1. Introduction
Clinical mastitis in primigravidae ewes (animals during their first gestation), i.e., prior to first lambing, is rare, and indeed, there is a paucity of relevant information in the international literature. Mastitis can have significant detrimental effects on the affected animals, reducing future animal production and potentially leading to early culling.
In heifers, in which there is a significant amount of information about the disorder, which is termed ‘heifer mastitis’, a variety of aerobic or anaerobic pathogens are implicated as aetiological agents. The most frequent causal bacteria are coagulase-negative staphylococci, whilst Staphylococcus aureus and environmental pathogens are responsible for fewer cases [
1].
The objective of the present report is to describe an outbreak of acute clinical mastitis in ewes during the period immediately before their first lambing. To the best of our knowledge, this is the first report of this pathological condition internationally.
2. Materials and Methods
The incident occurred in a dairy sheep farm in Central Greece during January 2023. The farm referred to an intensively managed flock with 802 high-yielding ewes and 98 primigravidae ewes (Lacaune-breed). No protocols for controlling the reproductive activity were applied in the farm. Vaccinations included anti-clostridial infection vaccination (one month prior to the outbreak described) and anti-contagious agalactia (Mycoplasma agalactiae) vaccination (seven months prior to the outbreak). Anthelmintic treatment involved administration of eprinomectin also one month prior to the outbreak. Feeding was based on a commercial concentrate feed (0.9 kg per animal daily) and ad libitum availability of alfalfa hay. Straw was provided as bedding.
Firstly, the farm experienced an outbreak of 6 cases of acute clinical mastitis in the flock of primigravidae ewes (98 ewes). This was followed by two similar cases of acute mastitis in the same flock, five days later, at which point the farmer asked for veterinary advice.
The farmer indicated that 8 cases developed in the flock of primigravidae ewes within the final week (7 to 4 days) before the estimated lambing. He reported that affected animals had enlarged and hot mammary glands, accompanied by systemic signs (fever, anorexia, dullness). The first six animals had died and their carcasses had been duly disposed, but the subsequent two animals were alive during the visit to the farm and were made available for examination. In the meantime (i.e., between the initial case and the visit to the farm), 19 ewes had lambed without problems. At the time of visit, the prevalence of the cases was 8.2% (8 cases in 98 primigravidae ewes).
A detailed clinical examination of these two animals was performed; blood samples were collected. Special attention was paid to the udder. A standardised clinical examination of the udder (observation, palpation, comparison between glands) was performed [
2,
3]. Mammary secretion samples were collected by the following aseptic techniques: Briefly, the teat apex and the lower (1 cm) part of the teat skin were subjected to a comprehensive disinfection process using a scrub solution of povidone iodine. All investigators involved in sampling procedures wore disposable, non-sterile latex gloves, which were changed when moving to examining the second animal. The first two squirts of secretion were drawn on the gloved hand of an assisting investigator and were assessed macroscopically. Clinical examinations were also performed on 10 randomly selected animals from the same group of primigravidae ewes, without any special findings.
During the visit, an inspection of the husbandry conditions was also performed. Recordings of environmental conditions were available on the farm and taken as an indicator. The animal houses were assessed and stocking burdens (surface and volume available per animal) were calculated. The frequency of litter removal was assessed during the discussion with the farmer. All samples were transported to the laboratory within 3 h of collection for testing.
Haematological examinations performed in blood samples were as follows: haematocrit and packed cell volume measurements, leucocyte and thrombocyte counts, leucocyte differential counts and fibrinogen concentration. Blood β-hydroxybutyrate concentration was measured using a portable ketone metre. An enzyme-linked immunosorbent assay (ELISA) for detection of antibodies against ovine lentivirus was also performed by using a commercial test (IDEXX CAEV/MVV Total Ab Test) [
4].
Cytological examination of mammary secretion was performed by means of the California Mastitis Test, as applied for sheep’s milk [
5].
Bacteriological examination of mammary secretion samples was carried by following standard techniques as previously described in detail [
3]. Bacteria isolated were tested for antibiotic resistance by means of the disc diffusion test [
5]. The following antibiotics were used: amoxycillin plus clavulanic acid, ampicillin, cefquinome, gentamicin, streptomycin, tetracycline and trimethoprim plus sulfamethoxazole. Results were evaluated by applying the EUCAST standards for assessing bacteria of veterinary interest and, accordingly, isolates were classified as susceptible, susceptible to increased exposure or resistant [
6].
