Abstract
Findings about CO2 dynamics in the Earth’s ancestral atmosphere have suggested much higher concentrations in past eras. Along this line, cyanobacteria are an early evolved photosynthetic group that is suggested to have experienced both high and low CO2 availability since their Precambrian origin, and therefore, it is reasonable to assume that these microbes have the potential to cope with these scenarios by rapidly adjusting to various carbon dioxide levels. Thus, in this work, we performed a short-term (72-h) investigation of the physiological parameters (cell growth, photosynthesis and saxitoxin production) of toxic and nontoxic strains of the cyanobacterium Raphidiopsis raciborskii challenged by an extremely high pCO2 (40,000 ppm). Additionally, the transcriptomic profile (regarding the carbon concentrating mechanism and photosynthesis) of selected toxic and nontoxic strains is also presented. We found that short-term exposure to extremely elevated CO2 concentrations did not affect R. raciborskii physiology regardless of toxin production. However, transcripts related to bicarbonate transporters and the RuBisCO enzyme indicated the upregulation of CCM and downregulation of the Calvin cycle, respectively. According to our findings, at least at the initial growth phase, R. raciborskii was able to cope with a very high CO2 level, which shed light on the understanding that this species might have the potential to cope with carbon dioxide in water above the predicted levels.
1. Introduction
Usually, CyanoHABs (cyanobacterial harmful algal blooms) are correlated with artificial eutrophication in inland and coastal waters impacted by anthropogenic activities. However, in recent decades, these phenomena have become more frequent and persistent worldwide due to the rise in CO2, which directly affects global temperatures and results in extreme climate changes [1,2]. According to the International Panel on Climate Change (IPCC), a threefold increase in the current CO2 concentration (410 ppm) is expected in the next hundred years [3], worsening global warming even more. Moreover, in addition to increasing global surface temperature, CO2 enhancement can also affect the water chemistry (e.g., pH and dissolved inorganic carbon dynamics) in both freshwater and marine environments, which together may impact the ecophysiology of several HAB species [4].
Over the past geological eras, the planet Earth has experienced many changes in atmospheric gas composition [5], with special emphasis on carbon dioxide. The highest CO2 concentrations date from the Precambrian and Paleozoic eras [6] and estimates suggest that carbon dioxide concentrations could have reached 6000 ppm during the Cambrian period and decreased until the Permian to reach the current values. However, more recently, Lehmer et al. [7] suggested an even higher estimated CO2 concentration by modeling iron oxidation of micrometeorites by CO2. The authors suggested that 2.7 billion years ago, our atmosphere could have been composed of a much higher concentration of carbonic gas (approximately 70%) [7]. These findings coincide with estimates of both the emergence of cyanobacteria and the atmospheric oxygenation around 2.7–3.8 billion years ago [8]. Therefore, these oxyphotosynthetic microbes must have coped with an extremely variable CO2 scenario up until the present conditions.
As a key enzyme in the photosynthetic process, CO2-fixing ribulose-1,5-biphosphate carboxylase/oxygenase (RuBisCO) has evolved in different phytoplankton groups in terms of CO2/O2 affinity [9]. For instance, in cyanobacteria, to sustain maximum CO2 fixation, RuBisCO was allocated to cellular microcompartments called carboxysomes [10]. In early evolved phytoplankton, such as cyanobacteria and dinoflagellates, RuBisCO enzymes had a low CO2 affinity since these microorganisms dated from a scenario with higher CO2 availability [9]. However, as the CO2 concentration declined, phytoplankton evolved carbon concentrating mechanisms (CCMs) [11] to allow a higher inorganic carbon (Ci) accumulation at the active site of carboxilation. CCMs consist of uptake systems (membrane transporters and enzymes) that enable cyanobacteria and some phytoplankton to take up Ci when CO2 levels are low. Five different Ci uptake systems have been described in cyanobacteria to date: two for CO2 uptake (NDH-I3 and NDH-I4) and three for HCO3− uptake (BCT1, BicA and SbtA) with differential bicarbonate affinity and flux rate [12,13]. Moreover, the enzyme carbonic anhydrase (CA) also plays a role in CCM functioning by catalyzing the interconversion between CO2 and HCO3- and preventing Ci leakage out of the cell [14].
Among bloom-forming cyanobacteria, Raphidiopsis raciborskii (formerly Cylindrospermopsis raciborskii) [15] stands out as an N2-fixing filamentous cyanobacterium known to tolerate a range of environmental conditions [16,17], in addition to being considered an invasive microbe, with recent expansion to temperate regions [18,19]. This cyanobacterium is also assumed to be a bicarbonate-user species with a CCM more suitable to acquire Ci as HCO3− [20,21], and its adaptive success has been related to high phenotypic plasticity [22]. Furthermore, some strains of R. raciborskii produce toxic metabolites, including bioactive alkaloids such as cylindrospermopsins (CYNs) or saxitoxins (STX—also referred to as paralytic shellfish toxins). STXs are guanidinium-containing neurotoxic alkaloids that block cellular ionic channels encompassing more than 50 analogs, according to the degree of sulfation (e.g., saxitoxin, gonyautoxins and C-toxins) and decarbamoylation (e.g., dicarbamoyl-saxitoxin) [23]. The CYN analogs (7-epicylindrospermopsin and 7-deoxycyclicylindrospermopsin) are tricyclic alkaloids of cytotoxic potential whose mechanism of action is the inhibition of protein synthesis, and genotoxic, immunotoxic and oxidative damage effects have been described [24].
