Head Sexual Characterization of Sanmartinero Creole Bovine Breed Assessed by Geometric Morphometric Methods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.3. Pictures
2.4. Geometric Morphometric Analysis
3. Results
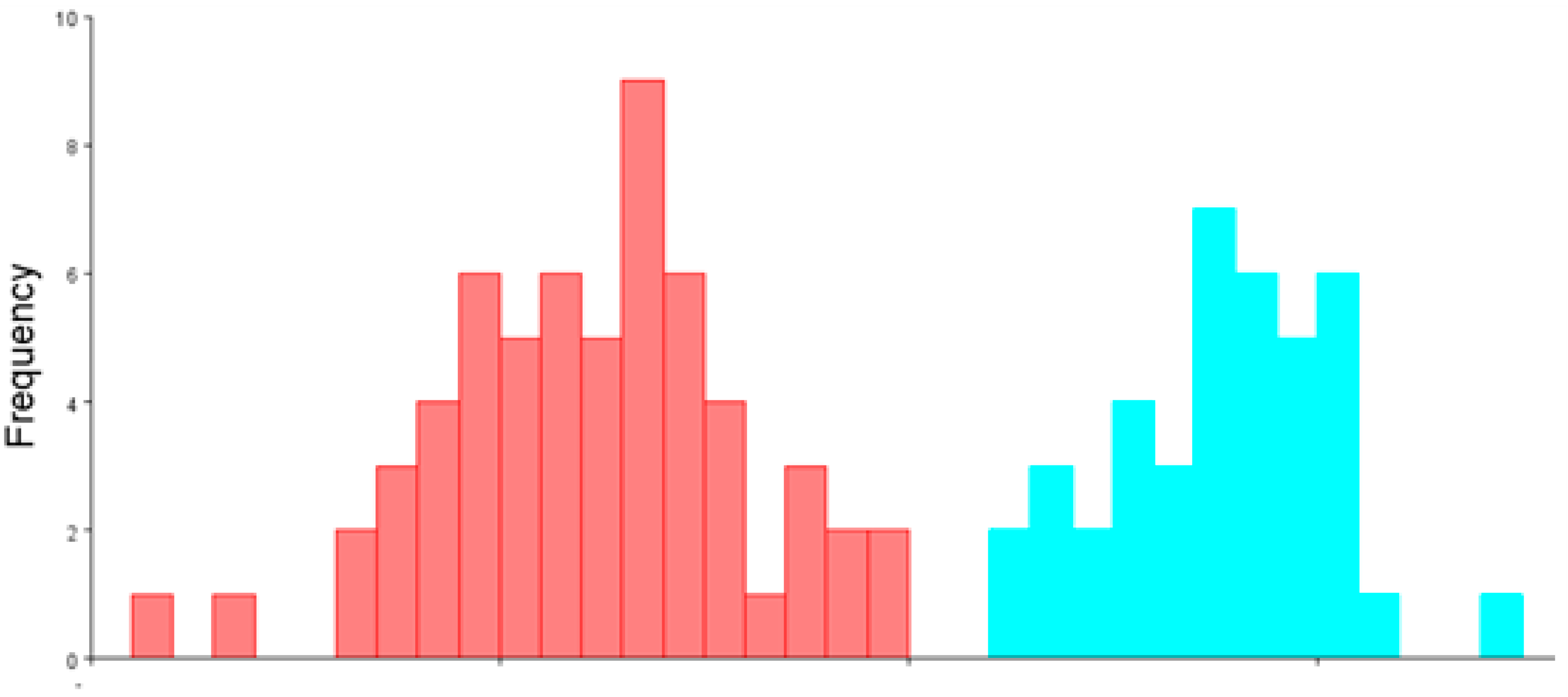
3.1. Sexual Dimorphism in Size
3.2. Allometry
3.3. Sexual Dimorphism in Shape
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, C.G.; Flórez, D.H.; Martínez-Villate, G.C. El ganado criollo colombiano Sanmartinero, su conservación y aporte sostenible a la producción bovina en la Orinoquia colombiana. Arch. Latinoam. Prod. Anim. 2020, 28, 69–86. [Google Scholar]
- Bookstein, F.L. Reworking geometric morphometrics into a methodology of transformation grids. Evol. Biol. 2023, 50, 275–299. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M. (Ed.) Introductory Chapter—Morphometric Studies: Beyond Pure Anatomical Form Analysis. In New Insights into Morphometry Studies; IntechOpen: Tokyo, Japan, 2017; Chapter 1; p. 96. [Google Scholar]
- Mitteroecker, P.; Gunz, P. Advances in Geometric morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef]
- Rohlf, F.J. The tps series of software. Hystrix It. J. Mamm. 2015, 26, 9–12. [Google Scholar]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef]
- Cooke, S.B.; Terhune, C. Form, function, and geometric morphometrics. Anat. Rec. 2015, 298, 5–28. [Google Scholar] [CrossRef]
- Lawing, A.M.; Polly, P.D. Geometric morphometrics: Recent applications to the study of evolution and development. J. Zool. 2010, 280, 1–7. [Google Scholar] [CrossRef]
- Gündemir, O.; Koungoulos, L.; Szara, T.; Duro, S.; Spataru, M.C.; Michaud, M.; Onar, V. Cranial morphology of Balkan and West Asian livestock guardian dogs. J. Anat. 2023, 243, 951–959. [Google Scholar] [CrossRef]
- Bookstein, F.L. Combining the tools of geometric morphometrics. In Advances in Morphometrics; Springer: Boston, MA USA, 1996; pp. 131–151. [Google Scholar]
- Günay, E.; Szara, T.; Çakar, B.; Deveci, E.I.; Coşkun, A.S.; Gün, G.; Yiğit, F.; Gündemir, O.; Duro, S.; Spataru, M.C. Geometric Morphometric Analysis of Sexual Dimorphism in the Bill of the White Stork (Ciconia ciconia). Animals 2025, 15, 1312. [Google Scholar] [CrossRef]
- Ağaç, K.D.; Onuk, B.; Gündemir, O.; Kabak, M.; Manuta, N.; Çakar, B.; Janeczek, M.; Crampton, D.A.; Szara, T. Comparative Cranial Geometric Morphometrics among Wistar Albino, Sprague Dawley, and WAG/Rij Rat Strains. Animals 2024, 14, 1274. [Google Scholar] [CrossRef]
- Polák, J.; Frynta, D. Patterns of sexual size dimorphism in cattle breeds support Rensch’s rule. Evol. Ecol. 2010, 24, 1255–1266. [Google Scholar] [CrossRef]
- Cordeschi, G.; Canestrelli, D.; Porretta, D. Sex-biased phenotypic plasticity affects sexual dimorphism patterns under changing environmental conditions. Sci. Rep. 2024, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Berns, C.M. The evolution of sexual dimorphism: Understanding mechanisms of sexual shape differences. In Sexual Dimorphism; Moriyama, H., Ed.; IntechOpen: Tokyo, Japan, 2013; pp. 1–15. [Google Scholar]
