Abstract
Tannins can be utilized to increase rumen undegradable protein (RUP) by their capacity to form complexes with diverse nutrients present in the feed. In that regard, high-performance ruminants demand elevated RUP levels. The objective of this study was to evaluate the effects of incorporating varying levels of tannin into three protein sources (cottonseed, peanut, and soybean meals) on ruminal kinetic parameters, ruminal fermentation, and intestinal digestibility. Thus, three in situ experiments were conducted to investigate the ruminal degradation kinetics, where Fraction A represents the soluble portion, Fraction B relates to the portion potentially degraded in the rumen, and kd denotes the degradation rate of Fraction B, for both dry matter (DM) and crude protein (CP) in the rumen. Additionally, the study assessed dry matter effective degradability (ED), rumen undegradable protein (RUP), and intestinal digestibility (ID). These experiments utilized three cannulated animals for the in situ incubations. Regarding cottonseed meal in terms of DM degradation kinetics, tannin inclusion had a quadratic effect on fraction A (p < 0.01), B (p = 0.10, trend), kd (p = 0.03), and ED (p < 0.01). Fraction A of CP had a cubic effect (p = 0.03), being greater for the control compared with the other treatments. The inclusion of tannin linearly increased RUP (p < 0.01). The RUP proportion increased 29, 33, and 45% when 20, 40, and 60 g/kg tannin were used, respectively, compared to the control. For peanut meal, the A fraction of protein and RUP responded quadratically as tannin was included in peanut meal (p < 0.01). However, tannin levels did not affect fraction B of protein and ID. Regarding soybean meal, fractions A and B of DM and ED had cubic effects (p < 0.01), being greater for the control compared with the other treatments, and responded quadratically as tannin increased. Also, tannin inclusion had a cubic effect on fractions A and B of protein, RUP, and ID (p < 0.01). The cubic behavior showed greater B fraction and ID and lower A fraction and RUP for the control compared other treatments (p < 0.01). Tannins offer a promising avenue for elevating RUP levels in diets featuring cottonseed and peanut meals. Nevertheless, no advantages were observed when treating soybean meal with tannin.
1. Introduction
Ruminant animals still exhibit significantly lower feed efficiency compared to poultry and swine, indicating the pressing need for further advancements in this area [1,2,3]. Furthermore, protein is the costliest component among the feed constituents [4]. Hence, there is a need for research endeavors to optimize the utilization of this nutrient, which in the case of ruminants is classified into rumen-degradable protein (RDP) and rumen-undegradable protein (RUP) [5]. The RDP undergoes conversion into amino acids by rumen microorganisms, thereby serving as the primary source of metabolizable protein for ruminants [4]. However, microbial protein alone is insufficient to meet the requirements of high-producing animals [6]. Thus, high-producing animals require a greater RUP contribution to fulfill their metabolizable protein needs.
Soybean, peanut, and cottonseed meals are the main protein sources used in feedlot cattle production in Brazil [7]. Although widely utilized, a significant portion of these feeds’ protein is fermented in the rumen [8,9]. Thus, researchers have sought processing techniques (physical and chemical) to enhance protein protection against ruminal fermentation and improve protein utilization [10]. Several processing methods have been developed to manipulate the ruminal degradation of protein sources with the objective to increase feedstuff RUP, including autoclaving [11], microwave treatment [12,13], toasting or irradiation [14], heating [15,16], malic acid [16], xylose [17], and tannins [18,19]. Among these processing techniques, the addition of condensed tannins is noteworthy. Condensed tannins, recognized for their anti-nutritional properties, can form stable complexes with protein when added at low concentrations, resulting in increased resistance to rumen microbial degradation [20,21]. Thus, studies have shown that the addition of condensed tannins, at low concentrations, can increase RUP [22]. However, the optimal levels of tannin utilization for protein protection are not yet well-established. It is important to note that this protein protection should not interfere with intestinal protein digestibility, should not cause harm to animals, and should not adversely affect the ruminal microbial population [23].
There are uncertainties regarding the use of tannins to increase RUP, such as the lack of specific dosages and their effects on different feed ingredients, such as peanut and cottonseed meals. Furthermore, there are limited studies on the effectiveness of increasing RUP supply on ruminal fermentation. In this regard, the use of in vitro techniques allows for controlled studies and provides relevant information on ruminal fermentation of feedstuffs [24,25]. Therefore, this study aimed to evaluate different levels of tannin inclusion in three protein sources (cottonseed, peanut, and soybean meals) on ruminal kinetic parameters, ruminal fermentation, and intestinal digestibility. Our hypothesis is that tannin treatment can effectively protect the protein in these feed ingredients against ruminal fermentation, leading to an increase in RUP and improving nutrient utilization efficiency.
2. Materials and Methods
2.1. Location and Ethical Approval
The experiments took place at the Beef Cattle Research Center, located at the Institute of Animal Science in Sertãozinho, São Paulo, Brazil. This study was conducted in full compliance with the guidelines set forth by the Animal Use Ethics Committee of the Institute of Animal Science. The committee approved the protocol for animal care and handling under the reference number 249-19.