In South America, toxic strains of R. raciborskii have been reported to be STX producers [21,25,26], while no strain has been characterized as producing CYN to date, although this alkaloid has been found in environmental samples [27]. In addition, strains lacking genes encoding STXs or CYN [28] can also compose R. raciborskii blooms, cooccurring with toxic blooms.
Studies regarding the effects of increasing CO2 on R. raciborskii usually focus on the scenario predicted by the IPCC (a threefold increase in 100 years or ~1300 ppm) and have demonstrated responses to high CO2 conditions by examining different parameters, such as photosynthesis [29], cellular Ci transporters [20], toxin production [30] and metabolism [31]. In general, the data suggest that this species can cope with the future CO2 scenario. However, little is known regarding extremely high CO2 concentrations, as reported for past geological eras, since the studies are restricted to the levels predicted for the future. Furthermore, there is a gap in the comprehension of the overall early responses underlying exposure to extreme CO2 levels, which are likely to determine the upcoming physiological responses. In addition to several studies that have examined only toxic strains, it is also important to assess whether the ability to produce toxins is a significant trait regarding the response to increasing CO2. Thus, we aimed to evaluate the physiological traits and the overall gene expression (transcriptome) of saxitoxin-producing and nonproducing strains of R. raciborskii under short-term exposure to extremely high CO2 conditions (40,000 ppm). Considering the CO2 scenario from which cyanobacteria have evolved, we tested the hypothesis that R. raciborskii can potentially cope with extreme CO2 conditions, regardless of toxin production.
2. Materials and Methods
2.1. Cyanobacterial Strains and Culture Conditions
Four Raphidiopsis raciborskii strains were used in this study: the saxitoxin-producing LETC-CY-05 (formerly CYRF-01) and T3, which produce mainly the nonsulfated analogs saxitoxin (STX) and neosaxitoxin (neoSTX), and their decarbamoylated variants [32,33]. The nonproducing strains were LETC-CY-01 (formerly CYLP-01) and LETC-CY-02 (formerly NPCS-1) [34]. These cyanobacterial strains were isolated from waterbodies from different Brazilian regions: LETC-CY-05 (Funil Reservoir, Rio de Janeiro, southeastern Brazil), T3 (Billings Dam, São Paulo, southeastern Brazil), LETC-CY-01 (Paranoá Lake, Federal District, midwestern Brazil) and LETC-CY-02 (Custódia Reservoir, Pernambuco, northeastern Brazil). Batch cultures were established in sterile ASM-1 medium [35] under constant aeration (≅0.041% CO2, air level) provided by an air injection device, initial pH 8.0, 23 ± 1 °C, light intensity of 40–50 μmol photons m−2·s−1 and a 12:12 h light:dark cycle. The stock cultures were kept at the exponential growth phase through weekly medium renovation with fresh liquid ASM-1.
2.2. Experimental Setup
Batch cultures (n = 4) of the R. raciborskii strains were inoculated to an initial cell concentration of approximately 105 cells mL−1 (≅15 mm3·L−1) in 2-L Erlenmeyer flasks filled with sterile ASM-1 culture medium. The cyanobacteria were grown in an extremely high CO2 environment obtained through a Thermco 8500 gas mixer (Thermco Instrument Corp, La Porte, IN, USA) coupled to a 100% CO2 cylinder adjusted to provide 4% CO2-rich air bubbling (≅40,000 ppm). The CO2-rich air bubbling system consisted of a cotton plug crossed by a glass pipette immersed in the culture medium and connected to a silicone hose, which in turn was attached to a 5 mL syringe filled with hydrophobic cotton as a filtration device through which the compressed air passed. Controls (n = 4) consisted of cultures established at air level CO2 conditions (≅410 ppm or 0.041% provided by an air compressor). The experiment was run over 72 h to examine the physiological responses and CCM and photosynthesis-related gene expression underlying cyanobacterial acclimation to the different CO2 environments. Samples were taken every 24 h to evaluate physiological parameters (growth and photosynthesis) and after 72 h for cyanotoxins and transcriptome analysis.
2.3. Growth and Photosynthesis Measurements
Cyanobacterial samples were preserved with 1% acetic Lugol’s solution, and cell counting was performed on a Fuchs–Rosenthal hemocytometer using an optical microscope (Olympus Trinocular Microscope BX series, Olympus, Center Valley, PA, USA). Given that R. raciborskii is a filamentous species, the measurement of at least 30 random trichomes and cells of each strain was carried out to establish the average cell number per trichome. Cell measurements were also used to estimate the mean cell volume (µm3) according to its respective geometric form and, together with the cell concentration, used to estimate the biovolume (mm3·L−1) [36,37]. The specific growth rate (µ·d−1) was calculated according to Reynolds [38], and the growth yield (fold change) was determined by the ratio between the final and the initial biovolume.
The photosynthetic yield (photosystem II quantum yield; Fv’/Fm’) was measured for each R. raciborskii strain by a fluorimeter Phytoplankton Analyzer equipped with a PHYTO-EDF sensor (Walz PHYTO-PAM- Heinz Walz, Effeltrich Germany).