- Turcotte, C.M.; Mann, E.H.J.; Stock, M.K.; Villamil, C.I.; Montague, M.J.; Dickinson, E.; Surratt, S.B.; Martinez, M.; Williams, S.A.; Anton, S.C. The ontogeny of sexual dimorphism in free-ranging rhesus macaques. Am. J. Biol. Anthr. 2022, 177, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bu, R.; Ning, M.; Yang, B.; Wu, Z.; Huang, H. Sexual Dimorphism in the Chinese Endemic Species Hynobius maoershanensis (Urodela: Hynobiidae). Animals 2022, 12, 1712. [Google Scholar] [CrossRef]
- Cox, R.M.; Kahrl, A.F. Sexual Selection and Sexual Dimorphism. In Reproductive Biology and Phylogeny of Lizards and Tuatara, 1st ed.; Rheubert, J.L., Siegel, D.S., Trauth, S.E., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 78–108. [Google Scholar]
- Kaliontzopoulou, A.; Carretero, M.A.; Llorente, G.A. Sexual dimorphism in traits related to locomotion: Ontogenetic patterns of variation in Podarcis wall lizards. Biol. J. Linn. Soc. 2010, 99, 530–543. [Google Scholar] [CrossRef]
- Scharf, I.; Meiri, S. Sexual dimorphism of heads and abdomens: Different approaches to ‘being large’ in female and male lizards. Biol. J. Linn. Soc. 2013, 110, 665–673. [Google Scholar] [CrossRef]
- Chirinos, Z.; Contreras, G.; Zambrano, S.; Molero, E.; Paéz, A. Caracterizacion del dimorfismo sexual en ganado criollo limonero mediante medidas corporales. Rev. Fac. Agron. (LUZ) 2011, 28 (Suppl. S1), 554–564. [Google Scholar]
- Lang, D.R.; Hansel, W. A Sexual Dimorphism in Three Somatic Tissues of Cattle. J. Dairy Sc. 1959, 42, 1330–1337. [Google Scholar] [CrossRef]
- Çakar, B.; Tandir, F.; Can Güzel, B.; Bakıcı, C.; Ünal, B.; Duro, S.; Szara, T.; Spataru, C.; Spataru, M.-C.; Gündemir, O. Comparison of Skull Morphometric Characteristics of Simmental and Holstein Cattle Breeds. Animals 2024, 14, 2085. [Google Scholar] [CrossRef]
- Gündemir, O.; Manuta, N.; Can Güzel, B.; Bakıcı, C.; Duro, S.; Ünal, B.; de Cakar, C.; Szara, T. Skull morphology in native and non-native cattle breeds in Türkiye. J. Anat. 2025, 247, 179–188. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix Ital. J. Mammal. 2013, 24, 7–14. [Google Scholar]
- Sañudo Astiz, C. Valoración Morfologica de los Animales Domésticos. Ministerio de Medio Ambiente y Medio Rural y Marino; Artegraf, Industrias Graficas S.A: Madrid, Spain, 2009; p. 865. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Res. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST v. 4.17c. Paleontological Statisticts Software Package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–229. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer; Elsevier Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Clutton-Brock, T. Sexual selection in males and females. Science 2007, 318, 1882–1885. