Abstract
The objective of this research was to evaluate the ruminal fermentation parameters and in vitro Greenhouse gas (GHG) production derived from the fermentation of a balanced sheep diet with the addition of vegetable oils (canola, corn, safflower, and sunflower) or glycerol at different proportions (0, 20, and 40 g/kg of dry matter, DM). The fermentations showed that the highest Gmax was obtained with the addition of 40 g/kg of sunflower oil and 20 g/kg of glycerol with values of 180.97 and 179.95 mL/g DM, respectively. The treatment with 40 g/kg DM of corn oil showed the lowest values in CH4 production (7.15 mL/g DM when compared to the control) and it seemed to be a potential feeding strategy for reducing GHG emissions without affecting gas production. However, the N-NH3 content for this treatment in both doses (1.90 and 1.88 mg/dL) indicated that some toxicity for the animal could be expected.
1. Introduction
Greenhouse gases (GHG) such as CO2, CH4, and N2O absorb infrared radiation and consequently impact global warming [1]. The enteric production of CH4 by livestock is considered an important source of GHG emissions from the agricultural sector, with ruminant livestock being responsible for the annual global production of approximately 80 million tons of CH4, which represents nearly 33% of anthropogenic CH4 emissions and is implicated as a driver of global climate change [2,3,4]; furthermore, the livestock sector is responsible for GHG emissions of 5.6–7.5 GtCO2e/year [5].
Methane has a 100 year global warming potential that is twenty-five times greater than the equivalent amount of CO2, whereas enteric methane is an end product of the microbial fermentation of food generated within the gastrointestinal tract, particularly in the rumen [6]. Ruminal degradation of food also produces short-chain fatty acids (in particular acetic, propionic, and butyric acids), as well as CO2 and H2, which are converted into CH4 by methanogenic bacteria. It is estimated that 6–18% of the gross energy intake is wasted as ruminal methane [7]; for this reason, the formation of CH4 also represents a loss of energy from the animal’s diet. Therefore, the development of mitigation strategies to reduce CH4 emissions from ruminants is currently the subject of scientific and public interest [3]. Studies indicate that an improvement in the quality of diet will reduce the emissions of CH4 [8]. However, several factors influence ruminal production, such as the consumption of dry matter, lipids supply, a fiber-free carbohydrate diet, ingredient digestibility, and forage/concentrate ratios [7]. Some research indicates that the addition of vegetable oils improves the mechanisms involved in the antimethanogenic effects in the rumen [6], since lipids have been recognized as effective dietary additives to reduce enteric methane production [9]. Corn, canola, sunflower, and safflower oils have different profiles of polyunsaturated fatty acids. Canola oil is rich in monounsaturated oleic acid and has a relatively high proportion of polyunsaturated linoleic acid (omega-6) and a low proportion of alpha-linolenic acid (omega-3). Sunflower oil has a higher proportion of linoleic acid and a low proportion of oleic acid. Corn oil has a high proportion of linoleic acid and a low proportion of oleic acid. Safflower oil has a relatively high proportion of linoleic acid and a low proportion of oleic acid, similar to sunflower oil [9].
The polyunsaturated fatty acids present in vegetable oils have a toxic effect on ciliated protozoa, which are the rumen microorganisms involved in the production of H2 (the main substrate for the enteric production of CH4); hence, a reduction in the number of ruminal protozoa is generally associated with a decrease in CH4 production due to a reduction in H2 availability in the rumen [10].
On the other hand, glycerol or crude glycerin is a by-product of biodiesel, which can be used as a feed ingredient in ruminant diets without affecting the performance of beef cattle in fattening diets [11]. Additionally, it could inhibit the degradation of fats by bacteria and promote the ruminal passage of the total lipid content, thus providing higher proportions of beneficial unsaturated fats. Moreover, an excess of glycerol can be absorbed by both the ruminal and intestinal mucosa, as it is a direct gluconeogenic source for the ruminant [12]. The gross energy of glycerin varies from 13 to 25 MJ/kg, depending on its composition [13].
