Milk Fat Depression in Dairy Cattle: Etiology, Prevention, and Recovery Approaches
Simple Summary
Abstract
1. Introduction
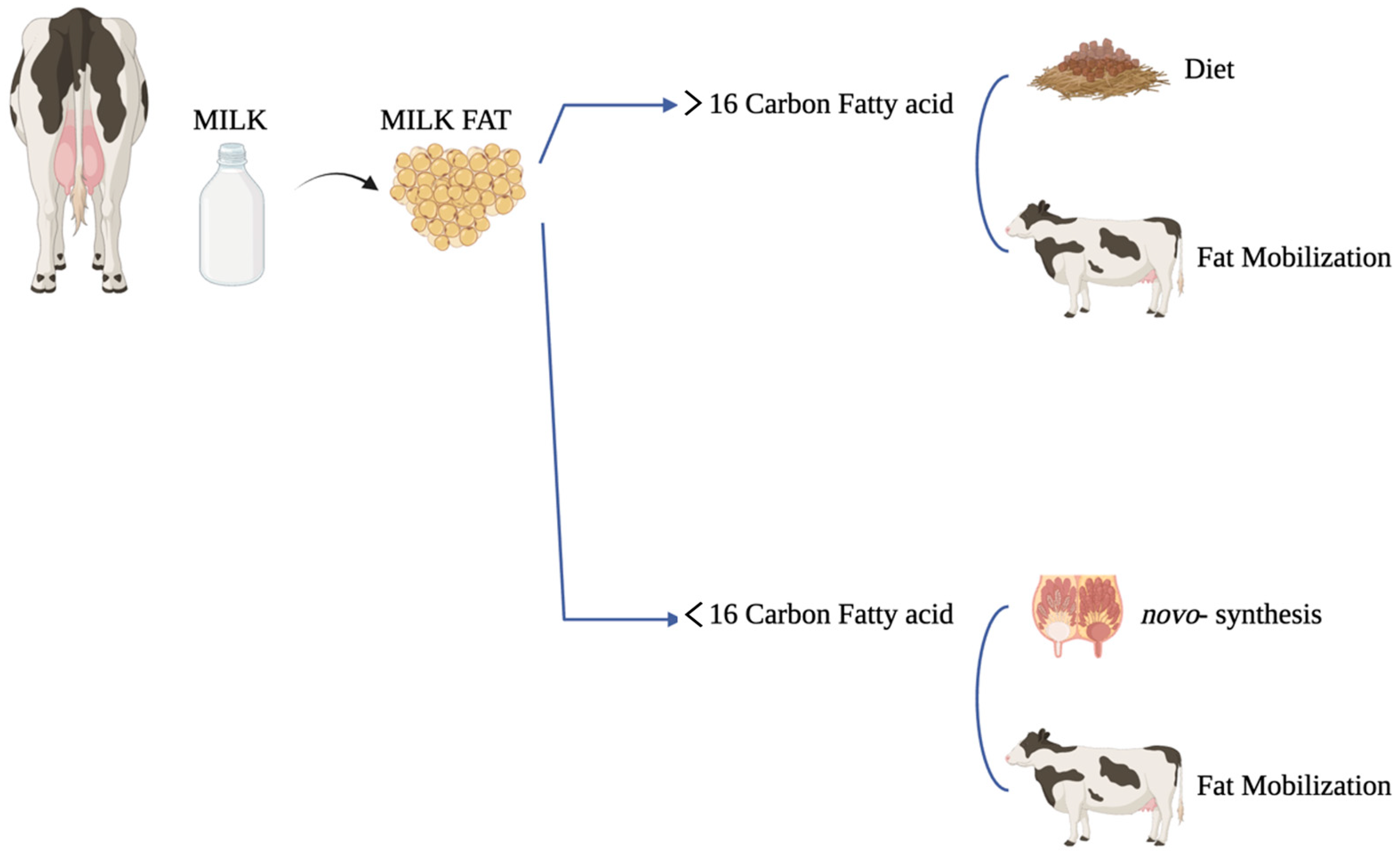
2. Milk Fat
3. Milk Fat Depression Syndrome
- Metabolic stressors, including the onset of lactation, inflammation, immune activation, and oxidative stress. Genetic background may further modulate these metabolic responses [42].
- Nutritional stressors, such as high-starch or high-oil diets.
- Non-nutritional stressors, including thermal stress (heat or cold), housing conditions, regrouping, and feeding behavior [20].