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Harvey, P.H.; Rudder, B. Sexual Dimorphism, Socionomic Sex Ratio and Body Weight in Primates. Nature 1977, 269, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Kamalakannan, M. Characterisations of Hair of Hoolock Gibbon Hoolock Hoolock (Harlan, 1834) (Hylobatidae: Primates: Mammalia). J. Entomol. Zool. Stud. 2017, 5, 986–988. [Google Scholar]
- Cassini, M.H. Role of Fecundity Selection on the Evolution of Sexual Size Dimorphism in Mammals. Anim. Behav. 2017, 128, 1–4. [Google Scholar] [CrossRef]
- Gündemir, O.; Szara, T.; Yalin, E.E.; Karabagli, M.; Mutlu, Z.; Yilmaz, O.; Büyükünal, S.K.; Blagojevic, M.; Parés-Casanova, P.M. Examination of Shape Variation of the Skull in British Shorthair, Scottish Fold, and Van Cats. Animals 2023, 13, 614. [Google Scholar] [CrossRef]
- Salamanca Carreño, A.; Crosby Granados, R.A. Estudio fenotípico del bovino criollo Casanare biotipo Araucano. Análsisi zoométrico. Zootec. Trop. 2013, 31, 201–208. [Google Scholar]
- Salamanca-Carreño, A.; Parés-Casanova, P.M.; Vélez Terranova, O.M.; Castro Rosa, G.; Jáuregui, R. Valoración morfométrica de una población de cerdos criollos araucanos (Colombia). Rev. Investig. Vet. Perú 2022, 33, 1–12. [Google Scholar] [CrossRef]
- Liao, W.B.; Liu, W.C.; Merilä, J. Andrew meets Rensch: Sexual size dimorphism and the inverse of Rensch’s rule in Andrew’s toad (Bufo andrewsi). Oecologia 2015, 177, 389–399. [Google Scholar] [CrossRef]
- Yan, C.; Ma, H.; Yang, Y.; Mi, Z. Sexual Dimorphism in the Limb Bones of Asiatic Toad (Bufo gargarizans) in Relation to Sexual Selection. Animals 2023, 13, 2638. [Google Scholar] [CrossRef]
- Dursun, C.; Gül, S.; Özdemir, N. Sexual size and shape dimorphism in Turkish common toads (Bufo bufo Linnaeus 1758). Anat. Record 2022, 305, 1548–1558. [Google Scholar] [CrossRef]
- Stirrat, M.; Perrett, D.I. Valid Facial Cues to Cooperation and Trust: Male Facial Width and Trustworthiness. Psychol. Sci. 2010, 21, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, R.; Mangiacotti, R.; Scali, S.; Storniolo, F.; Zuffi, M.A.L. Species-Specific Spatial Patterns of Variation in Sexual Dimorphism by Two Lizards Settled in the Same Geographic Context. Animals 2023, 13, 736. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.J.; Georgevsky, D.; Valenzuela, M.; McGreevy, P.D. A pilot study of sexual dimorphism in the head morphology of domestic dogs. J. Veter. Behav. 2014, 9, 43–46. [Google Scholar] [CrossRef]
- Sagrero-Del Moral, M.; Castañeda-Ortega, J.C. Dimorfismo sexual mediante el uso de la morfometría en Felis silvestris catus (Schreber, 1775). Rev. Dig. Act. Med. Vet. Zoot. México 2020, en línea. Available online: https://acmevez.mx/dimorfismo-sexual-mediante-el-uso-de-la-morfometria-en-felis-silvestris-catus-schreber-1775/ (accessed on 29 May 2025).