The final products of ruminal fermentation depend on the ingredients of the diet, which are volatile fatty acids (VFA) mainly composed of acetate, propionate, and butyrate with a lower acetate:propionate (A/P) ratio for concentrates compared to fibrous feeds. VFA supply an important part of the energy and carbon requirements of the ruminants and are largely absorbed through the rumen wall [3,7]. The higher amount of propionate formed during ruminal fermentation allows H2 capture, thus restricting the formation of CO2, which does not favor methanogenic bacteria. The production of acetate and butyrate releases CO2 and H2, which are precursors of CH4 in the ruminal environment [7]. Any glycerol that is not absorbed through the rumen epithelium increases the concentration of propionate, butyrate, and valerate in the rumen whilst decreasing acetate [4].
Ruminant nutrition studies are aimed at establishing diets that maximize microbial protein production in the rumen since they reduce the need to supplement animal feed with non-degradable protein sources in the rumen. From an ecological point of view, the increase in carbon fixation in microbial biomass reduces carbon losses in the form of CO2 and CH4 [14]. N-NH3 in the rumen is one of the most important variables that determine ruminal proper functioning [15], since it is essential for bacterial multiplication. An adequate amount of N-NH3 in the ruminal fluid is needed by the bacteria, mainly for the synthesis of their body proteins [16]. The availability of fermentable carbohydrates in a ruminal environment and maintaining optimal levels of N-NH3 in the ruminal medium during most of the day optimize the use of energy for microbial growth and increase metabolizable proteins [17,18,19]. It has been stated that the maximum microbial efficiency occurs when the concentration of ruminal N-NH3 is between 5 and 8 mg/100 mL since the synthesis of protein in the rumen reaches a maximum at such a range [20].
Livestock production must be considered by the global scientific community when addressing the challenge of climate change [5]. Limiting the increase in emissions from the livestock sector is certainly a challenge. However, there are opportunities to simultaneously increase productivity and decrease the intensity of emissions in such a manner that the economy and livelihoods in rural areas will not be hampered [21]. Therefore, the study of the intake in ruminants is becoming increasingly important in the search to improve the understanding of the digestive processes that occur in the previously mentioned multi-compartment system.
The current challenge is to increase the productivity of ruminants without negatively affecting the environment [2], which is why, in the present work, the effect of vegetable oils (canola, corn, safflower, and sunflower) or glycerol was evaluated (0, 20 and 40 g/kg DM) in an extruded diet based on the consumption of alfalfa for sheep by considering its response on the ruminal fermentation parameters (N-NH3 concentration and VFA production), the accumulated gas production, and the gaseous fraction using in vitro techniques.
2. Materials and Methods
The data obtained from in vitro gas production kinetics, methane, and CO2 production, as well as ruminal fermentation parameters were analyzed using a completely randomized design with doses [20 and 40 g/kg DM] and additives [corn, canola, safflower, sunflower, and glycerol] as factors. Each treatment was randomly selected and subjected to each analysis as a triplicate. The treatments consisted of a diet composed of 500 g/kg of a previously designed extrudate [22], 300 g/kg of corn, 190 g/kg of alfalfa, and 10 g/kg of minerals, to which were added 0, 20, and 40 g/kg of vegetable oils (canola, corn, safflower, and sunflower) in DM basis or crude glycerin. After extrusion processing, treatments were dried in a forced-air oven at 55 °C for 48 h and crushed in a Wiley-TP4274E70024 mill (Thomas Scientific, Morelia, Michoacán, México) with a 1 mm sieve looking for uniformity in particle size.
Three Suffolk breed sheep (80.5 ± 3.7 kg body weight) provided with a ruminal cannula were used as ruminal fluid donors. The sheep were housed in individual pens and fed twice a day (9:00 and 16:00 h) with a maintenance diet based on corn silage. Whole rumen contents were collected before the morning feeding and strained through a polyester monofilament fabric (250 μm mesh aperture) to remove solids. Inocula were obtained by mixing equal parts of rumen fluid from all animals, and 10 mL were immediately inoculated into 120 mL vials containing 30 mL of an anaerobic buffer solution kept at 39 °C [23]. The vials were incubated anaerobically at 39 °C for up to 24 h. The vials without substrate were used as controls.
The laboratory analyses were carried out within the hour of recollection according to [24], where the buffered mineral solution was prepared and kept at 39 °C under continuous CO2 gassing as an anaerobic medium. Gas production was determined according to the methodology described by [25].