2.2. Experimental Designs and Chemical Analysis
Three experiments were performed for individual feed evaluation: cottonseed (Exp.1), peanut (Exp.2), and soybean (Exp.3) meals. Within each experiment, feed were submitted to four levels of tannin (85% condensed and 15% hydrolyzed from Acacia mearnsii, Tanac SA, Montenegro, RS, Brazil) including: 0, 20, 40, and 60 g/kg (DM basis). Furthermore, a commercial soybean-based product was also evaluated as positive control (SoyPass®, Nutron Cargill, São Paulo, SP, Brazil) in Exp.3.
For in situ trials, three Nellore bulls (BW of 397 ± 51 kg, 24 months old, and a body condition score of 3.5), cannulated in the rumen, were used for ingredients incubation. The experimental design included a randomized complete block (animal). For each ingredient, simultaneously in each animal, bags were incubated in the rumen for 0, 2, 4, 8, 12, 24, or 48 h. Filter bags were incubated for each treatment in duplicate in each animal and timepoint, totaling 42 observations per treatment. In the in vitro trials, a setup comprising four 4-L digestion vessels (TE-150, Tecnal Equipamentos Científicos, Piracicaba, SP, Brazil) was employed. These vessels were equipped with a gentle rotational mechanism and a temperature controller and used in a 24 h fermentation batch. Thus, the experimental design included a randomized complete block (vessel), with a total of 26 filter bags (experimental units) incubated for each treatment.
The ingredients used in these studies underwent grinding using a 2 mm screen (Wiley mill; Thomson Scientific Inc., Philadelphia, PA, USA) for all incubations and analyses. Subsequently, the samples were subject to chemical analyses, including dry matter (DM; method G-003/1), ash (method M-001/1), crude protein (method N-001/1), and ether extract (method G-005/1), following the procedures outlined in Detmann et al. [26]. The organic matter (OM) content was calculated as the difference between DM and ash. To determine neutral detergent fiber, the samples were treated with alpha thermo-stable amylase, with the omission of sodium sulfite, in accordance with the method by Van Soest et al. [27] and adapted for the Ankom200 Fiber Analyzer (Ankom Technology, Macedon, NY, USA). Detailed information on the chemical compositions of the experimental ingredients can be found in Table 1. The tannin treatments were prepared as follows: 600 g of each sample was weighed using a digital scale (Mettler Toledo, model ME2002E, Polaris Parkway, Columbus, OH, USA) and placed in metal molds. Then, the tannin was weighed and homogenized with each sample. After homogenization, distilled water (in a 1:2 ratio) was added to the mixture, which was left to stand for 6 h. After resting, the sample was transferred to a ventilated oven at 60 °C for 72 h.

Table 1.
Chemical composition of protein sources.
2.3. In Situ Procedures and Calculations
The animals were situated in an enclosed barn and placed in individual tie stalls. They were provided with a diet consisting of 40% forage and 60% concentrate, which included a mixture of 60% corn silage, 24.9% dry ground corn, 13% soybean meal, 0.2% urea, and 1.9% mineral supplement. Bulls were adapted to this diet for 14 d before the commencement of the study, and they had continuous access to water. Each ingredient was weighed and then inserted into Nylon bags (Sefar Nitex, Switzerland, Fairport, NY, USA) with a porosity of 50 μm and a surface area of 400 cm2. These bags were subsequently placed inside each animal for incubation, ensuring a bag surface area to mass ratio of 15 mg/cm2. Samples were incubated in the rumen by attaching the bags to a steel chain with a weight at the end to allow for continual immersion within ruminal contents. Bags were placed into the rumen in the reverse order of incubation hours so that all bags were removed at the same time for washing.
After removal, the bags were immersed in an ice-cold saline solution for 15 min to halt microbial activity and detach bacteria from the feed fraction. Subsequently, the bags underwent a thorough washing in a washing machine using cold running tap water until the rinsing water became clear. Bags designated as “0 h” were not subjected to rumen incubation but were rinsed alongside the incubated bags. Following this, the bags were dried in an oven at 55 °C for 72 h. Upon completion of the drying process, each bag was individually weighed. The residues from each diet were carefully extracted from the bags and placed into labeled plastic bags to create a sample for each diet corresponding to each animal and incubation time. These residual samples from different time points in the bags were utilized to estimate the parameters of ruminal degradation.
The DM and CP degradation profiles were estimated asymptotic function [28]:
where Yt is the fraction degraded in time ‘t’, g/kg; A is the water-soluble fraction, g/kg; B is the potentially degradable water-insoluble fraction, g/kg; kd is the degradation rate of fraction b, h−1; and t is time, h.
Yt = A + B × (1 − e − (kd × t)),
The effective degradability (ED, g/kg) of DM was calculated using the model [29]:
where A is the water-soluble fraction, g/kg; B is the potentially degradable water-insoluble fraction, g/kg; kd is the degradation rate of fraction b, h − 1; t is time, h; and kp is the rumen passage rate (k) of 0.074 h−1, obtained from the equation developed for concentrates [30].
ED = A + [B × kd/(kd + kp) × e-kpt),
The rumen undegradable protein (RUP) content in ingredients was calculated as:
where B is the potentially degradable water-insoluble fraction, g/kg; kd is the degradation rate, h−1; kp = passage rate, h−1.