2.4. Transcriptomic Profile of CCM and Photosynthesis-Related Genes
We examined R. raciborskii LETC-CY-05 and LETC-CY-01 (saxitoxin-producing and nonproducing, respectively) transcriptomes to verify the effect of extremely high CO2 on the expression of genes related to the carbon concentration mechanism and photosynthesis in both toxic and nontoxic strains.
2.5. Sample Processing and RNA Extraction
A total of 500 mL samples (n = 3) were harvested after 72 h of incubation from both control and extremely high CO2 cultures. The samples were centrifuged in a Sorvall RC-5B refrigerated superspeed centrifuge (Du Pont Instruments, Wilmington, DE, USA) (10 min, 9148.2× g). The supernatant was discarded, and the pellet was immediately frozen in a Shell Freezer (Labconco, Kansas City, MO, USA). Frozen material was lyophilized (Labconco, Kansas City, USA), and RNA extraction was performed with freeze-dried cell biomass. Total RNA extraction was performed by adding 3 mL of TRIzol (Ambion, Austin, TX, USA) to the freeze-dried biomass and processed according to the manufacturer’s instructions. Ribosomal RNA depletion of the samples was obtained using a Ribo-Zero™ rRNA Removal kit (Epicenter, Denver, CO, USA).
2.6. Library, Sequencing and Gene Expression Analysis
Library preparation for massively parallel sequencing was performed using the Ion Total RNA-seq Kit v2 protocol and barcodes (RNA-Seq Barcode 1–16 kit, Thermo Fisher, Massachusetts, USA) to allow multiplex sequencing. Sequencing was performed using an Ion PI Hi-Q Sequencing 200 kit in a high-potency Ion ProtonTM sequencer (Thermo Fisher, USA) on an Ion PITM V3 semiconductor chip (100 gigabases of capacity). Sequencing reads were exported from Ion Server (Thermo Fisher, USA) in FASTQ format and uploaded to CLC Genomics Workbench v. 8.5 to proceed to sequence data analysis. Quality control was initially applied by removing low quality and size (<25 bp) sequences. Reads were mapped against the R. raciborskii reference genome CS-505, available in public databases. To identify genes in which transcripts varied significantly (fold change < −1.5 > 1.5; p value < 0.05), empirical analysis of differential gene expression (EDGE) was used [39]. To improve the confidence of comparative analysis between CO2 conditions, datasets were log-transformed [40]. Moreover, in this study, differential gene expression analysis focused on some aspects of inorganic carbon acquisition-related transcripts. Complete transcriptome data will be provided in a further publication (data not shown).
2.7. Saxitoxin Extraction and HPLC-FLD Analysis
After 72 h of incubation, 200 mL culture samples of each STX-producing strain (T3 and LETC-CY-05) were harvested for saxitoxin analysis. For STX extraction, the samples were freeze-dried, resuspended in 500 mM acetic acid solution and incubated for 1 h. Subsequently, the extract was centrifuged at 8 °C and 1937× g for 10 min, and the supernatant was preserved. The procedure was repeated twice. The pooled supernatants were filtered through a 0.45 µm polyvinylidene fluoride (PVDF) Whatman filter and stored in 1.5 mL vials at −20 °C until the subsequent cyanotoxin analysis.
Saxitoxin content was analyzed using high-performance liquid chromatography equipment (Shimadzu, Kyoto, Japan) coupled to a fluorescence detector (HPLC-FLD). Toxin separation was performed in a silica-based reversed-phase column (125.0 mm × 4.0 mm, 5 μm; Lichrospher 100 Reversed-Phase C18), and the analysis was carried out for nonsulfated saxitoxins (neosaxitoxin and saxitoxin) according to Oshima [41] under the following conditions: mobile phase of 2 mM heptanesulfonate in 30 mM ammonium phosphate buffer plus 5% acetonitrile running under isocratic conditions and at a flow rate of 0.8 mL/min. STX detection was performed using an RF-10 Alx fluorescence detector (Shimadzu, Japan) at excitation and emission wavelengths of 330 nm and 390 nm, respectively. The STXs were identified and quantified by comparison with STX and neoSTX standard solutions purchased from the Institute of Marine Bioscience, National Research Council of Canada (Halifax, NS, Canada). Total STX data (neoSTX + STX) were obtained in volumetric concentration (µg·L−1) but thereafter expressed as the total STX cell quota (fgSTX cell−1) for toxin production evaluation.
2.8. Statistical Analysis
A two-way analysis of variance (ANOVA) was applied to compare strain-specific responses to the CO2 effects on biovolume and photosynthetic yield. Student’s t-test was used to perform comparisons of the CO2 effect on the R. raciborskii growth rate, growth ratio and toxin production. All analyses and graphs were performed in GraphPad Prism 8.0 software, and the statistical significance assumed was a p value < 0.05.
3. Results
Incubation under an extremely high CO2 environment (or high partial CO2 pressure; pCO2) did not affect the growth of most of the tested R. raciborskii strains (Figure 1). However, only the nontoxic LETC-CY-01 showed a significant decrease in growth under the higher pCO2 (RM ANOVA, F(1,6) = 13.88; p < 0.01). In general, regardless of the ability to produce toxins and pCO2 effects, R. raciborskii displayed an intraspecific variability in growth pattern. For instance, although the toxic R. raciborskii T3 was accidentally inoculated at an approximately twofold higher initial cell concentration (Figure 1), this strain displayed either a growth ratio (biomass yield) or a specific growth rate similar to the nontoxic LETC-CY-02, which in turn was different from the nontoxic LETC-CY-01 (Figure 2).