3.1. Theories on MFD Syndrome
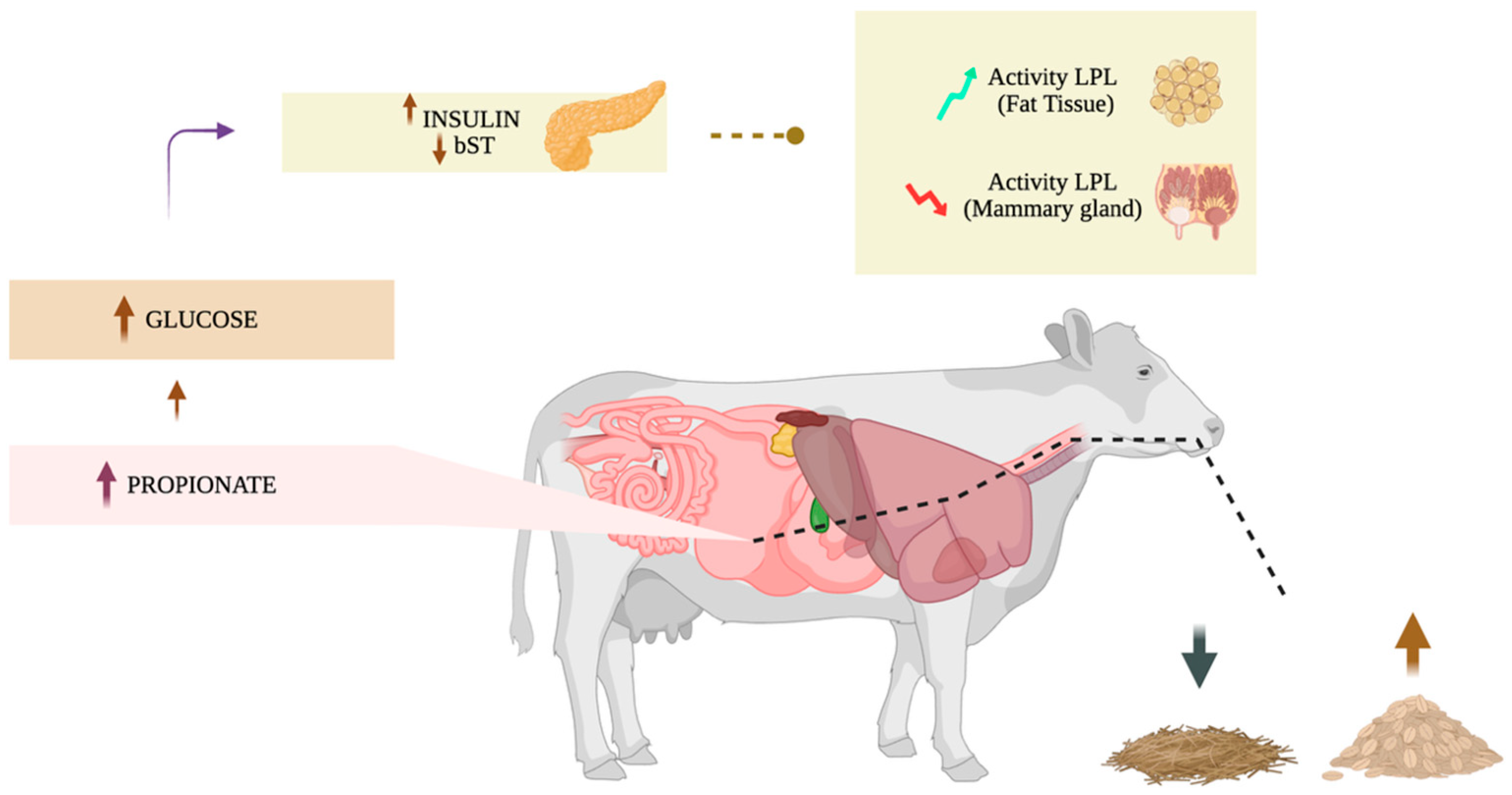
3.1.1. Milk Fat Depletion: Glucogenic Theory
3.1.2. Substances with an Inhibitory Capacity for Milk Fat Synthesis in the Mammary Gland: Trans Fatty Acid Theory
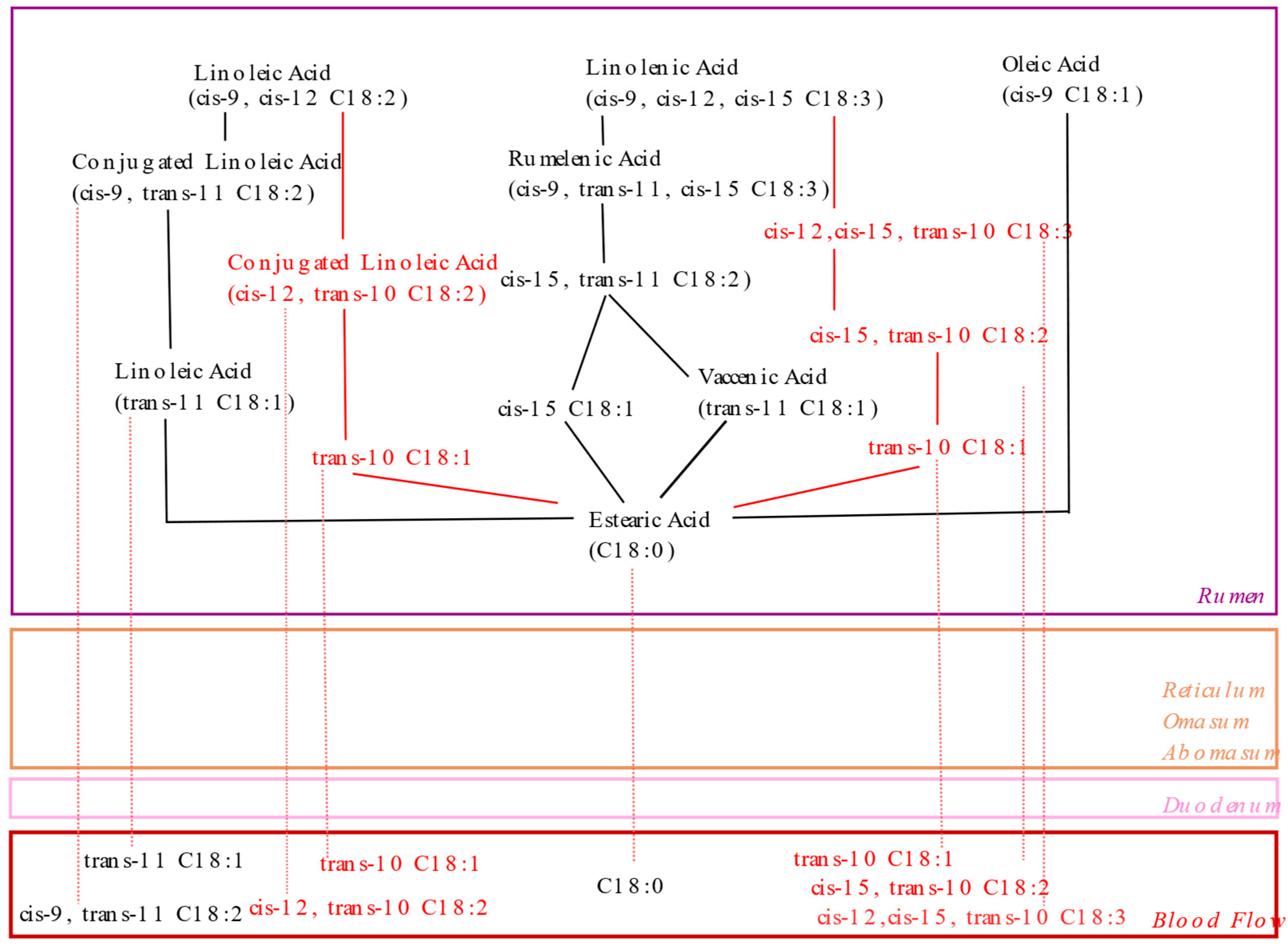
3.1.3. Substances with an Inhibitory Capacity for Milk Fat Synthesis in the Mammary Gland: Biohydrogenation Theory
Biohydrogenation Intermediates Associated with MFD: Trans-10, Cis 12 CLA
Biohydrogenation Intermediates Associated with MFD: Trans-10 18:1
Biohydrogenation Intermediates Associated with MFD: Cis-10, Trans-12 CLA
Biohydrogenation Intermediates Associated with MFD: Trans-9, Cis-11 CLA
4. Methods to Avoid and Mitigate MFD Syndrome
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization/Food Agricultural Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation, Geneva, 28 January–1 February 2002. 2003. Available online: https://www.who.int/publications/i/item/924120916X (accessed on 17 June 2025).