RUP = B × [kp/(kp + kd)],
2.4. Intestinal Digestibility Procedures and Calculations
In the context of in vitro trials, a setup was utilized that included four digestion vessels, each with a capacity of 4 L (TE-150, Tecnal Equipamentos Científicos, Piracicaba, SP, Brazil). These vessels were equipped with a controlled system to ensure slow rotation and maintained temperature, enabling a 24 h fermentation batch. To assess the intestinal digestion of RUP, a three-step in vitro procedure was employed [31,32]. In brief, 0.335 g (on a dry matter basis) from the 12 h timepoint of the in situ incubation for each ingredient was weighed and placed into R520 bags (Ankom Technology, Macedon, NY, USA). These bags were then sequentially incubated with constant rotation at 39 °C using a TE-150 incubator (Tecnal Equipamentos Científicos, Piracicaba, SP, Brazil). The incubation process involved the use of pepsin solution (P-7000, Sigma, St. Louis, MO, USA) for 1 h, followed by pancreatin solution (P-7545, Sigma, St Louis, MO, USA) for 24 h. Upon completion of the incubation period, the bags were rinsed with tap water until the effluent water became clear. Subsequently, the samples were dried in an oven at 60 °C for 48 h. The residues remaining in the bags were then analyzed to determine the dry matter (DM) and nitrogen (N) content.
2.5. Statistical Analysis
The DM and CP fractions, ED, RUP, and ID were first determined for each replication and compared using a completely randomized model design. The parameters were compared through a regression analysis, with differences considered statistically significant at a level of p ≤ 0.05 and trending when 0.05 < p ≤ 0.10. Thus, the levels of tannin inclusion were examined for linear, quadratic, and cubic responses using the following model:
where Yijk represents the response variable obtained from the ith level of tannin in the diet of the jth incubation and the kth experimental unit. The indices i, j, and k denote the levels of inclusion of tannin, the random factor of incubation, and the random factor of bottle, respectively. The regression parameters of the model are denoted as B0, B1, and B2. The Xi represents the effect of the ith level of the fixed quantitative factor (inclusion of tannin), Pj represents the effect of the level of the random factor incubation, Ak represents the effect of the level of the random factor, and eijkl represents the residual error, assumed to follow a normal distribution (0, s2). All analyses were run using the PROC GLIMMIX of SAS (SAS on Demand, online version).
3. Results
3.1. Cottonseed Meal
Ruminal degradation parameters of DM and CP, as well as protein ID can be found in Table 2 and Figure 1. Regarding DM degradation kinetics, tannin inclusion had linear and quadratic effects on fraction A (PLin. < 0.01; PQuad. < 0.01), B (PQuad. = 0.10, trend), kd (PLin. = 0.10, trend; PQuad. = 0.03), and ED (PQuad. < 0.01). Fraction A of CP had linear and cubic effects (PLin. < 0.01; PCub. = 0.03), being greater for the control compared with the other treatments and responded quadratically as tannin increased. Tannin levels did not affect Fraction B of CP (PLin. = 0.72; PQuad. = 0.77; PCub. = 0.87). The inclusion of tannin linearly increased RUP (p < 0.01). Compared to the control (0 g/kg tannin), the RUP proportion increased 29, 33, and 45% when 20, 40, and 60 g/kg tannin were used, respectively. Furthermore, ID had a cubic effect (p < 0.01), being greater for the control compared with the other treatments and responded quadratically as tannin increased.

Table 2.
Effects of tannin inclusion on rumen degradation parameters of cottonseed meal.
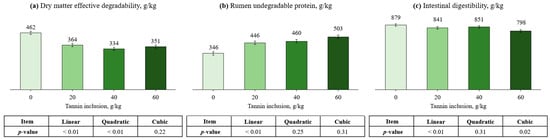
Figure 1.
Effects of tannin inclusion on dry matter effective degradability ((a), ED), rumen undegradable protein ((b), RUP), and intestinal digestibility (c), of cottonseed meal. ED = A + [B × kd/(kd + kp) × e-kt] [29]; RUP = B × [kp/(kp + kd)], where A is the water-soluble fraction, g/kg; B is the potentially degradable water-insoluble fraction, g/kg; kd is the degradation rate of fraction b, h−1; t is time, h; and kp is the rumen passage rate of 0.074 h−1 [30].
3.2. Peanut Meal
The effects of tannin treatment in peanut meal can be found in Table 3 and Figure 2. For DM kinetics, tannin inclusion had a quadratic effect on fraction A (p < 0.01). Furthermore, fraction B had a cubic effect (p < 0.01), being lower for the control compared with the other treatments and responded quadratically as tannin increased. On the other hand, tannin levels did not affect kd (PLin. = 0.56; PQuad. = 0.38; PCub. = 0.15). Nevertheless, the inclusion of tannin linearly decreased ED (p = 0.02). Regarding CP degradation kinetics, tannin levels did not affect fraction B (PLin. = 0.08; PQuad. = 0.08; PCub. = 0.29) and ID (PLin. = 0.37; PQuad. = 0.70; PCub. = 0.84). The A fraction (p < 0.01) and RUP (p = 0.05) responded quadratically as tannin was included in peanut meal.