3. Results
3.1. Clinical Findings
The two affected animals presented with dullness, depression, apathy and sternal recumbency. Clinical examination revealed fever (40.5 and 40.6 °C) and increased respiratory (45 and 47 breaths min−1) and heart (93 and 98 beats min−1) rates. The abdomen was greatly distended. Ballottement of the abdominal wall indicated the presence of a foetus. Body condition score was four (on the five-point scale). Mucous membranes were mildly congested.
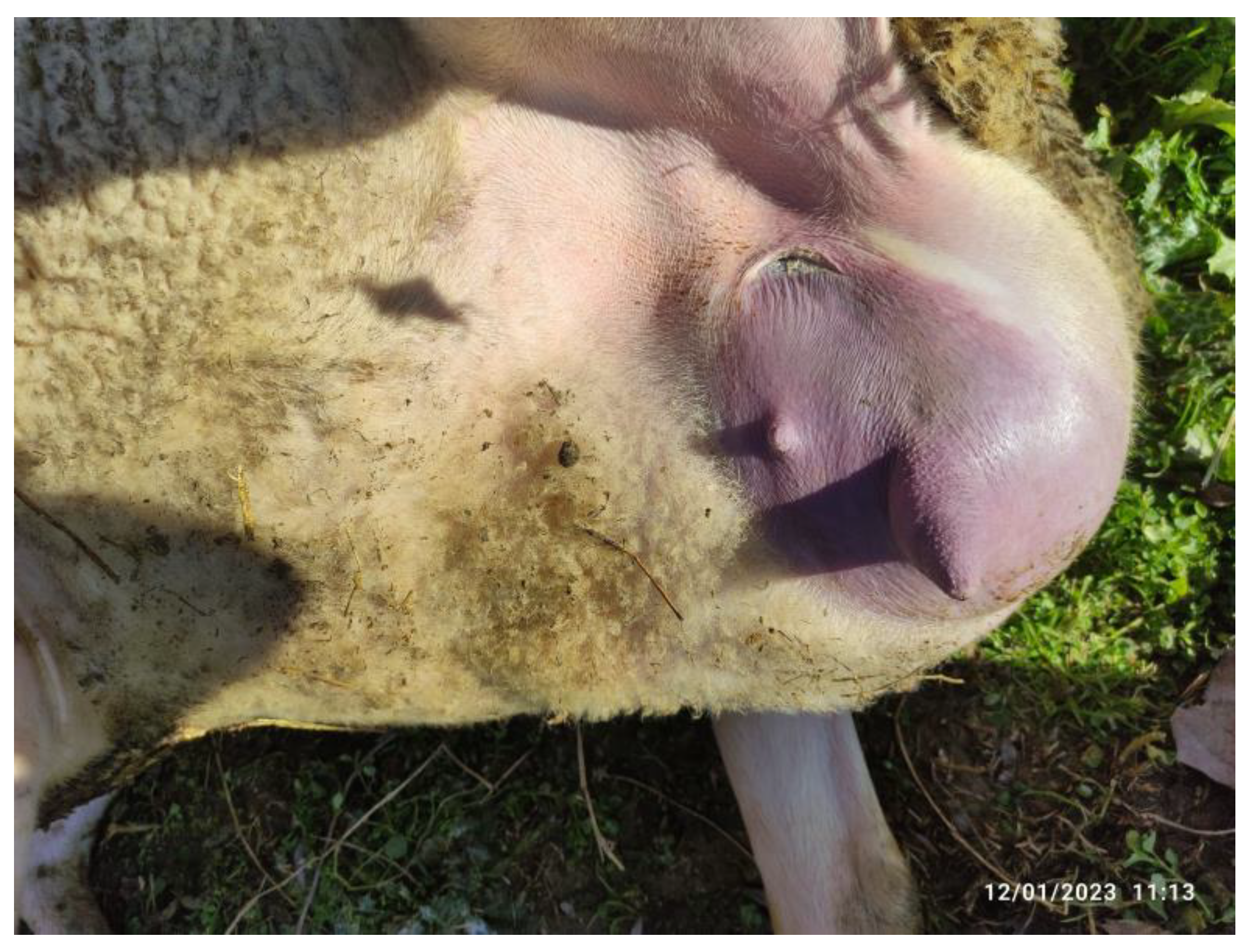
In both animals, only one mammary gland was affected. This was swollen to grossly enlarged, warm and painful during palpation. In one animal, the skin of the affected mammary gland was markedly reddened (
Figure 1). The ipsilateral supramammary lymph nodes were swollen and painful. Expression of mammary secretion was difficult. The secretion drawn was thick and purulent, with flakes and clots. In one of the animals, it was also red-tinted. The California Mastitis Test was positive (+++).