- Xiong, J.; Zhang, B.; Liu, Q.; Pan, T.; Gou, J. Sexual dimorphism in the Chinese endemic species Pachyhynobius shangchengensis Fei, Qu and Wu, 1983 (Urodela: Hynobiidae). PeerJ 2019, 7, e6408. [Google Scholar] [CrossRef]
- Aguilar-Moreno, M.; Rodríguez-Romero, F.J.; Aragón-Martínez, A.; Muñoz-Manzano, J.A.; Granados-González, G.; Hernández-Gallegos, O. Dimorfismo sexual de Aspidoscelis costata costata (Squamata: Teiidae) en el sur del Estado de México, México. Rev. Chil. Hist. Nat. 2010, 83, 585–592. [Google Scholar] [CrossRef]
- Borczyk, B.; Kusznierz, J.; Paśko, L.; Turniak, E. Scaling of the sexual size and shape skull dimorphism in the sand lizard (Lacerta agilis L.). Vertebr. Zool. 2014, 64, 221–227. [Google Scholar] [CrossRef]
- Punzalan, D.; Hosken, D.J. Sexual dimorphism: Why the sexes are (and are not) different. Curr. Biol. 2010, 20, 972–973. [Google Scholar] [CrossRef]
- Li, Y.-P.; Huang, Z.-P.; Yang, Y.; He, X.-B.; Pan, R.-L.; He, X.-M.; Yang, G.-W.; Wu, H.; Cui, L.-W.; Xiao, W. Ontogenetic Development of Sexual Dimorphism in Body Mass of Wild Black-and-White Snub-Nosed Monkey (Rhinopithecus bieti). Animals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Székely, T. Evolution of reproductive strategies: Sex roles, sex ratios and phylogenies. Biol. Futur. 2023, 74, 351–357. [Google Scholar] [CrossRef]
- Shingleton, A.W.; Vea, I.M. Sex-specific regulation of development, growth and metabolism. Semin. Cell Dev. Biol. 2023, 138, 117–127. [Google Scholar] [CrossRef]
- Milella, M.; Franklin, D.; Belcastro, M.G.; Cardini, A. Sexual differences in human cranial morphology: Is one sex more variable or one region more dimorphic? Anat. Rec. 2021, 304, 2789–2810. [Google Scholar] [CrossRef]
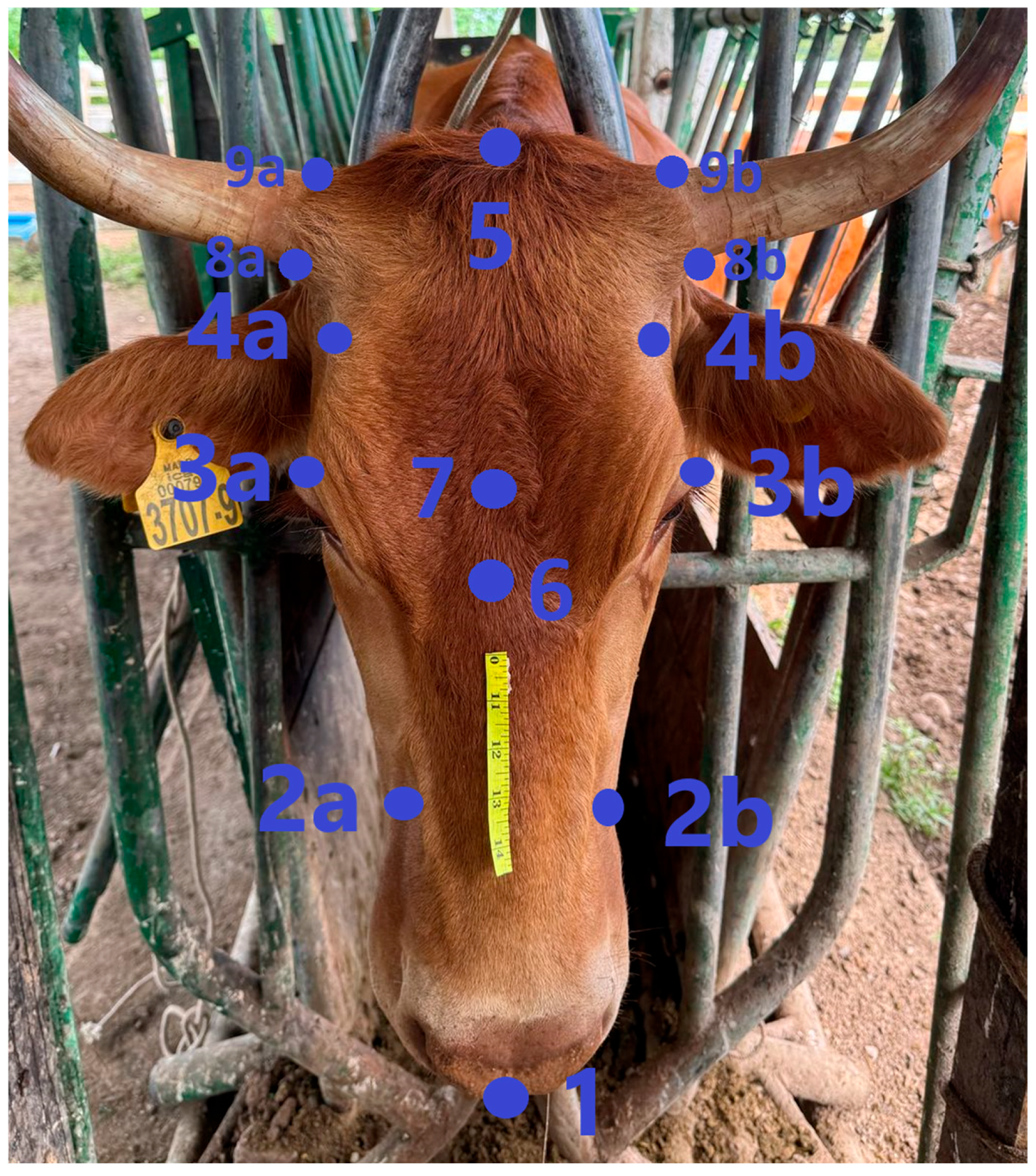


| 1 | Most Rostral Point of the Head (“Point of the Muzzle”) |
|---|---|
| 2a and 2b | Narrowest points of the muzzle |
| 3a and 3b | Most lateral margins of the orbits |