Table 3.
Effects of tannin inclusion on rumen degradation parameters of peanut meal.
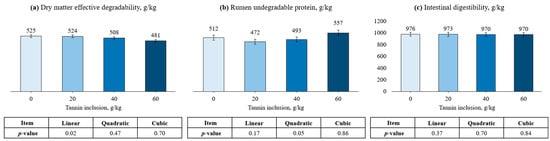
Figure 2.
Effects of tannin treatment on dry matter effective degradability ((a), ED), rumen undegradable protein ((b), RUP), and intestinal digestibility (c), of peanut meal. ED = A + [B × kd/(kd + kp) × e-kt] [29]; RUP = B × [kp/(kp + kd)], where A is the water-soluble fraction, g/kg; B is the potentially degradable water-insoluble fraction, g/kg; kd is the degradation rate of fraction b, h−1; t is time, h; and kp is the rumen passage rate of 0.074 h−1 [30].
3.3. Soybean Meal
Regarding DM degradation kinetics, fractions A and B, and ED had cubic effects (p < 0.01), being greater for control compared with the other treatments and responded quadratically as tannin increased (Table 4 and Figure 3). However, tannin levels did not affect kd (PLin. = 0.68; PQuad. = 0.41; PCub. = 0.34). For CP kinetics, tannin inclusion had a quadratic effect on fraction kd, heating the greatest value at 60 g/kg (p < 0.01). Also, tannin inclusion had a cubic effect on fractions A and B, RUP, and ID (p < 0.01). The cubic behavior showed greater B fraction and ID, and lower A fraction and RUP for control, compared other treatments. Then, tannin inclusion had a quadratic effect on these parameters.

Table 4.
Effects of tannin inclusion on rumen degradation parameters of soybean meal.
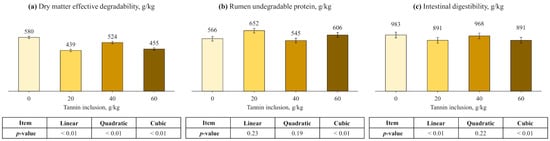
Figure 3.
Effects of tannin treatment on dry matter effective degradability ((a), ED), rumen undegradable protein ((b), RUP), and intestinal digestibility (c), of soybean meal. ED = A + [B × kd/(kd + kp) × e-kt] [29]; RUP = B × [kp/(kp + kd)], where A is the water-soluble fraction, g/kg; B is the potentially degradable water-insoluble fraction, g/kg; kd is the degradation rate of fraction b, h−1; t is time, h; and kp is the rumen passage rate of 0.074 h−1 [30].
4. Discussion
The rise of the rumen undegradable protein (RUP) content of a diet is essential to fulfilling the requirements of high-performance animals [6]. In that regard, tannins can be utilized to increase RUP by their capacity to form complexes with diverse nutrients present in the feed [21,33]. However, to our knowledge, the optimal levels of tannin inclusion on protecting the amino acids of common protein sources (cottonseed, peanut, and soybean meals) from ruminal fermentation remain unclear. Regarding ruminal kinetic parameters, fraction A is soluble and readily available for ruminal degradation, fraction B is potentially degraded in the rumen within a specific timeframe, and kd is the degradation rate of fraction B [34,35]. Thus, we hypothesized that the treatment of these feed with tannin could modify the ruminal kinetic parameters of DM and CP. Indeed, tannin inclusion reduced the soluble fraction of DM for all tested feed, reaching lower values at 40 g/kg for cottonseed and peanut meals and 60 g/kg for soybean meal. Interestingly, the tannin effect was higher on protein soluble fraction, where 60 g/kg was the treatment with lower values for all feeds. The explanation for these results lies in the fact that tannin treatment can facilitate the creation of complex compounds with low solubility in the rumen [36]. Moreover, hydrogen bond interactions between tannin and protein could be the reason for the higher effect on protein soluble fraction [37,38]. The inclusion of tannins has also resulted in a reduction in the percentage of protein degradation in the rumen over time for both cottonseed and soybean meals, at 60 and 20 g/kg, respectively. This reduction in Kd indicates a shorter duration for the protein ruminal fermentation; this, in turn, can facilitate the enhancement of amino acid flow and absorption in the animal’s intestine [23,39,40]. Thus, our results suggest that tannin treatment changed ruminal nutrient kinetics by protecting the feed from ruminal fermentation.