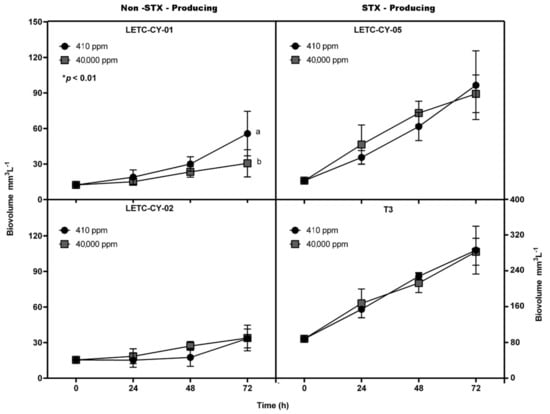
Figure 1.
Growth curves estimated by biovolume (mm3·L-1) for LETC-CY-01, LETC-CY-02, LETC-CY-05 and T3 strains of Raphidiopsis raciborskii exposed to extremely high pCO2 concentrations (≅40,000 ppm) and under control conditions (current pCO2 concentration ≅410 ppm). Different letters indicate significant differences (p < 0.05).
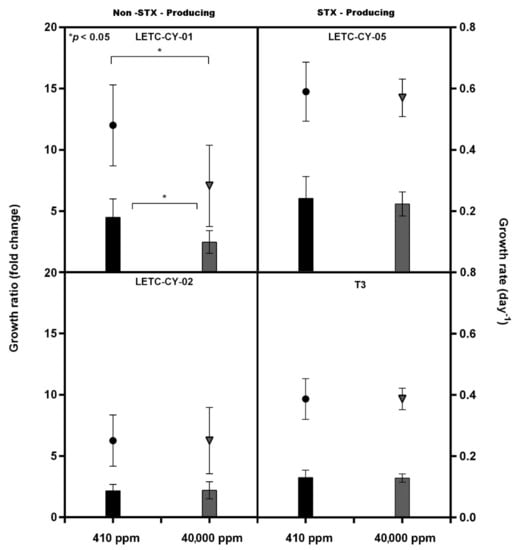
Figure 2.
Growth rates (diamond and circle; day-1) and growth ratio (bars; fold change) estimated by biovolume for LETC-CY-01, LETC-CY-02, LETC-CY-05 and T3 strains of Raphidiopsis raciborskii exposed to extremely high pCO2 concentrations (≅40,000 ppm) and under control conditions (current pCO2 concentration ≅410 ppm). Significant differences (*) = p < 0.05, Student’s t-test.
The decreased biovolume concentration shown by R. raciborskii LETC-CY-01 in response to the extremely high CO2 (Figure 1) also resulted in a significantly decreased biomass production and specific growth rate (t-test; p < 0.05), demonstrating by the fact that this cyanobacterium grew half as much in comparison to itself when grown under air-level CO2 concentration (Figure 2).
Furthermore, under a high pCO2, LETC-CY-01 also displayed a significant decrease in the quantum yield of photosystem II (Fv’/Fm’) after 24 h of incubation (Bonferroni’s test, p < 0.0001), accompanied by a slight but still significant increase (RM ANOVA, p < 0.05; Table 1). Overall, the CO2 environment had no impact on the photosynthetic yield of the other tested R. raciborskii strains (Table 1).

Table 1.
Photosynthetic yields (Fv’/Fm’) for LETC-CY-01, LETC-CY-02, LETC-CY-05 and T3 strains of Raphidiopsis raciborskii exposed to extremely high pCO2 concentrations (≅40,000 ppm) and under control conditions (current pCO2 concentration; ≅410 ppm). Different letters indicate significant differences (p < 0.05).
Regarding the STX-producing R. raciborskii strains, T3 was characterized as displaying a relatively higher total saxitoxin production (reaching ~12-fold; STX + neoSTX) than LETC-CY-05. Both toxic strains displayed significant changes in toxin production after 72 h of incubation (Figure 3; pairwise t-test, p < 0.05). However, saxitoxin production was not affected when these strains were challenged by extremely high pCO2.
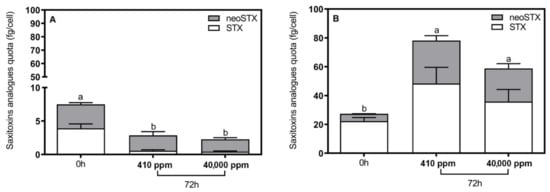
Figure 3.
Total saxitoxin quota (fg/cell) of Raphidiopsis raciborskii (A) LETC-CY-05 and (B) T3 exposed to extremely high pCO2 concentrations (≅40,000 ppm) and under control conditions (current pCO2 concentration, ≅410 ppm) at 0 and 72 h. a,b Significant differences = p < 0.05, ANOVA.
The transcriptome of the nontoxic LETC-CY-01 and the toxic LETC-CY-05 was examined for transcripts of CCM and photosynthesis-related genes by using the EDGE method. According to the transcriptomic analysis, both strains displayed an upregulation in genes encoding bicarbonate transport in response to the extremely higher pCO2 (Table 2). However, genes encoding NADPH-dependent glyceraldehyde-3-phosphate dehydrogenase, RuBisCO and related to CCM activity were downregulated in LETC-CY-01 after 72 h of incubation under a 40,000 ppm CO2 concentration (Table 2).