One gram of sample was added to each flask with 120 mL of ruminal fluid and a nutrient solution mixture in a 1:2 v/v ratio [26]; three replicates per treatment (thirty in total) and three fermentation flasks were used as blanks (containing ruminal fluid, nutrient solution, and one gram of the compound diet) and were incubated using a DaysiII incubator (ANKOM Technology, Macedon, NY, USA) for 96 h.
In vitro accumulated gas pressure was measured automatically for 96 h using a piece of automatic gas measurement equipment (ANKOM RF Gas Production System) equipped with a pressure transducer connected to each cylinder that transmits the gas cumulative pressure values by radiofrequency to a computer, and readings were taken at 0, 0.5, 1, 2, 3, 6, 12, 24, 36, 48, 72, and 96 h after inoculation. The obtained gas production profiles were adjusted to the Gompertz sigmoidal equation of three parameters [27]:
where PG = gas production (mL/g DM), Gmax = maximum gas production (mL/g DM), A = lag or adaptation phase (h), t = time (h), and k = gas production rate (h−1).
The gas samples of the fermenters were obtained from the headspace at 12 h to calculate the gas fraction (CO2 and CH4) using the Biogas 5000 equipment (Landtec, Dexter, MI, USA); the liquid samples were centrifugated at 2500 rpm for 5 min and filtered for the analysis of N-NH3 and VFA [28], and frozen immediately afterward. VFA were analyzed on a 6890 N gas chromatograph (Agilent Technologies, Wilmington, DE, USA) equipped with a flame ionization detector and an HP-Innowax polyethylene glycol capillary column (30 m × 0.32 mm × 0.15 µm, J&W Scientific, Folsom, CA, USA). The oven temperature was set from 80 °C (held for 1 min) to 120 °C at a rate of 20 °C/min to 205 °C at a rate of 10 °C/min and held for 2 min. Hydrogen was the carrier gas at a constant flow rate of 40 mL/min and 1 µL of the sample was injected in no split mode. The peaks were identified by comparing retention times with VFA standards. The results were expressed as mmol of each VFA per 100 mmol of total VFA detected.
The ruminal environment was evaluated with N-NH3, of which the samples were analyzed using a Genesys 10S VIS UV visible spectrophotometer (Thermo ScientificTM, Madison, WI, USA) at a wavelength of 630 nm [28].
The data obtained from animal performance were analyzed using a completely randomized design with a generalized linear model. Experimental data were adjusted to quadratic models and regression coefficients were obtained. Statistical significances of the regression terms were examined by variance analyses (ANOVA) for the three parameters of the Gompertz sigmoidal equation (p < 0.05).
The gas production of fermentations, composition of the generated gas, N-NH3, and VFA production were analyzed using the MIXED procedure of the statistical package SAS (SAS Institute, Inc., Cary, NC, USA), and the comparison of means (p < 0.05) and variance were performed using least-squares differences.
3. Results and Discussion
The formulation of the diet consisted of 12 g 100 g−1 soybean meal, 15 g 100 g−1 dried distillers’ grains with solubles (30% protein), 7 g 100 g−1 molasses, 30 g 100 g−1 nixtamalized corn (NC), and 27:9 g of cottonseed meal (Gossypium hirsutum L.): g of NC 100 g−1. The chemical composition of the evaluated diet is shown in Table 1.

Table 1.
Proximal chemical analysis of balanced optimal diet for sheep [22].
Total gas production after inoculation of all treatments is shown in Figure 1. These results might be attributed to an increment of amylolytic bacteria since some oils are toxic to cellulolytic bacteria and protozoa [30]. The differences among treatments could be the result of the type and amount of oils [9].
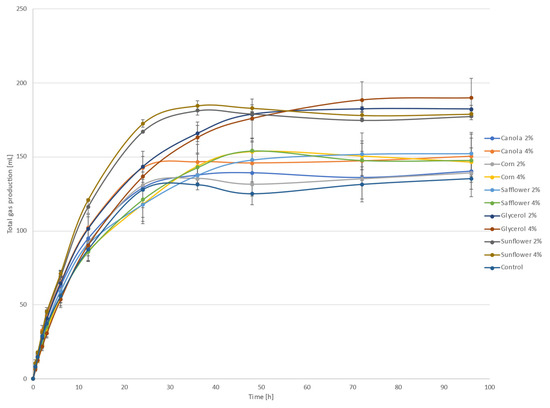
Figure 1.