The use of reduced concentrations of tannins in the diet can be an alternative for optimizing protein supply to ruminants [41]. This is attributed to the capacity of tannins to bind with proteins and form tannin–protein complexes. This alteration in fermentation kinetics promotes greater ruminal escape and consequently increases RUP levels [42]. Thus, the changes in the ruminal kinetics caused by tannin inclusion were reflected in the reduction in ED and an increase in RUP levels, as expected. Regarding ED, caution must be exercised when including tannins as their usage may lead to toxic effects on ruminal microorganisms [43]. This could result in a reduction in fiber degradation and organic matter digestibility [43,44]. The utilization of condensed tannins could lead to a reduction in substrate degradation by hindering the attachment of microbes to feed particles and by forming bonds with nutrients and microbial enzymes [37]. Furthermore, there is a possibility of tannin complexation with other compounds, such as starch [45]. The interaction between tannins and starch is considered pH-independent but can be influenced by factors like tannin solubility [45]. This could explain the results observed for peanut meal (a feed with higher starch content). In this case, there was a linear decrease in digestibility and a quadratic effect on RUP. Specifically, there was a reduction in digestibility for the first two tannin levels, followed by an increase in the 60 g/kg treatment. Thus, it appears that the highest tannin level offers the most favorable conditions for improving protein–tannin complexation in both cottonseed and peanut meals.
The increase in RUP implies a decrease in the quantity of substrates accessible for microbial fermentation within the ruminal environment [46,47]. However, it is important to emphasize that this protection should occur only at the ruminal level, leaving it susceptible to digestion and absorption in the other compartments of the animal’s gastrointestinal tract [48]. Thus, we hypothesized that tannin treatment could increase the RUP content of the tested feeds without impairing the intestinal digestibility of protein. Indeed, the lack of differences observed for the peanut meal treatments indicates that protein–tannin complexes were sufficiently robust to shield the protein from rumen fermentation but not strong enough to prevent their breakdown after passing through the rumen. The complexes formed for feed protection between protein and tannin should be reversible when exposed to pH values lower than the ruminal pH, such as those found in the abomasum and small intestine [21,49]. On the other hand, the cubic effect observed for the protein intestinal digestibility of cottonseed and soybean meals suggests that higher levels of tannin inclusion (60 g/kg) could also have a detrimental impact on post-rumen protein utilization.
Therefore, our results lead us to the conclusion that tannin treatment may enhance the RUP content of cottonseed and peanut meals. However, caution must be exercised regarding the levels of tannin inclusion as an excess of tannins could potentially impact the availability of amino acids for intestinal absorption, as observed with soybean meal. For this feed, the alteration in ruminal kinetics has also impacted the RUP, but the reductions in intestinal digestibility did not lead to an improvement in protein utilization. In fact, the control treatment exhibited 1.01%, 2.91%, and 3.36% more RUP being digested in the intestine compared to the increasing levels of tannin inclusion. Considering that the reactivity of tannins with proteins can be influenced by factors such as the chemical structure, molecular weight of tannins, and amino acid content of proteins [40,45,50], it appears that soybean meal was less affected by these treatments. Some studies observed that doses of up to 20 g/kg of dry matter (DM) resulted in reduced protein digestibility when Jersey steers were fed diets based on soybean meal [51]. According to these authors, this effect may be attributed to several factors, including the binding effect of tannins with proteins, the lack of dissociation of a portion of the tannin–protein complex in the abomasum, potential inactivation of intestinal enzymes, or re-binding to proteins.
In summary, the amount of RUP digested in the intestine was as follows: for cottonseed meal: 0 g/kg tannin inclusion (304 g/kg), 20 g/kg tannin inclusion (375 g/kg), 40 g/kg tannin inclusion (392 g/kg), and 60 g/kg tannin inclusion (401 g/kg); for peanut meal: 0 g/kg tannin inclusion (500 g/kg), 20 g/kg tannin inclusion (459 g/kg), 40 g/kg tannin inclusion (478 g/kg), and 60 g/kg tannin inclusion (540 g/kg). Thus, our results suggest the potential inclusion of tannins for each feed. Regarding both cottonseed and peanut meals, we highlight the inclusion of 60 g/kg of tannins, which resulted in 31.9% and 8.14% more RUP being digested in the intestine compared to the non-processed treatment, respectively. Subsequent actions will involve conducting studies to assess the rumen fermentation parameters of these ingredients.
5. Conclusions
Tannins present a promising alternative for enhancing RUP levels in diets containing cottonseed and peanut meals. However, the effectiveness of tannins is contingent upon their dosage. Therefore, the most favorable outcomes under these experimental conditions were observed with a tannin inclusion level of 60 g/kg for both feeds. This dosage indicates effective protection of protein from ruminal microbial degradation without compromising its absorption at the intestinal level. This suggests that tannins can serve as a viable alternative for processing these feedstuffs. However, no benefits were observed from treating soybean meal with tannin. Concerning the treated cottonseed and peanut meals, it is advisable to conduct additional research to assess the impact of these ingredients on ruminal fermentation parameters and animal performance.
Author Contributions
Conceptualization, P.D.B.B. and E.M.P.; methodology, K.E.L., D.A.B.P., F.R., E.M., E.A.B., and E.M.P.; formal analysis, P.D.B.B., M.I.M., and E.M.P.; investigation, K.E.L., D.A.B.P., P.D.B.B., and E.M.P.; resources, R.H.B. and E.M.P.; data curation, K.E.L., D.A.B.P., M.I.M., P.D.B.B., and E.M.P.; writing—original draft preparation, K.E.L., D.A.B.P., M.I.M., P.D.B.B., and E.M.P.; writing—review and editing, K.E.L., D.A.B.P., F.R., E.M., M.I.M., R.H.B., P.D.B.B., and E.M.P.; visualization, K.E.L., D.A.B.P., F.R., E.M., M.I.M., R.H.B., P.D.B.B., and E.M.P.; supervision, R.H.B., P.D.B.B., and E.M.P.; project administration, R.H.B. and E.M.P.; funding acquisition, R.H.B., P.D.B.B., and E.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the São Paulo Research Foundation (FAPESP) for funding the project (Grant# 2018/19743-7; Grant # 2017/50339-5). They also thank the members of the Laboratory of Nutrition and Ruminal Fermentation of the Beef Cattle Research Center of Instituto de Zootecnia for their assistance with sample collection and laboratorial analyses. The authors also acknowledge the funding received from the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC, Public notice number: 48/2022, grant: 2023TR000535).