Table 2.
Differential gene expression (EDGE) of R. raciborskii strains LETC-CY-01 and LETC-CY-05 under extremely high pCO2 (≅40,000 ppm).
4. Discussion
In this study, we investigated the short-term physiological and transcriptomic responses of STX-producing and nonproducing R. raciborskii to an extremely high carbon dioxide concentration. In general, when assessing physiological parameters, such as growth, photosynthesis and saxitoxin production, most of the R. raciborskii strains were not affected by increased CO2. However, transcriptomic analysis revealed an upregulation of CCM activity proteins, such as bicarbonate transporters, and downregulation of proteins related to the Calvin cycle, such as RuBisCO.
Overall, regardless of CO2 challenge and the ability to produce STXs, the strains maintained their growth (increased biovolume) during the examined three-day cycle. R. raciborskii LETC-CY-01 was the unique strain that showed a reduction in biomass production and growth kinetics when subjected to the extremely high CO2 concentration. Although most of the studies usually assess cyanobacterial growth over a longer cycle (1–4 weeks), the response to increased Ci availability can be rapidly evidenced in the first few days (<4 days), as reported by Holland et al. [20] and Vilar and Molica [21]. These authors have shown R. raciborskii to perform better under a high CO2 environment but much lower than the pCO2 tested in our experimental setup.
Furthermore, despite not being affected by increasingly high pCO2, most of the R. raciborskii strains displayed different growth rates, in addition to toxin production, which suggests intraspecific variability. Willis et al. [42] reported different growth rates and toxin quotas in 24 R. raciborskii strains. The authors highlighted that, in nature, several subpopulations constitute a ‘populational mosaic’ by occurring as different ecotypes. This rationale has also been pointed out by Saker and Neilan [43]. Indeed, subpopulations at different abundances and physiological traits at different levels (e.g., chlorophyll cell quota) may occur and have divergent environmental preferences, such as depth, light intensity, nutrient and organic matter sources [44,45,46].
In general, R. raciborskii photosynthesis was not affected by the extreme CO2 level. The efficient maintenance of photosynthesis under the different CO2 concentrations could be a result of a very adjustable photosynthetic apparatus. In a study with one R. raciborskii strain, the plasticity of photosystem II (PSII) was observed when the cyanobacterium was cultured under two distinct CO2 conditions (current and 1300 ppm) [29]. Exposed cells reorganized their PSII when submitted to higher concentrations of CO2, while the carbon concentration mechanism of R. raciborskii had a better performance under lower CO2 conditions [29]. Moreover, in a meta-analysis study with 20 phytoplankton strains, it was demonstrated that earlier groups (cyanobacteria and dinoflagellates) had a higher CO2 concentrating mechanism (CCM) plasticity because they may have evolved to compensate for the low specificity of RuBisCO for CO2 by diversifying their carbon transporters [9]. In fact, in our findings, although an extremely high CO2 concentration was introduced in the cultures, the slight mean pH difference observed between the experimental conditions (from 9.5 under 410 ppm to 8.2 under 40,000 ppm) (data not shown) was still in the range of bicarbonate availability; inorganic carbon has been assumed as the more suitable to be used by R. raciborskii [20]. A similar study did not find relevant variations in pH values when cultures were submitted to 400 and 1000 ppm CO2 [31]. Studies also demonstrated a diversity of CCM genes in the genomes of different R. raciborskii strains [31,47]. Here, we first report preliminary results of the transcriptomic response of R. raciborskii to extremely high pCO2.
Additionally, in our comparative transcriptomics analysis, we found an upregulation of the BicA transporter and downregulation of RuBisCO, carboxysome formation (ccmO and ccmN transcripts) and proteins related to the Calvin cycle after 72 h under extremely high pCO2. Although BicA is characterized as having a relatively low affinity for bicarbonate (K0.5 ~70–350 μmol·L−1) in contrast to a high flux rate [12], under increased Ci availability, a higher affinity to bicarbonate is less essential for Ci acquisition than a higher flux rate. However, Sandrini et al. [48] found a downregulation of bicarbonate transporters and no significant change in RuBisCO and carboxysome protein expression for Microcystis PCC 7806 immediately after 2 h when cultured under a sixfold higher pCO2. However, the authors demonstrated an upregulation of bicarbonate transporters at low Ci availability, which is argued to be induced CCM activity for better CO2 acquisition by the cyanobacterium. These short-term variations in transcript levels indicate that cyanobacteria can rapidly respond to CO2 dynamics with a likely physiological adjustment to cope with the pressure exerted by the environment.
The increased pCO2 had no significant effect on saxitoxin production by either R. raciborskii T3 or LETC-CY-05. This finding demonstrates that toxin production is probably not involved in immediate cellular responses to changes in CO2 concentration, at least in the first 72 h. However, the significant differences between the STX cell amounts displayed by the strains demonstrated that they have different potential effects on toxin production.