Total gas production after inoculation.
The fermentation kinetics of feed can be determined by the gas production and by the storage of short-chain volatile fatty acids [12]; therefore, to carry out the ruminal degradation of the substrate, it must undergo a colonization time or lag time that allows the enzymes to reach the substrate (lag or adaptation phase, A). In this research, the inclusion of different oils at different levels showed no significant differences (p > 0.05) for any treatment (Table 2), which could indicate that the gas production kinetics from the in vitro fermentation of vegetable oils or glycerol might not be affected by the colonization of microorganisms in the rumen [12]. Regarding the gas production profiles, an increase was obtained for all treatments compared to the control. The highest gas productions obtained were 180.97 and 179.95 mL/g DM for sunflower (at a rate of 40 g/kg DM) and glycerol (at a rate of 20 g/kg DM), respectively, thus representing a 40% increment compared to the control. For the glycerol treatment, the results obtained might indicate the presence of non-structural carbohydrates associated with glycerin, which are rapidly metabolized (between 4 and 6 h), thus providing greater synchronism with fast nitrogen sources degradation and, consequently, increased gas production [12]. Ref. [7] evaluated ruminal fermentation with added glycerin that showed a rapid fermentation during the first 12 h of incubation. However, in this research, in terms of the gas production rate (k), similar values with the control were obtained, except for the safflower (20 g/kg DM) and glycerol (20 and 40 g/kg DM) treatments, which resulted in a decrement of 38% compared to the control (0.11 h−1), indicating a longer time to achieve maximum gas production. Safflower oil is rich in polyunsaturated fatty acids and is a rich source of linoleic acid (0.76 of the total fatty acids) [31] with availability in the carbon chain that could be saturated with H2.

Table 2.
Parameters of the Gompertz model.
Table 3 indicates that the linear coefficients of the dose and all the vegetable oils and the glycerol had a negative significant effect (p < 0.05) on the lag phase, except for glycerol and sunflower. Regarding the maximum gas production and gas production rate, all the linear coefficients and their interactions with the dose showed a significant effect (p < 0.05). Such effects could also be attributed to the different profiles of the polyunsaturated fatty acids of the used oils and glycerol [6,9]. Canola and corn oil showed a negative significant effect (p < 0.05) on maximum gas production, which could be attributed to changes in the microbial community of rumen fluid and digesta associated to the total VFA concentration, the molar proportions of acetate, isobutyrate, butyrate, and total protozoa [32].

Table 3.
Regression coefficients of responses of gas production parameters.
The production of CH4 and CO2, as well as the CH4/CO2 ratio (Table 4), yielded 13.09 mL (50% increment, compared to the control) and 73.70 mL (34% increment, compared to the control) of CH4 and CO2, respectively, as maximum values with the 40 g/kg DM sunflower treatment. However, CH4/CO2 ratio presents values with significant differences for all treatments, showing the highest values for the safflower treatment 20 g/kg DM (0.190), representing an increment of 20% compared to the control. Additionally, it was observed that the treatments with added corn oil in both doses presented the lowest CH4 production, indicating decreases of 7 and 18% for 20 and 40 g/kg DM treatments, respectively, compared to the control. A lower H2 production per unit of fermented feed resulting in a lower CH4 formation might have occurred, which is associated with an increase in the amount of propionate among the final fermentation products in the rumen [3].

Table 4.
Gas fraction resulting from the in vitro gas production kinetics (mL).
According to [7,9], increasing the propionate amount and decreasing the A/P ratio results in lower H2 production in the rumen and, therefore, lower CH4 production due to the net consumption of H2 in propionate synthesis, possibly improving the efficiency of the energy use of feed.