Institutional Review Board Statement
This study was carried out in strict accordance with the recommendations of the Animal Use Ethics Committee of the Instituto de Zootecnia. Animal care and handling protocols were approved by this committee (Protocol Number: 249-19).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the farm crew at the Experimental Station (Instituto de Zootecnia) for animal feeding and care.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albino, F.T. Frango de Corte—Manual Prático de Manejo e Produção; Aprenda Fácil: Viçosa, MG, Brasil, 1998; p. 72. [Google Scholar]
- Pires, A.V. Bovinocultura de Corte—Volume I; FEALQ: Piracicaba, SP, Brasil, 2010; Volume 1, p. 1760. ISBN 978-85-7133-069-6. [Google Scholar]
- Rostagno, H.; Albino, L.F.T.; Donzele, J.P.; Gomes, P.C.; Oliveira, R.F.D.; Lopes, D.C.; Ferreira, A.S.; Barreto, S.L.d.T.; Euclides, R.F. Tabelas Brasileiras Para Aves e Suínos, 3rd ed.; UFV: Viçosa, MG, Brasil, 2011; p. 252. ISBN 978-85-6024-97-2-5. [Google Scholar]
- Silva, F.A.S.; Benedeti, P.D.B.; Silva, L.F.C.E.; Rotta, P.P.; Menezes, A.C.B.; Marcondes, M.I.; Valadares Filho, S.C. Protein and Amino Acids Requirements for Beef Cattle. In Nutrient Requirements of Zebu and Crossbred Cattle, 4th ed.; Valadares Filho, S.C., Saraiva, D.T., Benedeti, P.D.B., Silva, F.A.S., Chizzotti, M.L., Eds.; Independent Production: Visconde de Rio Branco, MG, Brazil, 2023; pp. 201–232. [Google Scholar] [CrossRef]
- Yousefinejad, S.; Fattahnia, F.; Kazemi-Bonchenari, M.; Nobari, B.; Ghaffari, M.H. Effects of Protein Content and Rumen-Undegradable to Rumen-Degradable Protein Ratio in Finely Ground Calf Starters on Growth Performance, Ruminal and Blood Parameters, and Urinary Purine Derivatives. J. Dairy Sci. 2021, 104, 8798–8813. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine, Nutrient Requirements of Beef Cattle: Eighth Revised Edition; National Academy Press: Washington, DC, USA, 2016.
- Pinto, A.C.J.; Millen, D.D. Nutritional Recommendations and Management Practices Adopted by Feedlot Cattle Nutritionists: The 2016 Brazilian Survey. Can. J. Anim. Sci. 2019, 99, 392–407. [Google Scholar] [CrossRef]
- Molosse, V.L.; Pereira, D.A.B.; Rigon, F.; Loregian, K.E.; Magnani, E.; Marcondes, M.I.; Branco, R.H.; Benedeti, P.D.B.; Paula, E.M. Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: (2) Cottonseed Meal. Animals 2022, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Rigon, F.; Pereira, D.A.B.; Loregian, K.E.; Magnani, E.; Marcondes, M.I.; Branco, R.H.; Benedeti, P.D.B.; Paula, E.M. Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: (1) Peanut Meal. Animals 2022, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.S.; Jordaan, L.; Dreyer, O. Effect of Extrusion with Molasses on the Rumen Undegradable Protein Fraction of Canola Oilcake Meal and Sweet Lupins. S. Afr. J. Anim. Sci. 2022, 52, 792–801. [Google Scholar] [CrossRef]
- Samadi; Yu, P. Dry and Moist Heating-Induced Changes in Protein Molecular Structure, Protein Subfraction, and Nutrient Profiles in Soybeans. J. Dairy Sci. 2011, 94, 6092–6102. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.S.R.; Brodie, G.; Cullen, B.; Kaur, R.; Cho, E.; Cheng, L. Microwave Heat Treatment Induced Changes in Forage Hay Digestibility and Cell Microstructure. Appl. Sci. 2020, 10, 8017. [Google Scholar] [CrossRef]
- Paya, H.; Taghizadeh, A.; Janmohammadi, H.; Moghaddam, G.A.; Khani, A.H.; Alijani, S. Effects of microwave irradiation on in vitro ruminal fermentation and ruminal and post-ruminal disappearance of safflower seed. J. Biodivers. Environ. Sci. 2014, 5, 349–356. [Google Scholar]
- Akbarian, A.; Khorvash, M.; Ghorbani, G.R.; Ghasemi, E.; Dehghan-Banadaky, M.; Shawrang, P.; Hosseini Ghaffari, M. Effects of Roasting and Electron Beam Irradiating on Protein Characteristics, Ruminal Degradability and Intestinal Digestibility of Soybean and the Performance of Dairy Cows. Livest. Sci. 2014, 168, 45–52. [Google Scholar] [CrossRef]
- Mojarrad, M.T.; Seidavi, A.; Dadashbeiki, M.; Roca-Fernández, A.I. Short communication. Effect of soybean meal heat procedures on growth performance of broiler chickens. Span. J. Agric. Res. 2014, 12, 180–185. [Google Scholar] [CrossRef]
