Abstract
Ionizing radiation induces many different types of DNA lesions. But one of its characteristics is to produce complex DNA damage, of which tandem DNA damage has received much attention, owing to its promise of distinctive biological properties. Oxidative stresses in response to inflammation in tissues and metal-catalyzed reactions that result in generation of radicals also form these DNA lesions. In this minireview, we have summarized the formation of the tandem lesions as well as the replication and repair studies carried out on them after site-specific synthesis. Many of these lesions are resistant to the traditional base excision repair, so that they can only be repaired by the nucleotide excision repair pathway. They also block DNA replication and, when lesion bypass occurs, it may be significantly error-prone. Some of these tandem DNA lesions may contribute to ageing, neurological diseases, and cancer.
1. Introduction
Tandem DNA damage and multiply damaged sites (MDS): An interesting feature of ionizing radiation-induced DNA damage is that energy deposition is not homogeneous, and a single energy deposition event may generate several free radical species from water [1,2]. The most common mode of fission of H2O upon exposure to ionizing radiation involves generation of hydroxyl radicals (•OH) that induce many types of DNA damages [3]. Hydroxyl radicals are also generated in cells by several other mechanisms [3]. In addition to isolated single DNA lesions, single- and double-strand breaks, and apurinic/apyrimidinic sites, formation of clustered DNA lesions within a short stretch of DNA is a well-established characteristic of ionizing radiation [4,5,6,7,8,9]. For the sake of convenience, MDS have been defined as two or more lesions within ~20 base pairs, of which tandem lesions, with damage to two contiguous bases, constitute an important fraction [7,10,11,12]. Generation of radicals by metal ions also results in MDS [13,14]. Free radicals produced by the Fenton reaction, for example, yield tandem lesions [10]. Additionally, the carbonate radical anion (CO3•−), an oxidant derived from the oxidation of bicarbonate anions and nitrosoperoxocarboxylate anions in mammalian cells, forms tandem lesions [15,16,17]. The MDS present a greater challenge for the DNA repair systems than any individual DNA lesion [1,6,18,19]. They are also mutagenic and genotoxic [12,14]. The mechanism of formation of clustered DNA lesions, however, typically involves multiple radical hits, whereas most tandem lesions are generated by a single radical event, frequently initiated by a hydroxyl radical [8,20]. The distinct mechanisms for the generation of tandem lesions can be briefly outlined as follows.
I. A common mechanism of generation of a peroxyl radical, such as 1 (Scheme 1), is initiated by a hydroxyl radical attack at the pyrimidine C5 (or C6), followed by O2 addition. The peroxyl radical can abstract a hydrogen atom from a neighboring sugar (such as from C1′) (to form 2) or add on to the C8 position of an adjacent guanine (discussed in Section 2). An example of H-atom abstraction at C1′ of the 2-deoxyribose moiety by the peroxyl radical is the formation of 2-deoxyribonolactone with concomitant release of the base, while the 3′ thymine base is converted to thymine glycol (discussed in Section 2.2) [21].
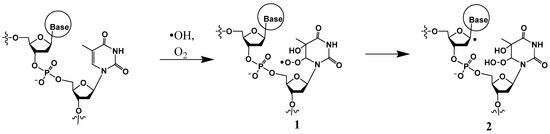
Scheme 1.
Hydroxyl radical-induced formation of 6-(5-hydroxy-5,6-dihydrothyminyl)peroxyl radical 1 followed by abstraction of a hydrogen atom from C1′ of the neighboring sugar to generate 2.
II. Hydroxyl radical-induced hydrogen atom abstraction from the 5′ position of the 2-deoxyribose (to form 3) or the methyl group of thymine (or 5-methylcytosine) (to form 4) (Scheme 2) constitutes additional pathways to form tandem DNA lesions. Radical 3 can result in the formation of a 8,5′-cyclo-2′-deoxyribonucleoside (discussed in Section 3.3) [22,23], whereas radical 4 can give rise to G[8,5-Me]T and T[5-Me,8]G intra-strand cross-links (discussed in Section 3.1) [24]. It is noteworthy that substantial concentrations of tandem lesions are generated in these pathways only in low-oxygen conditions.
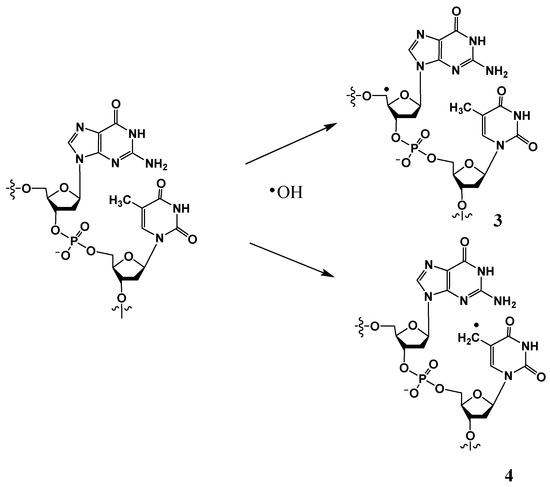
Scheme 2.