The contralateral mammary glands appeared to be enlarged and distended but otherwise clinically normal. There were traces of mammary secretion present at the teat orifice. Mammary secretion was difficult to draw and thick, but no abnormalities (e.g., flakes) were evident therein.
3.2. Housing Conditions
Primigravidae ewes and lactating ewes lived in the same barn under the same conditions but were separated by wooden dividers. During the visit, the following recordings of the indicator of environmental conditions were noted: the outside temperature was 12 °C, and the inside temperature was 14 °C, with a relative humidity of 44%. The ventilation system was deemed to be unsatisfactory, as there were no ventilators, and the strong smell of ammonia within the animal house was almost unbearable for people. The animal house had wall-openings covered with plastic, which were closed at the time of the visit. According to the farmer, this had remained from the previous week, when outside temperatures were as low as −6 to −4 °C for a series of days, in an attempt to maintain a warmer environment for the animals. The presence of plastic coverings on the wall-openings prevented the circulation of fresh air and resulted in a build-up of ammonia levels, causing discomfort for both animals and humans. Stocking rate was calculated to be 1.5 m2 and 5.25 m3 per animal. As observed during the visit, there was a high population of flies in the farm, and it was noted that there was no apparent strategy in place for pest control.
As reported by the farmer, straw bedding had been last removed four days before the visit because the previous bedding had become excessively soiled, wet and muddy.
3.3. Laboratory Results
In both animals, haematological examination revealed mild anaemia (packed cell volume < 24.0%), increased leucocyte counts (>16,000 leucocytes μL
−1) with mature neutropaenia and left shift, and marked thrombocytosis (>980 × 10
3 cells μL
−1). Additional laboratory data have been included in the
supplementary file.
Blood β-hydroxybutyrate concentration was within the reference intervals (<0.78 mmol L−1). The results of the serological examination conducted to detect small ruminant lentivirus infection indicate that both samples exhibited a considerably high S/P percentage value. The first and second samples displayed S/P values of 233.80% and 247.50%, respectively, surpassing the established cut-off point of 60% for a positive diagnosis
Bacteriological examinations yielded Streptococcus dysgalactiae in one sample and Escherichia coli in heavy growth (>5 × 104 cfu/mL) in the other. Streptococcus dysgalactiae isolate was found to be resistant to gentamicin, streptomycin and trimethoprim plus sulfamethoxazole, whilst no resistance or susceptibility to increased exposure was found for the E. coli isolate.
A review of the milk quality records of the farm, as provided by the dairy company that collected the milk, indicated that somatic cell counts (SCC) in the bulk-tank milk, during the previous fortnightly assessment, was 0.65 × 106 cells mL−1.
3.4. Recommendations and Follow-Up
Because of the severity of the clinical condition of the two affected animals, the reduced likelihood for full recovery and return to maximum productivity and the availability of a countrywide insurance scheme for small ruminants affected with mastitis, the two affected ewes were euthanized.
Recommendations included the increase of ventilation within the animal house through the continuous opening of the side panels and the relocation of 40 primigravidae animals to another animal house in order to decrease the stocking rate. Both were followed by the farmer.
No more cases of the disorder developed. Overall, the prevalence of the cases was 8.2% (8 cases in 98 primigravidae ewes in a period of about 1 month).
4. Discussion
The report presents, for the first time internationally, an outbreak of mastitis in primigravidae ewes at the end of gestation and during the last week before lambing. The disorder is a common and serious problem in dairy cattle farms but has not been reported in sheep. As in cattle, similar conditions are described by the term “heifer mastitis”; in a direct analogy, we propose the use of the term “ewe-lamb mastitis” for this pathological condition.
We postulated that the mammary glands of the affected ewes became distended (as seen in clinical examination) due to the increased amount of milk present in there, which leaked (as corroborated by the detection of traces of milk at the teat orifice), causing opening of the teat. The animals were of a high-yielding breed (Lacaune) and in good condition due to the high plane of feeding (body condition score 4), which led to excessive growth and productivity of the mammary gland. The opening of the teat and the flow of mammary secretion provided potential portals of entry to environmental organisms (as evidenced by the causal agents, which were
Streptococcus dysgalactiae. and
E. coli) [
7,
8].