- Ventto, L.; Leskinen, H.; Kairenius, P.; Stefański, T.; Bayat, A.R.; Vilkki, J.; Shingfield, K.J. Diet-induced milk fat depression is associated with alterations in ruminal biohydrogenation pathways and formation of novel fatty acid intermediates in lactating cows. Br. J. Nutr. 2017, 117, 364–376. [Google Scholar] [CrossRef]
- Ferlay, A.; Doreau, M.; Chilliard, Y.; Bauchart, D. Ruminant milk fat plasticity: Nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Priyashantha, H.; Vidanarachchi, J.K.; Kiani, A.; Holman, B.W.B. Effects of nutritional factors on fat content, fatty acid composition, and sensorial properties of meat and milk from domesticated ruminants: An overview. Animals 2024, 14, 840. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of fatty acids in milk fat and the influence of selected factors on their variability—A review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Timlin, M.; Tobin, J.T.; Brodkorb, A.; Murphy, E.G.; Dillon, P.; Hennessy, D.; O’Donovan, M.; Pierce, K.M.; O’Callaghan, T.F. The impact of seasonality in pasture-based production systems on milk composition and functionality. Foods 2021, 10, 607. [Google Scholar] [CrossRef]
- Dewanckele, L.; Toral, P.G.; Vlaeminck, B.; Fievez, V. Invited review: Role of rumen biohydrogenation intermediates and rumen microbes in diet-induced milk fat depression: An update. J. Dairy Sci. 2020, 103, 7655–7681. [Google Scholar] [CrossRef]
- Atalay, A.B.; Atalay, A.I. Determination of fat depression levels in cow milk obtained from Edirne and Tekirdağ provinces in May. J. Agric. 2022, 5, 2. [Google Scholar] [CrossRef]
- Bailey, K.W.; Jones, C.M.; Heinrichs, A.J. Economic returns to Holstein and Jersey herds under multiple component pricing. J. Dairy Sci. 2005, 88, 2269–2280. [Google Scholar] [CrossRef]
- Rico, D.E.; Harvatine, K.J. Induction of and recovery from milk fat depression occurs progressively in dairy cows switched between diets that differ in fiber and oil concentration. J. Dairy Sci. 2013, 96, 6621–6630. [Google Scholar] [CrossRef]
- Leskinen, H.; Ventto, L.; Kairenius, P.; Shingfield, K.J.; Vilkki, J. Temporal changes in milk fatty acid composition during diet-induced milk fat depression in lactating cows. J. Dairy Sci. 2019, 102, 5148–5160. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th revised ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Parodi, P. Milk fat in human nutrition. Aust. J. Dairy Technol. 2004, 59, 3–59. [Google Scholar]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.; Messens, K.; Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef] [PubMed]
- MacGibbon, A.K.H.; Taylor, M.W. Composition and Structure of Bovine Milk Lipids. In Advanced Dairy Chemistry Volume 2 Lipids, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2006; pp. 1–42. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Valeur, J.; Aas, A.-M. Short-chain fatty acids as a link between diet and cardiometabolic risk: A narrative review. Lipids Health Dis. 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Lock, A.L.; Bauman, D.E. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 2004, 39, 1197–1206. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef]
- Razzaghi, A.; Ghaffari, M.H.; Rico, D.E. The impact of environmental and nutritional stresses on milk fat synthesis in dairy cows. Domest. Anim. Endocrinol. 2023, 83, 106784. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Beaulieu, A.D.; Barbano, D.M. Feed and animal factors influencing milk fat composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Månsson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Bauman, D.E.; Griinari, J.M. Regulation and nutritional manipulation of milk fat: Low-fat milk syndrome. Livest. Prod. Sci. 2001, 70, 15–29. [Google Scholar] [CrossRef]
- de Vries, M.J.; Veerkamp, R.F. Energy balance of dairy cattle in relation to milk production variables and fertility. J. Dairy Sci. 2000, 83, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bruckmaier, R.M. Milk ejection during machine milking in dairy cows. Livest. Prod. Sci. 2001, 70, 121–124. [Google Scholar] [CrossRef]
