Santos, J.P.; Torres, P.B.; dos Santos, D.Y.A.C.; Motta, L.B.; Chow, F. Seasonal effects on antioxidant and anti-HIV activities of Brazilian seaweeds. J. Appl. Phycol. 2019, 31, 1333–1341. [Google Scholar] [CrossRef]
- Guiry, M.D.; AlgaeBase. World-Wide Electronic Publication. Available online: https://www.algaebase.org (accessed on 14 April 2022).
- Armisen, R. Agar. In Thickening and Gelling Agents for Food; Imeson, A.P., Ed.; Springer: Boston, MA, USA, 1997; pp. 1–21. [Google Scholar]
- Torres, P.; Pires, J.; Chow, F.; Santos, D.Y.A.C. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Arulkumar, A.; Rosemary, T.; Paramasivam, S.; Rajendran, R.B. Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis of red seaweeds (Gracilaria Corticata and Gracilaria Edulis) from Palk Bay, India. Biocatal. Agric. Biotechnol. 2018, 15, 63–71. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Helidoniotis, F.; Shaw, K.J.; Svoronos, D. Distribution of bromophenols in species of marine algae from Eastern Australia. J. Agric. Food Chem. 1999, 47, 2367–2373. [Google Scholar] [CrossRef]
- Guaratini, T.; Lopes, N.P.; Marinho-Soriano, E.; Colepicolo, P.; Pinto, E. Antioxidant activity and chemical composition of the non polar fraction of Gracilaria Domingensis (Kützing) Sonder Ex Dickie and Gracilaria Birdiae (Plastino & Oliveira). Braz. J. Pharmacogn. 2012, 22, 724–729. [Google Scholar] [CrossRef]
- Torres, P.; Chow, F.; Furlan, C.M.; Mandelli, F.; Mercadante, A.; dos Santos, D.Y.A.C. Standardization of a protocol to extract and analyze chlorophyll a and carotenoids in Gracilaria tenuistipitata Var. Liui. Zhang and Xia (Rhodophyta). Braz. J. Oceanogr. 2014, 62, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Collén, P.N.; Camitz, A.; Hancock, R.D.; Viola, R.; Pedersén, M. Effect of nutrient deprivation and resupply on metabolites and enzymes related to carbon allocation in Gracilaria tenuistipitata (Rhodophyta). J. Phycol. 2004, 40, 305–314. [Google Scholar] [CrossRef]
- Nylund, G.M.; Weinberger, F.; Rempt, M.; Pohnert, G. Metabolomic assessment of induced and activated chemical defence in the invasive red alga Gracilaria vermiculophylla. PLoS ONE 2011, 6, 0029359. [Google Scholar] [CrossRef]
- Torres, P.; Nagai, A.; Teixeira, D.I.A.; Marinho-Soriano, E.; Chow, F.; dos Santos, D.Y.A.C. Brazilian native species of Gracilaria (Gracilariales, Rhodophyta) as a source of valuable compounds and as nutritional supplements. J. Appl. Phycol. 2019, 31, 3163–3173. [Google Scholar] [CrossRef]
- Torres, P.B.; Chow, F.; Ferreira, M.J.P.; dos Santos, D.Y.A.C. Mycosporine-like Amino acids from Gracilariopsis tenuifrons (Gracilariales, Rhodophyta) and its variation under high light. J. Appl. Phycol. 2016, 28, 2035–2040. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Analysis of antioxidant capacity and bioactive compounds in marine macroalgal and lichenic extracts using different solvents and evaluation methods. Cienc. Mar. 2016, 42, 271–288. [Google Scholar] [CrossRef]
- Saunders, G.W. Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1879–1888. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Carneiro, M.A.A.; Soriano, J.P. Manual de Identificação das Macroalgas Marinhas do Litoral do Rio Grande do Norte; UFRN: Natal, RN, Brazil, 2009. [Google Scholar]
- Bartz, R.; Li, W.-H.; Venables, B.; Zehmer, J.K.; Roth, M.R.; Welti, R.; Anderson, R.G.W.; Liu, P.; Chapman, K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 2007, 48, 837–847. [Google Scholar] [CrossRef]
- Torres, P.; Novaes, P.; Ferreira, L.G.; Santos, J.P.; Mazepa, E.; Duarte, M.E.R.; Noseda, M.D.; Chow, F.; dos Santos, D.Y.A.C. Effects of extracts and isolated molecules of two species of Gracilaria (Gracilariales, Rhodophyta) on early growth of lettuce. Algal Res. 2018, 32, 142–149. [Google Scholar] [CrossRef]