- Vanegas, J.L.; González, J.; Carro, M.D. Influence of Protein Fermentation and Carbohydrate Source on In Vitro Methane Production. J. Anim. Physiol. Anim. Nutr. 2017, 101, e288–e296. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Cheng, K.-J.; Beauchemin, K.A.; Bailey, D.R.C.; Pickard, M.D.; Gilbert, R.P. Use of Lignosulfonate to Decrease the Rumen Degradability of Canola Meal Protein. Can. J. Anim. Sci. 1993, 73, 211–215. [Google Scholar] [CrossRef]
- Lima, P.R.; Apdini, T.; Freire, A.S.; Santana, A.S.; Moura, L.M.L.; Nascimento, J.C.S.; Rodrigues, R.T.S.; Dijkstra, J.; Garcez Neto, A.F.; Queiroz, M.A.Á.; et al. Dietary Supplementation with Tannin and Soybean Oil on Intake, Digestibility, Feeding Behavior, Ruminal Protozoa and Methane Emission in Sheep. Anim. Feed Sci. Technol 2019, 249, 10–17. [Google Scholar] [CrossRef]
- Roca-Fernández, A.I.; Dillard, S.L.; Soder, K.J. Ruminal fermentation and enteric methane production of legumes containing condensed tannins fed in continuous culture. J. Dairy Sci. 2020, 103, 7028–7038. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, R.; Paulino, P.V.R.; Detmann, E.; Valadares Filho, S.C.; Paulino, M.F.; Monnerat, J.P.I.S.; Duarte, M.S.; Silva, L.H.P.; Moura, L.S. Influence of Condensed Tannin on Intake, Digestibility, and Efficiency of Protein Utilization in Beef Steers Fed High Concentrate Diet. Livest. Sci. 2011, 141, 1–11. [Google Scholar] [CrossRef]
- Verma, S.; Taube, F.; Malisch, C.S. Examining the Variables Leading to Apparent Incongruity between Antimethanogenic Potential of Tannins and Their Observed Effects in Ruminants—A Review. Sustainability 2021, 13, 2743. [Google Scholar] [CrossRef]
- Kelln, B.M.; Penner, G.B.; Acharya, S.N.; McAllister, T.A.; Lardner, H.A. Impact of Condensed Tannin-Containing Legumes on Ruminal Fermentation, Nutrition, and Performance in Ruminants: A Review. Can. J. Anim. Sci. 2021, 101, 210–223. [Google Scholar] [CrossRef]
- Broderick, G.A.; Wallace, R.J.; Ørskov, E.R. Control of Rate and Extent of Protein Degradation. In Physiological Aspects of Digestion and Metabolism in Ruminants: Proceedings of the Seventh International Symposium on Ruminant Physiology; Tsuda, T., Sasaki, Y., Kawashima, R., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 541–592. [Google Scholar] [CrossRef]
- Benedeti, P.D.B.; Valadares Filho, S.D.C.; Zanetti, D.; Fonseca, E.; Silva, F.; Silva, B.D.C.; Alhadas, H.M.; Vieira Pereira, J.M.; Pacheco, M.V.C.; Pucetti, P.; et al. Prediction of In Vivo Organic Matter Digestibility of Beef Cattle Diets from Degradation Parameters Estimated from In Situ and In Vitro Incubations. J. Agric. Sci. 2019, 157, 711–720. [Google Scholar] [CrossRef]
- Billman, E.D.; Dillard, S.L.; Roca-Fernández, A.I.; Soder, K.J. Supplementation of Oilseeds to an Herbage Diet High in Condensed Tannins Affects Methane Production with Minimal Impact on Ruminal Fermentation in Continuous Culture. Fermentation 2022, 8, 109. [Google Scholar] [CrossRef]
- Detmann, E.; Souza, M.S.; Valadares Filho, S.C.; Queiroz, A.; Berchielli, T.; Saliba, E.O.; Cabral, L.S.; Pina, D.S.; Ladeira, M.; Azevedo, J. Métodos Para Análise de Alimentos. In INCT; Suprema: Visconde do Rio Branco, MG, Brazil, 2012; p. 214. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Orskov, E.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Denham, S.C.; Morantes, G.A.; Bates, D.B.; Moore, J.E. Comparison of Two Models Used to Estimate In Situ Nitrogen Disappearance. J. Dairy Sci. 1989, 72, 708–714. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001; ISBN 0309069971. [Google Scholar]
- Calsamiglia, S.; Stern, M.D. A Three-Step In Vitro Procedure for Estimating Intestinal Digestion of Protein in Ruminants. J. Anim. Sci 1995, 73, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, S.; Calsamiglia, S.; Ferret, A. Technical Note: A Modified Three-Step In Vitro Procedure to Determine Intestinal Digestion of Proteins. J. Anim. Sci 2006, 84, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, R.; Paulino, P.V.R.; Detmann, E.; Teixeira, C.R.V.; Alves, L.C.; Assunção, R.N. Tannin on Non-Degradable Digestible Protein from Proteic Sources in Cattle Rumen. Acta Sci. Anim. Sci. 2015, 37, 389. [Google Scholar] [CrossRef]