Vilar and Molica [21] first reported the effect of Ci availability on R. raciborskii saxitoxin production and evidenced a decreased STX amount in contrast to a higher biomass produced under a high Ci level. In contrast to our experimental setup, the authors provided a higher Ci environment by culturing the cyanobacterium in an aerated medium enriched with sodium bicarbonate (0.2 mM NaHCO3). In an approach similar to our study, Pierangelini et al. [30] analyzed the cylindrospermopsin pool size in R. raciborskii under light and CO2 variations, concluding that the production of this toxin is constitutive and is not affected by light or high CO2 (1300 ppm). We observed a similar effect with two STX-producing R. raciborskii, even when challenged by a much greater CO2 concentration (40,000 ppm). Additionally, the remarkable differences in the regular toxin production by the R. raciborskii strains compared to the absence of an effect induced by the experimental challenge suggest that, to a certain extent, the intraspecific variability in toxin production might overlap with the changes promoted by different CO2 levels.
Furthermore, short-term exposure (72 h) was important to evaluate a likely genetic background to rapidly respond to a challenging CO2 environment and enable cells to maintain their growth. Strain acclimatization to the higher pCO2 prior to the experiment was not made as we aimed to observe any shift in parameters involved with the plasticity of R. raciborskii, since this species is known for its small genotype variability and high environmental plasticity [28].
In addition, this study evaluated the direct impacts of a greenhouse gas. Studies encompassing indirect impacts, such as dissolved inorganic carbon dynamics in water, and global climate changes, such as temperature increases (e.g., [21,31]), cannot be disregarded once, e.g., warming can allow R. raciborskii the optimum conditions for expansion and occurrence in environments where this species usually does not occur [49]. Similarly, a continental-scale study with water bodies in Europe demonstrated that temperature is indeed involved in the distribution of cyanotoxins or toxic cyanobacteria [50]. Additionally, Willis et al. [31] demonstrated that temperature is more important than pCO2 concentration (750 and 1000 ppm) for two R. raciborskii strains. The authors also observed contrasting strain responses for the two tested CO2 levels.
Impacts similar to those found for freshwater cyanobacteria were also observed for marine phytoplankton, where elevated CO2 concentrations did not affect marine cyanobacteria [51,52], emphasizing the rationale that solely increased CO2 is not as relevant for predicting future impacts on aquatic environments, especially if considering the different levels of CO2 with which cyanobacteria have coped over the past geological eras [5,6,7] as well as the extensive framework of genes selected by evolutionary forces due to its prevalence and ancient origin [8]. Additionally, a recent review by Ma and Wang [53] evidenced, by analyzing field data, that phytoplankton biomass was correlated with CO2 rise, but there was a lack of correlation when considering only cyanobacterial biomass. The authors highlight the complexity of simultaneous phenomena occurring in aquatic environments, such as competition, stressors, nutrient cycling and grazing, and compared them to laboratory results to demonstrate the gaps and predictability between the different approaches [53]. Additionally, it has been demonstrated that speculations about CCMs functioning under saturation of CO2 being able to affect cyanobacterial growth are not corroborated by field observations as cyanobacteria have displayed a good performance under any CO2 condition [53].
5. Conclusions
According to our findings, at least at the initial growth phase, R. raciborskii was able to cope with a very high CO2 level, which shed light on the understanding that this species might have the potential to cope with carbon dioxide in water above the predicted levels.
Additionally, STX production was not altered in the presence of extremely high pCO2, nor there was difference in the production of the two types of STX analyzed (neoSTX and STX). Different concentrations were observed only between different strains regardless of pCO2 conditions.
Finally, only LETC-CY-01, a nontoxic strain, had its growth altered in the presence of an extremely high pCO2 concentration. Our findings extend our comprehension of the plasticity of the cyanobacterium Raphidiopsis raciborskii.
Author Contributions
Conceptualization, R.R.P., L.H., R.C.P. and S.M.F.O.A.; data curation, R.R.P., M.V., R.S. and S.M.F.O.A.; formal analysis, R.R.P., M.V. and L.H.; funding acquisition, R.C.P. and S.M.F.O.A.; investigation, R.R.P.; methodology, R.R.P., L.H. and T.B.; project administration, S.M.F.O.A.; software, L.H. and T.B.; supervision, R.S., R.C.P. and S.M.F.O.A.; writing—original draft, R.R.P., M.V. and L.H.; writing—review and editing, M.V., T.B., R.S., R.C.P. and S.M.F.O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 446216/2015-1). A postgraduate fellowship was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES BR).
Data Availability Statement
When requested, authors may provide raw data to verification and/or to improve and compose other studies like meta-analysis studies.