Hydroxyl radical-induced H atom abstraction from C5′ of 2-deoxyribose or 5-methyl group of thymine to generate 3 or 4, respectively.
III. Another mechanism of tandem lesion formation involves one-electron oxidation of a nucleobase (most frequently the guanine moiety) generating a radical cation (Scheme 3), which is susceptible to form a covalent bond with the N3 position of a neighboring thymine (discussed in Section 3.2) [15,16,25,26,27]. Unlike the lesions described in II, these intra-strand cross-links are formed in the presence of oxygen. It is well-established that guanine is the most easily oxidizable nucleobase, whereas cytosine is most resistant to one-electron oxidation [3,28]. The guanine radical cation is highly susceptible to hydration via the nucleophilic addition of a H2O molecule at its C8 position [29,30]. Hydration of the guanine radical cation followed by deprotonation generates the 8-hydroxy-7,8-dihydroguanin-7-yl radical (•GOH), which predominantly gives rise to 8-oxo-7,8-dihydroguanine (8-OxoG) via one-electron oxidation and 2,6-diamino-4-hydroxy-5-formamidopyrimidine (Fapy•G) via one-electron reduction [30]. A hydroxyl radical also forms •GOH by adding on to the C-8 position of guanine, leading to the generation of 8-OxoG and Fapy•G lesions [3]. Specific to one-electron oxidants, the guanine cation radical or its conjugate base (i.e., (G-H)•) is also involved in the formation of the intra-strand cross-links between the C8 of guanine and the N3 of thymine, DNA–protein cross-links, and inter-strand DNA–DNA cross-links [28,31].
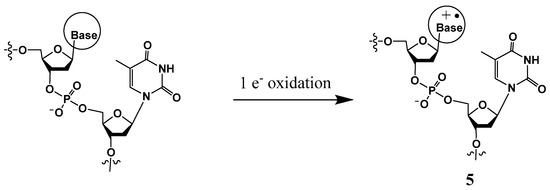
Scheme 3.
One-electron oxidation of a base to generate a radical cation.
Tandem lesions can be broadly divided into two types: one in which two neighboring DNA bases are converted into two discrete lesions, and another type where the two modified bases are linked by one or more covalent bonds. UV-induced thymine–thymine photodimers [32] and 6-4 photoproducts [33] are examples of the latter type that have been studied for many decades and will not be discussed here. Reviews of the tandem lesions formed by pyrimidinyl, 2-deoxyribosyl peroxyl and other radicals, with mechanistic and kinetic insights in their generation, have recently been published [20,34]. In the present minireview, we shall prioritize the site-specific studies of the tandem DNA lesions.
2. Tandem Lesions Containing Two Discrete DNA Modifications
In the early 1990s, Box and coworkers were the first to show that in an X-irradiated oxygenated aqueous solution, in addition to conversion of guanine to 8-OxoG and degradation of a pyrimidine nucleotide to a formamido remnant (formylamine) (F), tandem lesions containing these two modifications also were generated (e.g., structure A in Scheme 4) [24,35]. The hydroxyl radical generated from a water molecule is the primary reactant in this process. The mechanism of formation of these tandem lesions has been proposed to involve a single hydroxyl radical that can induce two distinct DNA damages on two adjacent bases. Using rigorous analytical methods, Cadet showed that an intramolecular addition of a thymine peroxyl radical to vicinal guanine results in the formation of tandem lesions comprised of 8-OxoG, either 5′ or 3′ to formylamine (8-OxoG-F or F-8-OxoG) [36]. First, a hydroxyl radical adds to the C5-C6 double bond of thymine, generating a radical at C6. Next, this radical reacts with oxygen to form a peroxyl radical, which adds on to the C8 position of the neighboring guanine. Rearrangement of the resulting peroxide yields 8-OxoG, whereas the 5-hydroxy-6-oxyl-5,6-dihydrothymine radical thus formed undergoes fragmentation to generate the formamido residue F (Scheme 4).
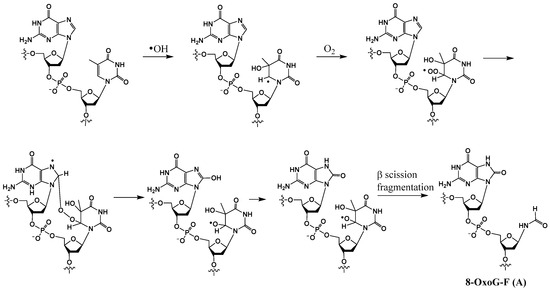
Scheme 4.