The VFA obtained values are presented in Table 5. The highest value of acetic acid was registered for the sunflower treatment of 40 g/kg DM with an increase of 41% compared to the control, with an acetate yield as the end product of the fermentation that exceeded propionate production. However, the obtained results indicate that for both glycerol concentrations, acetate and butyrate values increased compared to the control, while propionate concentrations decreased, which is consistent with an increase in CH4 production [33]. In the case of propionic acid, all treatments resulted in values below the control. Yet, the highest values correspond to the treatment of corn 40 g/kg DM (18.35%) and safflower 20 g/kg DM (17.18%); consequently, from the obtained values of acetic and propionic acid, the A/P ratio showed the lowest values for both corn 40 g/kg DM (3.41%) and safflower 20 g/kg DM (3.61%) treatments without significant differences (p > 0.05) to the control. The 40 g/kg DM corn treatment presented the lowest values both in the A/P ratio and in CH4/CO2 ratio (in this case, obtaining the lowest value even below the control). On the other hand, the 20 g/kg DM safflower treatment presented the second lowest value in the A/P ratio, but also showed the highest amount of CH4 per mL of CO2 produced. Regarding butyric acid, no significant differences (p > 0.05) were found among treatments.

Table 5.
Volatile fatty acid concentrations (%).
The optimal N-NH3 concentrations of 5.06 and 5.15 mg/dL (Table 6) were obtained with the 20 g/kg DM safflower and 40 g/kg DM sunflower treatments, respectively. In contrast, 20 g/kg DM (1.90 mg/dL) and 40 g/kg DM (1.88 mg/dL) corn oil treatments showed lower concentrations than those registered for the control treatment. N-NH3 maximum concentration was generally reached approximately two hours after the protein intake provided by feeding [20]. Samples were analyzed 12 h after fermentation. In in vitro experimentation, N-NH3 concentration is an indicator of protein degradability because there is no nitrogen uptake or recycling compared to those obtained using in vivo ruminal media [29], as indicated by [13]. Something similar could have occurred with the obtained N-NH3 values from the other treatments since the protein had been mostly degraded by the time the analysis sample was collected. The authors of [30] obtained values ranging from 10.8 to 13.8 mg/dL of ammonia nitrogen in ruminal fermentations with organic oils added at three levels to their evaluated diets: 2% fish oil; 2% fish oil and 1.5% soybean oil; and 2% fish oil and 3% soybean oil, which were higher than the levels and results from this research.

Table 6.
Ammoniacal nitrogen concentrations (mg/dL).
4. Conclusions
In this research, an effective ruminal fermentation process was presented with an adaptation phase without significant differences among the treatments tested herein, and with an increase in gas production which was favorable for all treatments compared to the control. The obtained values were consistent with the gaseous fraction observed due to GHG production and showed a decrease of 7 and 9.5% in the methane ratio for each mL of CO2 for the treatments with 20 g/kg and 40 g/kg of added corn oil, respectively. The treatment with corn oil showed the lowest value in CH4 production, which could represent a potential feeding strategy for reducing GHG emissions without affecting gas production.
Author Contributions
Conceptualization, G.A.P.-C. and D.R.-J.; methodology, V.H.-V. and E.H.-T.; software, C.S.C.-R.; validation, E.E.A.-R. and G.A.P.-C.; formal analysis, D.R.-J. and G.A.P.-C.; investigation, H.M.-R.; resources, J.B.P.-L.; data curation, G.A.P.-C.; writing—original draft preparation, C.S.C.-R.; writing—review and editing, D.R.-J.; visualization, G.A.P.-C.; supervision, D.R.-J.; project administration, H.M.-R.; funding acquisition, D.R.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Committee for the Foment and Animal Protection of Durango State (Mexican official norm NOM-062-ZOO-1999 at the ninth day of November of 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chung, R.; Kang, E.Y.; Shin, Y.J.; Park, J.J.; Park, P.S.; Han, C.H.; Kim, B.; Moon, S.I.; Park, J.; Chung, P.S. Development of a consolidated anaerobic digester and microbial fuel cell to produce biomethane and electricity from cellulosic biomass using bovine rumen microorganisms. J. Sustain. Bioenergy Syst. 2019, 9, 17–28. [Google Scholar] [CrossRef]