Acknowledgments
We thank the anonymous reviewers for the comments to improve this manuscript and the agencies CNPq and CAPES for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microb. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, R.K.L.; Meyer, G.K.; Plattner, T.; Stocker, T.F. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2015. [Google Scholar]
- Raven, J.A.; Gobler, C.J.; Hansen, P.J. Dynamic CO2 and pH levels in coastal, estuarine, and inland waters: Theoretical and observed effects on harmful algal blooms. Harmful Algae 2020, 91, 101594. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F.; Howard, M.T. Atmospheric composition and climate on the early Earth. Philos. Trans. R. Soc. B Biol. Sci. 2006, 3611474, 1733–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, N.M.; Lenton, T.M.; Watson, A.J. COPSE: A new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci. 2004, 3045, 397–437. [Google Scholar] [CrossRef]
- Lehmer, O.R.; Catling, D.C.; Buick, R.; Brownlee, D.E.; Newport, S. Atmospheric CO2 levels from 2.7 billion years ago inferred from micrometeorite oxidation. Sci. Adv. 2020, 6, eaay4644. [Google Scholar] [CrossRef] [Green Version]
- Altermann, W. The Early Earth’s Record of Supposed Extremophilic Bacteria and Cyanobacteria, at 3.8 to 2.5 GA. In Algae and Cyanobacteria in Extreme Environments; Springer: Dordrecht, The Netherlands, 2007; pp. 759–778. [Google Scholar]
- Van de Waal, D.B.; Brandenburg, K.M.; Keuskamp, J.; Trimborn, S.; Rokitta, S.; Kranz, S.A.; Rost, B. Highest plasticity of carbon-concentrating mechanisms in earliest evolved phytoplankton. LO Lett. 2019, 42, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Burnap, R.L.; Hagemann, M.; Kaplan, A. Regulation of CO2 concentrating mechanism in cyanobacteria. Life 2015, 5, 348–371. [Google Scholar] [CrossRef] [Green Version]
- Hagemann, M.; Kern, R.; Maurino, V.G.; Hanson, D.T.; Weber, A.P.; Sage, R.F.; Bauwe, H. Evolution of photorespiration from cyanobacteria to land plants, considering protein phylogenies and acquisition of carbon concentrating mechanisms. J. Exp. Bot. 2016, 6710, 2963–2976. [Google Scholar] [CrossRef] [Green Version]
- Price, G.D.; Badger, M.R.; Woodger, F.J.; Long, B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 2008, 59, 1441–1461. [Google Scholar] [CrossRef]
- Sandrini, G.; Ji, X.; Verspagen, J.M.; Tann, R.P.; Slot, P.C.; Luimstra, V.M.; Schuurmans, J.M.; Matthijs, H.C.P.; Huisman, J. Rapid adaptation of harmful cyanobacteria to rising CO2. Proc. Natl. Acad. Sci. USA 2016, 113, 9315–9320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, J.A.; Giordano, M.; Beardall, J.; Maberly, S.C. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth. Res. 2011, 109, 281–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, A.; Gómez, E.B.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Burford, M.A.; Mcneale, K.L.; Mckenzi-Smith, F.J. The role of nitrogen in promoting the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir. Freshw. Biol. 2006, 5111, 2143–2153. [Google Scholar] [CrossRef] [Green Version]
- Chonudomkul, D.; Yongmanitchai, W.; Theeragool, G.; Kawachi, M.; Kasai, F.; Kaya, K.; Watanabe, M.M. Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii Nostocales, Cyanobacteria strains from Thailand and Japan. FEMS Microbiol. Ecol. 2004, 483, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Antunes, J.; Leão, P.; Vasconcelos, V. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, D.P.; Pantorno, A.; Orr, P.T.; Stojkovic, S.; Beardall, J. The impacts of a high CO2 environment on a bicarbonate user: The cyanobacterium Cylindrospermopsis raciborskii. Water Res. 2012, 465, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Vilar, M.C.P.; Molica, R.J.R. Changes in pH and dissolved inorganic carbon in water affect the growth, saxitoxins production and toxicity of the cyanobacterium Raphidiopsis raciborskii ITEP-A1. Harmful Algae 2020, 97, 101870. [Google Scholar] [CrossRef]
- Bonilla, S.; Aubriot, L.; Soares, M.C.S.; Gonzalez-Piana, M.; Fabre, A.; Huszar, V.L.; Lürling, M.; Antoniades, D.; Padisák, J.; Kruk, C. What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiol. Ecol. 2012, 793, 594–607. [Google Scholar] [CrossRef]
- Wiese, M.; D’agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drug 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [Green Version]
- Scarlett, K.R.; Kim, S.; Lovin, L.M.; Chatterjee, S.; Scott, J.T.; Brooks, B.W. Global scanning of cylindrospermopsin: Critical review and analysis of aquatic occurrence, bioaccumulation, toxicity and health hazards. Sci. Total Environ. 2020, 738, 139807. [Google Scholar] [CrossRef]
- Lagos, N.; Onodera, H.; Zagatto, P.A.; Andrinolo, D.; Azevedo, S.M.; Oshima, Y. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon 1999, 3710, 1359–1373. [Google Scholar] [CrossRef]
- Molica, R.; Onodera, H.; García, C.; Rivas, M.; Andrinolo, D.; Nascimento, S.; Meguro, H.; Oshima, Y.; Azevedo, S.M.F.O.; Lagos, N. Toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii Cyanophyceae isolated from Tabocas reservoir in Caruaru, Brazil, including demonstration of a new saxitoxin analogue. Phycologia 2002, 41, 606–611. [Google Scholar] [CrossRef]
- Lorenzi, A.S.; Cordeiro-Araújo, M.K.; Chia, M.A.; Bittencourt-Oliveira, M.C. Cyanotoxin contamination of semiarid drinking water supply reservoirs. Environ. Earth Sci. 2018, 77, 1–8. [Google Scholar] [CrossRef]