- Rivero, M.J. Milk fat depression syndrome and the particular case of grazing cows: A review. Acta Agric. Scand. A Anim. Sci. 2015, 65, 1–10. [Google Scholar] [CrossRef]
- Vafa, T.S.; Naserian, A.A.; Heravi Moussavi, A.R.; Valizadeh, R.; Mesgaran, M.D. Effect of supplementation of fish and canola oil in the diet on milk fatty acid composition in early lactating Holstein cows. Asian-Australas. J. Anim. Sci. 2012, 25, 311–319. [Google Scholar] [CrossRef]
- Altenhofer, C.; Spornraft, M.; Kienberger, H.; Rychlik, M.; Herrmann, J.; Meyer, H.H.; Viturro, E. Effects of rapeseed and soybean oil dietary supplementation on bovine fat metabolism, fatty acid composition and cholesterol levels in milk. J. Dairy Res. 2014, 81, 120–128. [Google Scholar] [CrossRef]
- Alves, S.P.; Bessa, R.J.B. The trans-10,cis-15 18:2: A missing intermediate of trans-10 shifted rumen biohydrogenation pathway? Lipids 2014, 49, 527–541. [Google Scholar] [CrossRef]
- Dewanckele, L.; Jing, L.; Stefańska, B.; Vlaeminck, B.; Jeyanathan, J.; Van Straalen, W.M.; Koopmans, A.; Fievez, V. Distinct blood and milk 18-carbon fatty acid proportions and buccal bacterial populations in dairy cows differing in reticulorumen pH response to dietary supplementation of rapidly fermentable carbohydrates. J. Dairy Sci. 2019, 102, 4025–4040. [Google Scholar] [CrossRef]
- Ferlay, A.; Chilliard, Y. Effect of linseed, sunflower, or fish oil added to hay- or corn silage-based diets on milk fat yield and trans-C18:1 and conjugated linoleic fatty acid content in bovine milk fat. Livest. Sci. 2020, 235, 104005. [Google Scholar] [CrossRef]
- Davis, C.L.; Brown, R.E. Low-fat milk syndrome. In Physiology of Digestion and Metabolism in the Ruminant, Proceedings of the Third International Symposium, Cambridge, UK, 8–12 August 1969; National Dairy Council: Chicago, IL, USA, 1970; pp. 545–565. [Google Scholar]
- Gaynor, P.J.; Waldo, D.R.; Capuco, A.V.; Erdman, R.A.; Douglass, L.W.; Teter, B.B. Milk fat depression, the glucogenic theory, and trans-C18:1 fatty acids. J. Dairy Sci. 1995, 78, 2008–2015. [Google Scholar] [CrossRef]
- Emery, R.S. Milk fat depression and the influence of diet on milk composition. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Garnsworthy, P.C. The influence of nutrient balance on milk yield and composition. In Nutrition and Lactation in the Dairy Cow; Garnsworthy, P.C., Ed.; Elsevier: London, UK, 1988; pp. 97–119. [Google Scholar]
- Sutton, J.D. Altering milk composition by feeding. J. Dairy Sci. 1989, 72, 2801–2814. [Google Scholar] [CrossRef]
- Gallardo, M. El inicio de la primavera en el tambo: ¿Por qué baja la grasa en leche? In Proyecto Nacional de Lechería INTA; EEA Rafaela: Santa Fe, Argentina, 2002; Available online: https://www.produccion-animal.com.ar/produccion_bovina_de_leche/produccion_bovina_leche/33_baja_produccion_grasa.pdf (accessed on 9 July 2025).
- Gallardo, M. Importancia de la fibra en otoño. La Chacra Supl. Tambo 1999, 2, 1–4. Available online: https://www.produccion-animal.com.ar/informacion_tecnica/manejo_del_alimento/35-importancia_de_la_fibra_en_otono.pdf (accessed on 9 July 2025).
- Bauman, D.E. Update on milk fat: Identification of rumen biohydrogenation intermediates that inhibit synthesis. In Proceedings of the Cornell Nutrition Conference for Feed Manufacturers, Syracuse, NY, USA, 18–20 October 2006; pp. 59–65. Available online: https://www.academia.edu/21296352/ (accessed on 6 July 2025).
- Bauman, D.E. Milk Fat Synthesis: The Role of the Mammary Gland and Nutritional Regulation. Proceedings of the 3rd Strategic Dairy Nutrition Conference, Ontario, Canada, 25–26 April 2006; Available online: http://www.dairyweb.ca/Resources/3SDNC2006/Bauman.pdf (accessed on 9 July 2025).
- Cornell University. Palmitic Acid and Milk Fat; Cornell eCommons: Ithaca, NY, USA, 2021; Available online: https://ecommons.cornell.edu/server/api/core/bitstreams/b50fface-6a58-46fd-97d7-8968f41080a6/content (accessed on 5 May 2024).