- Castelán-Ortega, O.A.; Ku-Vera, J.C.; Estrada-Flores, J.G. Modeling methane emissions and methane inventories for cattle production systems in Mexico. Atmósfera 2014, 27, 185–191. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed. Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Karlsson, J.; Ramin, M.; Kass, M.; Lindberg, M.; Holtenius, K. Effects of replacing wheat starch with glycerol on methane emissions, milk production, and feed efficiency in dairy cows fed grass silage-based diets. J. Dairy Sci. 2019, 102, 7927–7935. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Gerber, P.; Vellinga, T.; Garnett, T.; Leip, A.; Opio, C.; Westhoek, H.J.; Thornton, P.K.; Olesen, J.; Hutchings, N.; et al. Livestock and greenhouse gas emissions: The importance of getting the numbers right. Anim. Feed. Sci. Technol. 2011, 166–167, 779–782. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Martin, C.; Jouany, J.; Ranilla, M.J. Rumen protozoa and methanogenesis: Not a simple cause-effect relationship. Br. J. Nutr. 2012, 107, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Homem, J.A.C.; Bertocco, E.J.M.; Ruiz, F.V.; Costa, A.M.T.; Paschoaloto, J.R.; Pastori, D.A.; Barbosa, C.V.; Faleiros, N.B.; Fernandes, C.L. Methane production by in vitro ruminal fermentation of feed ingredients. Semin. Cienc. Agrar. 2017, 38, 877–884. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Clark, D.A. Greenhouse gas mitigation opportunities with immediate application to pastoral grazing for ruminants. Int. Congr. Ser. 2006, 1293, 107–110. [Google Scholar] [CrossRef]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Villar, M.L.; Hegarty, R.S.; Nolan, J.V.; Godwin, I.R.; McPhee, M. The effect of dietary nitrate and canola oil alone or in combination on fermentation, digesta kinetics and methane emissions from cattle. Anim. Feed. Sci. Technol. 2020, 259, 114294. [Google Scholar] [CrossRef]
- Fiorentini, G.; Messana, J.D.; José-Neto, A.; Sgobi, E.G.; Castagnino, P.S.; Berchielli, T.T. Performance and meat quality of Nellore bulls fed crude glycerin combined with soybean oil. Anim. Feed. Sci. Technol. 2018, 241, 45–54. [Google Scholar] [CrossRef]
- Ortega-Cerrilla, M.E.; Hidalgo-Hernández, U.; Herrera-Haro, J.G.; Ramírez-Mella, M.; Zetina-Córdoba, P. Glicerol una alternativa para la alimentación de rumiantes. Agroproductividad 2018, 11, 124–129. Available online: https://revista-agroproductividad.org/index.php/agroproductividad/article/view/386 (accessed on 22 October 2022).
- Peripolli, V.; Prates, Ê.R.; Barcellos, O.J.; Costa, J.J.B.; Lopes, R.B.; Camargo, C.M. Partial replacement of corn with glycerin: Digestibility and ruminal fermentation kinetics by in vitro gas production. Rev. Colomb. De Cienc. Pecu. 2016, 29, 218–225. [Google Scholar] [CrossRef]
- Rodríguez, R.; Sosa, A.; Rodríguez, Y. La síntesis de proteína microbiana en el rumen y su importancia para los rumiantes. Rev. Cuba. De Cienc. Agrícola 2007, 41, 303–331. Available online: https://www.redalyc.org/pdf/1930/193017712001.pdf (accessed on 28 November 2022).
- Conde, P.A.; Cuesta, P.A.; Morales, V.C.J. Funcionamiento ruminal y consumo voluntario en ovinos alimentados con fibra de palma de aceite amonificada con sulfato de amonio 11%. Palmas 2004, 25, 288–294. Available online: https://publicaciones.fedepalma.org/index.php/palmas/article/view/1043 (accessed on 12 October 2022).
- Troncoso, A.H. Engormix 2018. Uso de la Urea en la Alimentación de los Rumiantes. Available online: https://www.engormix.com/ganaderia-carne/articulos/uso-urea-alimentacion-rumiantes-t42253.htm (accessed on 18 February 2023).