- Abreu, V.; Popin, R.V.; Alvarenga, D.O.; Schaker, P.D.C.; Hoff-Risseti, C.; Varani, A.M.; Fiore, M.F. Corrigendum: Genomic and genotypic characterization of Cylindrospermopsis raciborskii: Toward an intraspecific phylogenetic evaluation by comparative genomics. Front. Microbiol. 2018, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Pierangelini, M.; Stojkovic, S.; Orr, P.T.; Beardall, J. Elevated CO2 causes changes in the photosynthetic apparatus of a toxic cyanobacterium, Cylindrospermopsis raciborskii. J. Plant Physiol. 2014, 17112, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Pierangelini, M.; Sinha, R.; Willis, A.; Buford, M.A.; Orr, P.T.; Beardall, J.; Neilan, B.A. Constitutive cylindrospermopsin pool size in Cylindrospermopsis raciborskii under different light and CO2 partial pressure conditions. J. Appl. Environ. Microbiol. 2015, 819, 3069–3076. [Google Scholar] [CrossRef] [Green Version]
- Willis, A.; Chuang, A.W.; Orr, P.T.; Beardall, J.; Burford, M.A. Subtropical freshwater phytoplankton show a greater response to increased temperature than to increased pCO2. Harmful Algae 2019, 90, 101705. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.M.; Ger, K.A.; Silva, L.H.; Soares, M.C.S.; Faassen, E.J.; Lürling, M. Toxicity overrides morphology on Cylindrospermopsis raciborskii grazing resistance to the calanoid copepod Eudiaptomus gracilis. Microb. Ecol. 2016, 71, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Vilar, M.C.; Rodrigues, T.F.; Silva, L.O.; Pacheco, A.B.F.; Ferrão-Filho, A.S.; Azevedo, S.M. Ecophysiological aspects and sxt genes expression underlying induced chemical defense in STX-producing Raphidiopsis raciborskii (cyanobacteria) against the zooplankter Daphnia gessneri. Toxins 2021, 13, 406. [Google Scholar] [CrossRef]
- Marinho, M.M.; Souza, M.B.G.; Lürling, M. Light and phosphate competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa is strain dependent. Microb. Ecol. 2013, 66, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Gorham, P.; McLachlan, J.; Hammer, U.T.; Ki, W.K. Isolation and culture of toxic strains of Anabaena flos-aquae lyngb. Bréb SIL Proc. 1964, 15, 796. [Google Scholar]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; 435p. [Google Scholar]
- Robinson, M.D.; Smyth, G.K. Small sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 2008, 9, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Baggerly, K.A.; Deng, L.; Morris, J.S.; Aldaz, C.M. Differential expression in SAGE: Accounting for normal between-library variation. Bioinformatics 2003, 19, 1477–1483. [Google Scholar] [CrossRef]
- Oshima, Y. Postcolumn derivatization liquid chromatographic method for paralytic shellfish toxins. J. AOAC Int. 1995, 782, 528–532. [Google Scholar] [CrossRef]
- Willis, A.; Chuang, A.W.; Woodhouse, J.N.; Neilan, B.A.; Burford, M.A. Intraspecific variation in growth, morphology and toxin quotas for the cyanobacterium, Cylindrospermopsis raciborskii. Toxicon 2016, 119, 307–310. [Google Scholar] [CrossRef]
- Saker, M.L.; Neilan, B.A. Varied diazotrophies, morphologies, and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia. Appl. Environ. Microbiol. 2001, 67, 1839–1845. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.; Rocap, G.; Chisholm, S.W. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature 1998, 393, 464–467. [Google Scholar] [CrossRef]
- Ivars-Martinez, E.; Martin-Cuadrado, A.B.; D’Auria, G.; Mira, A.; Ferriera, S.; Johnson, J.; Friedman, R.; Rodriguez-Valera, F. Comparative genomics of two ecotypes of the marine planktonic copiotroph Alteromonas macleodii suggests alternative lifestyles associated with different kinds of particulate organic matter. ISME J. 2008, 2, 1194–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, C.A.; Morris, R.; Parsons, R.; Treusch, A.H.; Giovannoni, S.J.; Vergin, K. Seasonal dynamics of SAR11 populations in the euphotic and mesopelagic zones of the northwestern Sargasso Sea. ISME J. 2009, 3, 83–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, D.V.N.; Pacheco, A.B.F.; Goulart, C.L.; Azevedo, S.M.F.O. Physiological responses of Raphidiopsis raciborskii (Cyanobacteria) strains to water conductivity: Effect of sodium and magnesium ions. Hydrobiologia 2020, 847, 2449–2464. [Google Scholar] [CrossRef]
- Sandrini, G.; Cunsolo, S.; Schuurmans, J.M.; Matthijs, H.C.; Huisman, J. Changes in gene expression, cell physiology and toxicity of the harmful cyanobacterium Microcystis aeruginosa at elevated CO2. Front. Microbiol. 2015, 6, 401. [Google Scholar] [CrossRef]
- Sinha, R.; Pearson, L.A.; Davis, T.W.; Buford, M.A.; Orr, P.T.; Neilan, B.A. Increased incidence of Cylindrospermopsis raciborskii in temperate zones–is climate change responsible? Water Res. 2012, 465, 1408–1419. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Domis, L.S.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 465, 1349–1363. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R.; Campbell, J.; Craft, J.D.; Langdon, C. Algal chemical ecology in a changing ocean. Planta Med. 2014, 80, IL11. [Google Scholar] [CrossRef]
- Ma, J.; Wang, P. Effects of rising atmospheric CO2 levels on physiological response of cyanobacteria and cyanobacterial bloom development: A review. Sci. Total Environ. 2021, 754, 141889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