| 4a and 4b | Narrowest points of the front |
| 5 | Most caudal point of the head (protuberantia intercornualis) |
| 6 | Midline between most rostral points of the orbitas |
| 7 | Midline between most caudal points of the orbitas |
| 8a and 8b | Ventral point of horn base |
| 9a and 9b | Dorsal point of horn base |
| Effect | Sum of Squares | Mean Square | Degrees of Freedom | F | p |
|---|---|---|---|---|---|
| Individual | 0.305452 | 0.000519 | 588 | 4.84 | <0.0001 |
| Side | 0.004745 | 0.000395 | 12 | 3.69 | <0.0001 |
| Individual * Side | 0.063045 | 0.000107 | 588 | 10.94 | <0.0001 |
| Error | 0.011757 | 9.8 × 10−6 | 1200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca-Carreño, A.; Parés-Casanova, P.M.; Vélez-Terranova, M.; Rangel-Pachón, D.E.; Martínez-Correal, G.; Rosero-Alpala, J. Head Sexual Characterization of Sanmartinero Creole Bovine Breed Assessed by Geometric Morphometric Methods. Ruminants 2025, 5, 33. https://doi.org/10.3390/ruminants5030033
Salamanca-Carreño A, Parés-Casanova PM, Vélez-Terranova M, Rangel-Pachón DE, Martínez-Correal G, Rosero-Alpala J. Head Sexual Characterization of Sanmartinero Creole Bovine Breed Assessed by Geometric Morphometric Methods. Ruminants. 2025; 5(3):33. https://doi.org/10.3390/ruminants5030033
Chicago/Turabian StyleSalamanca-Carreño, Arcesio, Pere M. Parés-Casanova, Mauricio Vélez-Terranova, David E. Rangel-Pachón, Germán Martínez-Correal, and Jaime Rosero-Alpala. 2025. "Head Sexual Characterization of Sanmartinero Creole Bovine Breed Assessed by Geometric Morphometric Methods" Ruminants 5, no. 3: 33. https://doi.org/10.3390/ruminants5030033
APA StyleSalamanca-Carreño, A., Parés-Casanova, P. M., Vélez-Terranova, M., Rangel-Pachón, D. E., Martínez-Correal, G., & Rosero-Alpala, J. (2025). Head Sexual Characterization of Sanmartinero Creole Bovine Breed Assessed by Geometric Morphometric Methods. Ruminants, 5(3), 33. https://doi.org/10.3390/ruminants5030033