- Ojeda, A.; Reyes, M.; Rodríguez, W. Efecto de la liberación controlada de nitrógeno sobre la fermentación y la degradabilidad in situ de Cynodon dactylon. Rev. MVZ Córdoba 2012, 17, 3133–3139. [Google Scholar] [CrossRef][Green Version]
- Satter, L.D.; Slyter, L.L. Effect of ammonia concentration on rumen microbial protein production in vitro. Br. J. Nutr. 1974, 32, 199. [Google Scholar] [CrossRef]
- González, G.H.; Martínez, D.L.R.R.; Orozco, E.A.; Perea, N.H.; López, M.B.; Holguin, L.C.; Hernández, C.H.E. 2011. Efecto del Tipo de Dieta y del Grupo Racial Sobre el Comportamiento Digestivo en Borregos: Efecto del Nivel de Consumo y de la Relación Forraje: Concentrado Sobre el Comportamiento Digestivo en Borregos. Universidad Autónoma de Ciudad Juárez. Colección Reportes Técnicos de Investigación. ISBN 978-607-7953-80-7. Serie ICB, Vol. 2. Available online: http://www3.uacj.mx/DGDCDC/SP/Documents/RTI/RTI/9.%20Efecto%20del%20tipo%20de%20dieta.pdf (accessed on 10 July 2022).
- Garriz, M.; López, A. Suplementación Con Nitrógeno No Proteico en Rumiantes. Sitio Argent. De Prod. Anim. 2002, 1–24. Available online: http://www.produccion-animal.com.ar/informacion_tecnica/suplementacion_proteica_y_con_nitrogeno_no_proteico/07-suplementacion_con_nitrogeno.pdf (accessed on 2 March 2023).
- Herrero, M.; Henderson, B.; Havlík, P.; Thornton, P.K.; Conant, R.; Smith, P.; Wirsenius, S.; Hristov, A.N.; Gerber, P.; Gill, M.; et al. Greenhouse gas mitigation potentials in the livestock sector. Nat. Clim. Chang. 2016, 6, 452–461. [Google Scholar] [CrossRef]
- Ortiz-Romero, N.; Delgado, E.; Antonio Pámanes-Carrasco, G.; Medrano-Roldán, H.; Hernández-Vargas, V.; Reyes-Jáquez, D. Development and Evaluation of an Extruded Balanced Food for Sheep Based on Cottonseed Meal (Gossypium hirsutum). In Cotton, 1st ed.; Abdurakhmonov, I.Y., Ed.; IntechOpen Limited: London, UK, 2022; Volume 1, pp. 1–14. [Google Scholar] [CrossRef]
- Goering, H.K.; VanSoest, P.J. Forage Fibre Analysis; Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1970. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. Available online: https://www.scienceopen.com/document?vid=e1859372-e696-424a-85fb-d305b0b594bc (accessed on 12 December 2022).
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed. Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- González-Arreola, A.; Murillo-Ortiz, M.; Pámanes-Carrasco, G.; Reveles-Saucedo, F.; Herrera-Torres, E. Nutritive quality and gas production of corn silage with the addition of fresh and fermented prickly pear cladodes. J. Anim. Plant Sci. 2019, 40, 6544–6553. [Google Scholar]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Galyean, M.L. Laboratory Procedures in Animal Nutrition Research; Department of Animal and Food Sciences, Texas Tech University: Lubbock, TX, USA, 2010; Available online: https://www.depts.ttu.edu/afs/home/mgalyean/lab_man.pdf (accessed on 10 February 2023).
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Valenzuela-Rodríguez, E.I.; Pámanes-Carrasco, G.A.; Mata-Escobedo, M.I.; Medrano-Roldan, H.; Reyes-Jáquez, D. An in vitro and in situ evaluation of a diet for cattle added with organic oils. Agro Product. 2021, 14, 135–143. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Eun, J.S.; Young, A.J.; Bergman, J.W. Nutritive merits of whole Nutrasaff safflower seed when fed to Holstein dairy cows during mid-lactation. Anim. Feed. Sci. Technol. 2010, 156, 26–36. [Google Scholar] [CrossRef]
- Gruninger, R.J.; Zhang, X.M.; Smith, M.L.; Kung, L., Jr.; Vyas, D.; McGinn, S.M.; Kindermann, M.; Wang, M.; Tan, Z.L.; Beauchemin, K.A. Application of 3-nitrooxypropanol and canola oil to mitigate enteric methane emissions of beef cattle results in distinctly different effects on the rumen microbial community. Anim. Microbiome 2022, 4, 35. [Google Scholar] [CrossRef]
- Syahniar, T.M.; Ridla, M.; Samsudin, A.A.B.; Jayanegara, A. Glycerol as an Energy Source for Ruminants: A Meta-Analysis of In Vitro Experiments. Media Peterakan 2016, 39, 189–194. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).