- Palmquist, D.; Harvatine, K. Origin of Fatty Acids and Influence of Nutritional Factors on Milk Fat. In Origin of Fatty Acids and Influence of Nutritional Factors on Milk Fat; Springer: Cham, Switzerland, 2020; pp. 33–66. [Google Scholar] [CrossRef]
- Harvatine, K.J.; Boisclair, Y.R.; Bauman, D.E. Recent advances in the regulation of milk fat synthesis. Animal 2009, 3, 40–54. [Google Scholar] [CrossRef]
- Gama, M.A.S.; Garnsworthy, P.C.; Griinari, J.M.; Leme, P.R.; Rodrigues, P.H.M.; Souza, L.W.O.; Lanna, D.P.D. Diet-induced milk fat depression: Association with changes in milk fatty acid composition and fluidity of milk fat. Livest. Sci. 2008, 115, 319–331. [Google Scholar] [CrossRef]
- Peterson, D.G.; Matitashvili, E.A.; Bauman, D.E. Diet-induced milk fat depression in dairy cows results in increased trans-10, cis-12 CLA in milk fat and coordinate suppression of mRNA abundance for mammary enzymes involved in milk fat synthesis. J. Nutr. 2003, 133, 3098–3102. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Reynolds, C.K.; Hervás, G.; Griinari, J.M.; Grandison, A.S.; Beever, D.E. Examination of the persistency of milk fatty acid composition responses to fish oil and sunflower oil in the diet of dairy cows. J. Dairy Sci. 2006, 89, 714–732. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Corl, B.A.; Dwyer, D.A.; Saebø, A.; Bauman, D.E. Identification of the conjugated linoleic acid isomer that inhibits milk fat synthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R179–R184. [Google Scholar] [CrossRef]
- Onetti, S.G.; Reynal, S.M.; Grummer, R.R. Effect of alfalfa forage preservation method and particle length on performance of dairy cows fed corn silage-based diets and tallow. J. Dairy Sci. 2004, 87, 652–664. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Hornick, J.L.; Chentouf, M.; Cabaraux, J.F. Effects of sulla flexuosa hay as alternative feed resource on goat’s milk production and quality. Animals 2023, 13, 709. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, C.; Sun, D.; Seddik, H.; Mao, S. The ruminal microbiome and metabolome alterations associated with diet-induced milk fat depression in dairy cows. Metabolites 2019, 9, 7. [Google Scholar] [CrossRef]
- Zou, C.; Gu, Q.; Zhou, X.; Xia, Z.; Muhammad, W.I.; Tang, Q.; Liang, M.; Lin, B.; Qin, G. Ruminal microbiota composition associated with ruminal fermentation parameters and milk yield in lactating buffalo in Guangxi, China-A preliminary study. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1374–1379. [Google Scholar] [CrossRef]
- Kepler, C.R.; Hirons, K.P.; McNeill, J.J.; Tove, S.B. Intermediates and products of the biohydrogenation of linoleic acid by Butyrivibrio fibrisolvens. J. Biol. Chem. 1966, 241, 1350–1354. [Google Scholar] [CrossRef]
- Verhulst, A.; Janssen, G.; Parmentier, G.; Eyssen, H. Isomerization of polyunsaturated long chain fatty acids by propionibacteria. Syst. Appl. Microbiol. 1987, 9, 12–15. [Google Scholar] [CrossRef]
- Xue, M.; Sun, H.; Wu, X.; Guan, L.L.; Liu, J. Assessment of rumen microbiota from a large dairy cattle cohort reveals the pan and core bacteriomes contributing to varied phenotypes. Appl. Environ. Microbiol. 2018, 84, e00970-18. [Google Scholar] [CrossRef]
- Buitenhuis, B.; Lassen, J.; Noel, S.J.; Plichta, D.R.; Sørensen, P.; Difford, G.F.; Poulsen, N.A. Impact of the rumen microbiome on milk fatty acid composition of Holstein cattle. Genet. Sel. Evol. 2019, 51, 23. [Google Scholar] [CrossRef]
- Wang, L.; Liu, K.; Wang, Z.; Bai, X.; Peng, Q.; Jin, L. Bacterial community diversity associated with different utilization efficiencies of nitrogen in the gastrointestinal tract of goats. Front. Microbiol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Xue, M.-Y.; Sun, H.-Z.; Wu, X.-H.; Liu, J.-X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Si, B.; Liu, K.; Huang, G.; Chen, M.; Yang, J.; Wu, X.; Li, N.; Tang, W.; Zhao, S.; Zheng, N.; et al. Relationship between rumen bacterial community and milk fat in dairy cows. Front. Microbiol. 2023, 14, 1247348. [Google Scholar] [CrossRef]