- O’Connor, J.D.; Sniffen, C.J.; Fox, D.G.; Chalupa, W. A Net Carbohydrate and Protein System for Evaluating Cattle Diets: IV. Predicting Amino Acid Adequacy. J. Anim. Sci 1993, 71, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Bannink, A.; Crompton, L.A.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.K.; Bayat, A.R.; Yáñez-Ruiz, D.R.; et al. Invited Review: Nitrogen in Ruminant Nutrition: A Review of Measurement Techniques. J. Dairy Sci 2019, 102, 5811–5852. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Westreicher-Kristen, E.; Henke, A.; Diaby, M.; Susenbeth, A.; Dickhoefer, U. In Vitro Microbial Protein Synthesis, Ruminal Degradation and Post-Ruminal Digestibility of Crude Protein of Dairy Rations Containing Quebracho Tannin Extract. J. Anim. Physiol. Anim. Nutr. 2018, 102, e77–e86. [Google Scholar] [CrossRef]
- Min, B.R.; Castleberry, L.; Allen, H.; Parker, D.; Waldrip, H.; Brauer, D.; Willis, W. Associative Effects of Wet Distiller’s Grains plus Solubles and Tannin-Rich Peanut Skin Supplementation on In Vitro Rumen Fermentation, Greenhouse Gas Emissions, and Microbial Changes. J. Anim. Sci 2019, 97, 4668–4681. [Google Scholar] [CrossRef]
- Dijkstra, J.; France, J.; Forbes, J.M. Introduction: Quantitative aspects of ruminant digestion and metabolism. In Quantitative Aspects of Ruminant Digestion and Metabolism, 2nd ed.; Dijkstra, J., Forbes, J.M., France, J., Eds.; CABI publishing: Washington, DC, USA, 2005; pp. 13–22. ISBN 0851998143. [Google Scholar]
- Yanza, Y.R.; Fitri, A.; Suwignyo, B.; Elfahmi; Hidayatik, N.; Kumalasari, N.R.; Irawan, A.; Jayanegara, A. The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis. Animals 2021, 11, 3317. [Google Scholar] [CrossRef]
- Ahnert, S.; Dickhoefer, U.; Schulz, F.; Susenbeth, A. Influence of Ruminal Quebracho Tannin Extract Infusion on Apparent Nutrient Digestibility, Nitrogen Balance, and Urinary Purine Derivatives Excretion in Heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals 2019, 9, 856. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Thantsha, M.S. Preparation of Acacia Tannin Loaded Lipid Microparticles by Solid-in-Oil-in-Water and Melt Dispersion Methods, Their Characterization and Evaluation of Their Effect on Ruminal Gas Production In Vitro. PLoS ONE 2018, 13, e0206241. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.M.; Beauchemin, K.A. Effect of Feeding Condensed Tannins in High Protein Finishing Diets Containing Corn Distillers Grains on Ruminal Fermentation, Nutrient Digestibility, and Route of Nitrogen Excretion in Beef Cattle. J. Anim. Sci 2018, 96, 4398–4413. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of Tannins and Other Sorghum Phenolic Compounds with Starch and Effects on In Vitro Starch Digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of Feeding Plant By-Products: A Review of the Implications for Ruminant Meat Production. Anim. Feed Sci. Technol 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Chamadia, B.; Grewal, R.S.; Lamba, J.S.; Kaur, J.; Kashyap, N. Effect of Varying Levels of Tannins Treatment on In Vitro Degradability of Soybean Meal. Int. J. Curr. Microbiol.App. Sci 2020, 9, 3991–4000. [Google Scholar] [CrossRef]
- Lavrenčič, A.; Levart, A. In Vitro Dry Matter and Crude Protein Rumen Degradation and Abomasal Digestibility of Soybean Meal Treated with Chestnut and Quebracho Wood Extracts. Food Sci. Nutr. 2021, 9, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and Ruminant Nutrition. Pan. J. Agric. Res. 2004, 2, 191. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The Effect of Condensed Tannins on the Nutrition and Health of Ruminants Fed Fresh Temperate Forages: A Review. Anim. Feed Sci. Technol 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Fischer, M.L.; Anschau, F.A.; Venturini, T.; Tinini, R.C.R.; Dessbesell, J.G.; Faciola, A.P. Effects of Black Wattle (Acacia Mearnsii) Condensed Tannins on Intake, Protozoa Population, Ruminal Fermentation, and Nutrient Digestibility in Jersey Steers. Animals 2020, 10, 1011. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).