- Santos, K.P.; Sedano-Partida, M.D.; Sala-Carvalho, W.R.; Loureiro, B.O.S.J.; da Silva-Luz, C.L.; Furlan, C.M. Biological activity of Hyptis Jacq. (Lamiaceae) is determined by the environment. Ind. Crops Prod. 2018, 112, 705–715. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of Mycosporine-like Amino Acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Urrea-Victoria, V.; Pires, J.; Torres, P.B.; dos Santos, D.Y.A.C.; Chow, F. Ensaio Antioxidante em Microplaca do Poder de Redução do Ferro (FRAP) para Extratos de Algas, 1st ed.; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2016. [Google Scholar]
- Harb, T.B.; Torres, P.B.; Pires, J.S.; Santos, D.Y.A.C.; Chow, F. Ensaio em Microplaca do Potencial Antioxidante Através do Sistema Quelante de Metais para Extratos de Algas, 1st ed.; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2016. [Google Scholar]
- Pires, J.S.; Torres, P.B.; Santos, D.Y.A.C.; Chow, F. Ensaio em Microplaca de Substâncias Redutoras pelo Método do Folin-Ciocalteu para Extratos de Algas, 1st ed.; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2017; ISBN 978-85-85658-70-0. [Google Scholar]
- Le Tutour, B. Antioxidative activities of algal extracts, synergistic effect with vitamin E. Phytochemistry 1990, 29, 3759–3765. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Sotira, S.; Rebucci, R.; Reggi, S.; Castiglioni, B.; Rossi, L. In vitro evaluation of antimicrobial and antioxidant activities of algal extracts. Ital. J. Anim. Sci. 2020, 19, 103–113. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr. Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Cho, K.-H.; Hong, J.-H.; Lee, K.-T. Monoacylglycerol (MAG)-oleic acid has stronger antioxidant, anti-atherosclerotic, and protein glycation inhibitory activities than MAG-palmitic acid. J. Med. Food 2010, 13, 99–107. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Huang, H.-W.; Wu, Y.-C.; Chung, C.-C.; Yuan, S.-S.F.; Chang, F.-R.; Chang, H.-W. Antioxidant potential of solvent partitioned extraction from aqueous extract of Gracilaria tenuistipitata. Curr. Org. Chem. 2014, 19, 39–44. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant Potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef]
- Narasimhan, M.K.; Pavithra, S.K.; Krishnan, V.; Chandrasekaran, M. In vitro analysis of antioxidant, antimicrobial and antiproliferative activity of Enteromorpha antenna, Enteromorpha linza and Gracilaria corticata extracts. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 151–159. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Fitzgerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant Activity and phenolic content of pressurised liquid and solid-liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Pang, J.R.; Goh, V.M.J.; Tan, C.Y.; Phang, S.M.; Wong, K.H.; Yow, Y.Y. Neuritogenic and in vitro antioxidant activities of malaysian Gracilaria manilaensis Yamamoto & Trono. J. Appl. Phycol. 2018, 30, 3253–3260. [Google Scholar] [CrossRef]
- De La Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant activity of Mycosporine-like Amino Acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, P.; Chow, F.; dos Santos, D.Y.A.C. A Chemical Investigation of the Antioxidant Capacity of Extracts from Red Macroalga Gracilaria domingensis. Phycology 2022, 2, 332-343. https://doi.org/10.3390/phycology2030018
Torres P, Chow F, dos Santos DYAC. A Chemical Investigation of the Antioxidant Capacity of Extracts from Red Macroalga Gracilaria domingensis. Phycology. 2022; 2(3):332-343. https://doi.org/10.3390/phycology2030018
Chicago/Turabian StyleTorres, Priscila, Fungyi Chow, and Deborah Yara Alves Cursino dos Santos. 2022. "A Chemical Investigation of the Antioxidant Capacity of Extracts from Red Macroalga Gracilaria domingensis" Phycology 2, no. 3: 332-343. https://doi.org/10.3390/phycology2030018
APA StyleTorres, P., Chow, F., & dos Santos, D. Y. A. C. (2022). A Chemical Investigation of the Antioxidant Capacity of Extracts from Red Macroalga Gracilaria domingensis. Phycology, 2(3), 332-343. https://doi.org/10.3390/phycology2030018