- McClymont, G.L.; Vallance, S. Depression of blood glycerides and milk fat synthesis by glucose infusion. Proc. Aust. Soc. Anim. Prod. 1962, 21, xi. [Google Scholar]
- Lanier, J.S.; Corl, B.A. Challenges in enriching milk fat with polyunsaturated fatty acids. J. Anim. Sci. Biotechnol. 2015, 6, 26. [Google Scholar] [CrossRef]
- Matamoros, C.; Klopp, R.N.; Moraes, L.E.; Harvatine, K.J. Meta-analysis of the relationship between milk trans-10 C18:1, milk fatty acids <16 C, and milk fat production. J. Dairy Sci. 2020, 103, 10195. [Google Scholar] [CrossRef]
- Ba, C.; Ba, L.H.; Da, D.; Griinari, J.M.; Pariza, M.W.; Bauman, D.E. The role of Delta(9)-desaturase in the production of cis-9, trans-11 CLA. J. Nutr. Biochem. 2001, 12, 622–630. [Google Scholar] [CrossRef]
- Griinari, J.M.; Dwyer, D.A.; McGuire, M.A.; Bauman, D.E.; Palmquist, D.L.; Nurmela, K.V.V. Trans-Octadecenoic Acids and Milk Fat Depression in Lactating Dairy Cows. J. Dairy Sci. 1998, 81, 1251–1261. [Google Scholar] [CrossRef]
- Peterson, D.G.; Waldo, D.R.; Erdman, R.A.; Teter, B.B.; Bauman, D.E. Mammary lipogenic enzyme activity, trans fatty acids and conjugated linoleic acids are altered in lactating dairy cows fed a milk fat-depressing diet. J. Nutr. 2000, 130, 2568–2574. [Google Scholar] [CrossRef]
- Offer, N.W.; Marsden, M.; Phipps, R.H. Effect of oil supplementation of a diet containing a high concentration of starch on levels of trans fatty acids and conjugated linoleic acids in bovine milk. Anim. Sci. 2001, 73, 533–540. [Google Scholar] [CrossRef]
- Loor, J.J.; Herbein, J.H. Reduced Fatty Acid Synthesis and Desaturation Due to Exogenous trans-10, cis-12-CLA in Cows Fed Oleic or Linoleic Oil. J. Dairy Sci. 2003, 86, 1354–1369. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Bernard, L.; Belenguer, A.; Rouel, J.; Hervás, G.; Chilliard, Y.; Frutos, P. Comparison of ruminal lipid metabolism in dairy cows and goats fed diets supplemented with starch, plant oil, or fish oil. J. Dairy Sci. 2016, 99, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Sangster, J.K.; Bauman, D.E. Milk Fat Synthesis in Dairy Cows Is Progressively Reduced by Increasing Supplemental Amounts of trans-10, cis-12 Conjugated Linoleic Acid (CLA). J. Nutr. 2001, 131, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Säfers, S.; von Soosten, D.; Meyer, U.; Drong, C.; Frahm, J.; Kluess, J.; Raschka, C.; Rehage, J.; Tröscher, A.; Pelletier, W.; et al. Influence of conjugated linoleic acid and vitamin E on performance, energy metabolism, and change of fat depot mass in transitional dairy cows. J. Dairy Sci. 2017, 100, 3193–3208. [Google Scholar] [CrossRef]
- Bayat, A.R.; Razzaghi, A.; Sari, M.; Kairenius, P.; Tröscher, A.; Trevisi, E.; Vilkki, J. The effect of dietary rumen-protected trans-10,cis-12 conjugated linoleic acid or a milk fat-depressing diet on energy metabolism, inflammation, and oxidative stress of dairy cows in early lactation. J. Dairy Sci. 2022, 105, 3032–3048. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Griinari, J.M. Role of biohydrogenation intermediates in milk fat depression. Eur. J. Lipid Sci. Technol. 2007, 109, 799–816. [Google Scholar] [CrossRef]
- Lock, A.L.; Tyburczy, C.; Dwyer, D.A.; Harvatine, K.J.; Destaillats, F.; Mouloungui, Z.; Candy, L.; Bauman, D.E. Trans-10 Octadecenoic Acid Does Not Reduce Milk Fat Synthesis in Dairy Cows. J. Nutr. 2007, 137, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sæbø, A.; Sæbø, P.-C.; Griinari, J.M.; Shingfield, K.J. Effect of abomasal infusions of geometric isomers of 10,12 conjugated linoleic acid on milk fat synthesis in dairy cows. Lipids 2005, 40, 823–832. [Google Scholar] [CrossRef]
- Perfield, J.W.; Lock, A.L.; Griinari, J.M.; Sæbø, A.; Delmonte, P.; Dwyer, D.A.; Bauman, D.E. Trans-9, cis-11 conjugated linoleic acid reduces milk fat synthesis in lactating dairy cows. J. Dairy Sci. 2007, 90, 2211–2218. [Google Scholar] [CrossRef]
- Maxin, G.; Rulquin, H.; Glasser, F. Response of milk fat concentration and yield to nutrient supply in dairy cows. Animal 2011, 5, 1299–1310. [Google Scholar] [CrossRef]