Poor housing conditions (e.g., overstocking, limited ventilation, irregular manure removal) and/or inadequate disinfection programs could predispose to increased risk of mastitis development due to environmental pathogens [
9,
10]. In this context, Caroprese [
11] reported that a decrease in the space allowed to sheep within an animal house from 2.0 to 1.5 to 1.0 m
−2 per animal led to increased populations of total microorganisms (from 18 × 10
3 to 30 × 10
3 c.f.u. m
−3) and coliforms (from 0.5 × 10
3 to 0.9 × 10
3 c.f.u. m
−3) within the animal houses; in turn, this contributed to an increased incidence of mastitis in ewes in the flock and consequently to increased SCC and total bacterial counts in milk produced at the farm [
12,
13]. Moreover, it was reported that an airspace below 8 m
3 per animal could contribute to higher accumulation of staphylococci and other mesophilic bacteria within the animal buildings, which again can increase the incidence of mastitis [
11]. The potential increase in bacterial presence and circulation within the animal houses could have contributed to the infection of the animals.
Further, the teat’s local defence systems might not have been fully functional, as the mammary gland’s function had not yet started. The teat duct epithelium acts as a barrier between the mammary gland and the external environment in sheep. Unlike in other animals, the ovine teat does not have smooth muscles or a Furstenberg’s rosette. This suggests that local cellular responses are essential in protecting the mammary gland. Previous studies have shown increased numbers of leucocytes in the mammary parenchyma and teat cistern of ewes, indicating the importance of cellular immune mechanisms in these areas. It is an area where inductive lymphoid nodules are strategically developed at the border between the teat duct and teat cistern. These nodules can provide protection against invading microorganisms [
7,
8]. This lack of function may have allowed bacteria to colonise the teat duct and ascend to the mammary parenchyma. The documented general relaxation of immunity during the peri-parturient period [
14,
15,
16] allowed the establishment and development of the infection. Additionally, according to the serological results, the affected animals presented high titers of antibodies against lentivirus (Maedi–Visna). Based on the tropism of lentivirus to the monocyte/macrophages and dendritic cells of the mammary gland, Maedi–Visna Virus (MVV) should not be ruled out as a potential predisposing factor for clinical mastitis even in young animals [
17]. In our case, histopathological confirmation of the indurative non-suppurative interstitial mastitis caused by MVV was not feasible, and therefore the synergistic action of lentivirus with environmental pathogens cannot be asserted with certainty.
It is noted that the incidence of mastitis in primigravidae cattle ranges between 30% and 75%. Three types of risk factors can be associated with the disorder: animal-related factors (e.g., loss of the keratin plug during the last stage of gestation [
18] or udder edema [
19]), herd-related factors (e.g., the season of the year when parturitions are taking place [
20]) and management-related factors (e.g., a prolonged grazing season (>8 months) or no application of a fly control scheme [
21]). In the present case, during the visit to the farm and subsequent clinical work, several notable findings were observed which may have contributed to the outbreak of mastitis in primigravidae ewes. Among these findings was the presence of udder and teat edema, which indicates potential inflammation and swelling of the mammary gland and teat tissue. Additionally, increased humidity within the barn was observed, which could promote the growth and spread of bacteria known to cause mastitis. Furthermore, inadequate fly control measures were noted, which may have contributed to the spread of bacterial infections carried by flies.
The increased somatic cell counts in the bulk-tank milk indicate a prevalence of subclinical mastitis in the lactating animals in the flock [
12,
21]. This is turn refers to an increased circulation of pathogens within the flock, i.e., making easier their dissemination to susceptible animals. This is in full accordance with relevant findings in cattle [
21,
22], which have reported the concurrent presence of increased cell counts in bulk-tank milk and the high incidence of clinical mastitis in primigravidae animals.
The fact that
Streptococcus dysgalactiae isolate was found to be resistant to certain antibiotics, specifically gentamicin, streptomycin, and trimethoprim plus sulfamethoxazole, is of great concern as these antibiotics are commonly used for mastitis treatment in sheep. This highlights the importance of prudent use of antibiotics in the treatment of mastitis [
23].
5. Conclusions
This report presents, for the first time internationally, cases of mastitis in ewes before their first lambing. The prevalence of the cases was 8.2%. The outbreak developed in a dairy flock with increased prevalence of subclinical mastitis in already lactating animals and housing conditions that favoured pathogen dissemination. Primigravidae animals were affected, likely as the result of teat opening, leading consequently to udder distension with milk. Reduced immune competence of the animals, due to the reproductive stage (peri-parturient period), precipitated the infection. There were some similarities with the respective condition in cattle. Preventing such pathological disorders requires vigilance and adherence to thorough scientific recommendations by livestock farmers. This includes regular monitoring and assessment of animal health, proper farm hygiene and management practices, effective pest control strategy, and prompt identification and treatment of any signs of disease or disorder.