- Boivin, M.; Gervais, R.; Chouinard, P.Y. Effect of grain and forage fractions of corn silage on milk production and composition in dairy cows. Animal 2013, 7, 245–254. [Google Scholar] [CrossRef]
- Razzaghi, A.; Vakili, A.R.; Khorrami, B.; Ghaffari, M.H.; Rico, D.E. Effect of dietary supplementation or cessation of magnesium-based alkalizers on milk fat output in dairy cows under milk fat depression conditions. J. Dairy Sci. 2022, 105, 2275–2287. [Google Scholar] [CrossRef]
- Ding, L.; Shen, Y.; Jawad, M.; Wu, T.; Maloney, S.K.; Wang, M.; Chen, N.; Blache, D. Effect of arginine supplementation on the production of milk fat in dairy cows. J. Dairy Sci. 2022, 105, 8115–8129. [Google Scholar] [CrossRef]
- Razzaghi, A.; Malekkhahi, M.; Valizadeh, R.; Parand, E.; Bayat, A.-R. Modulation of ruminal pH, milk fat secretion, and biohydrogenation intermediates by alkalizing agents in dairy cows fed starch-rich diets. Livest. Sci. 2021, 248, 104485. [Google Scholar] [CrossRef]
- AlZahal, O.; Odongo, N.E.; Mutsvangwa, T.; Or-Rashid, M.M.; Duffield, T.F.; Bagg, R.; Dick, P.; Vessie, G.; McBride, B.W. Effects of monensin and dietary soybean oil on milk fat percentage and milk fatty acid profile in lactating dairy cows. J. Dairy Sci. 2008, 91, 1166–1174. [Google Scholar] [CrossRef]
- CimaVet. CIMA-Vet: KEXXTONE 32,4 g DISPOSITIVO INTRARRUMINAL DE LIBERACION CONTINUA PARA BOVINO. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Available online: https://cimavet.aemps.es/cimavet/publico/detalle.html?nregistro=EU/2/12/145/001 (accessed on 10 June 2025).
- Pottier, J.; Focant, M.; Debier, C.; De Buysser, G.; Goffe, C.; Mignolet, E.; Froidmont, E.; Larondelle, Y. Effect of Dietary Vitamin E on Rumen Biohydrogenation Pathways and Milk Fat Depression in Dairy Cows Fed High-Fat Diets. J. Dairy Sci. 2006, 89, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, H.M.M.; Santos, J.; Casal, S.; Alves, M.R.; Oliveira, M.B.P.P. Fat-soluble vitamin (A, D, E, and β-carotene) contents from a Portuguese autochthonous cow breed—Minhota. J. Dairy Sci. 2012, 95, 5476–5484. [Google Scholar] [CrossRef] [PubMed]
- Donkin, S.S.; Varga, G.A.; Sweeney, T.F.; Muller, L.D. Rumen-protected methionine and lysine: Effects on animal performance, milk protein yield, and physiological measures. J. Dairy Sci. 1989, 72, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; He, T.; Wan, X.; Liu, S.; Dong, Y.; Qu, Y. Meta-analysis of rumen-protected methionine in milk production and composition of dairy cows. Animals 2022, 12, 1505. [Google Scholar] [CrossRef]
| Rumen Environment Alteration | PUFAs |
|---|---|
| Low pH/low peNDF | Amount ingested (specially of linoleic acid (C18:2)) |
| Feed particle size | Availability |
| Fiber (amount and quality) | PUFA/SFA ratio |
| Starch (non-structural carbohydrates) | Feeding pattern |
| Rumensin (monensin) 2 | Variation in fat content and FA composition in feed |
| Feeding pattern |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, E.N.; Muíño, R.; Hernández Bermúdez, J.; Díaz González, L.; Benedito, J.L.; Castillo, C. Milk Fat Depression in Dairy Cattle: Etiology, Prevention, and Recovery Approaches. Ruminants 2025, 5, 38. https://doi.org/10.3390/ruminants5030038
Martínez EN, Muíño R, Hernández Bermúdez J, Díaz González L, Benedito JL, Castillo C. Milk Fat Depression in Dairy Cattle: Etiology, Prevention, and Recovery Approaches. Ruminants. 2025; 5(3):38. https://doi.org/10.3390/ruminants5030038
Chicago/Turabian StyleMartínez, Elena Niceas, Rodrigo Muíño, Joaquín Hernández Bermúdez, Lucia Díaz González, Jose Luis Benedito, and Cristina Castillo. 2025. "Milk Fat Depression in Dairy Cattle: Etiology, Prevention, and Recovery Approaches" Ruminants 5, no. 3: 38. https://doi.org/10.3390/ruminants5030038
APA StyleMartínez, E. N., Muíño, R., Hernández Bermúdez, J., Díaz González, L., Benedito, J. L., & Castillo, C. (2025). Milk Fat Depression in Dairy Cattle: Etiology, Prevention, and Recovery Approaches. Ruminants, 5(3), 38. https://doi.org/10.3390/ruminants5030038