Postulated mechanism of formation of 8-OxoG-F tandem lesion from G-T by hydroxyl radical.
It is noteworthy that X-irradiation of short oligonucleotides in oxygenated aqueous solutions was used in these experiments, when clustered lesions are unlikely to be formed and the major DNA damage includes single and tandem lesions. In a γ-irradiated aerated aqueous solution of calf thymus DNA, 8-OxoG-F is formed at higher rate than F-8-OxoG [37]. Ravanat and coworkers showed that in hydroxyl radical-induced reactions, about 50% of 8-OxoG and 8-OxoA are part of tandem DNA damages but are strongly dependent on the secondary structure of DNA [38]. Carter and Greenberg have determined that tandem lesions are the major product from pyrimidine nucleobase radicals [39].
Additional tandem lesions with two discrete modifications, such as thymine glycol adjacent to 8-OxoG (Tg-8-OxoG (C) (and 8-OxoG-Tg) [40], 2-deoxyribonolactone 5′ to thymine glycol (dL-Tg) (D) [21], 8-OxoG 5′ to 5-formyluracil (8-OxoG-5fU) (E) [41], Fapy•G 5′ to 5-formyluracil (Fapy•G-5fU) (F), and 8-OxoG 5′ to 5-hydroxymethyluracil (8-OxoG-5hmU) (G) [41] (Figure 1), formed via various radical-mediated processes, have been reported.
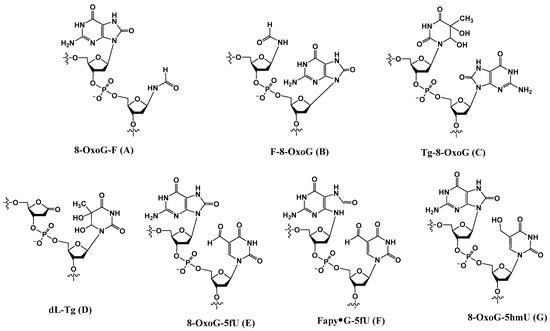
Figure 1.
Structures of a subset of tandem lesions containing two adjacent discrete lesions.
2.1. Tg-8-OxoG (C)
A high frequency of formation of Tg-8-oxoG (C) (and 8-oxoG-Tg) in calf thymus DNA upon exposure to Cu(II)/ascorbate along with H2O2 or γ-rays was reported by Wang and coworkers [40]. Evidently, Tg-8-oxoG can form in DNA in any site where thymine is located 5′ to a guanine. But Tg can also result from the deamination of 5-methylcytosine glycol following a hydroxyl radical attack at 5-methylcytosine. As a result, a Tg-8-oxoG tandem lesion may arise from an attack of reactive oxygen species at the methylated CpG site.
2.2. dL-Tg (D)
Hydroxyl radical addition on the C5-C6 π bond of thymine forms a radical at either C5 or C6, which, under aerobic conditions, is converted to the corresponding peroxyl radical. The peroxyl radical can selectively abstract the C1′-hydrogen atom from the 5′-adjacent nucleotide (such as 2 in Scheme 1) [42]. The C1′-radical is transformed into 2-deoxyribonolactone dL via an O2-dependent process, whereas the 3′ 6(5)-hydroperoxy-5(6)-hydroxy-5,6-dihydrothymine is converted to thymine glycol. Tandem dL-Tg (D) is produced via a reaction with a single hydroxyl radical [21].
2.3. 8-OxoG-5fU (E), Fapy•G-5fU (F), and 8-OxoG-5hmU (G)
Thymine 5-methyl radicals (T•) are produced by HO• and other radicals that can abstract H atoms from the methyl group. T• is also generated by deprotonation of pyrimidine radical cations. In an oxygenated environment, T• reacts with O2 to produce the corresponding methylperoxyl radical (TOO•). Robert and Wagner recently reported the formation of tandem lesions induced by TOO• in GT sequence, which comprise either Fapy•G or 8-OxoG in tandem with 5-formyluracil (5fU) [41]. They are formed by an initial attack of a TOO• radical to the C8 position of guanine, resulting in a cross-linked N7-aminyl radical endoperoxide that can undergo either oxidation or reduction to give rise to 8-OxoG-5fU (E) or Fapy•G-5fU (F), respectively (Scheme 5). In this investigation, 8-OxoG in tandem with 5-hydroxymethyluracil (8-OxoG-5hmU) (G) was also isolated.
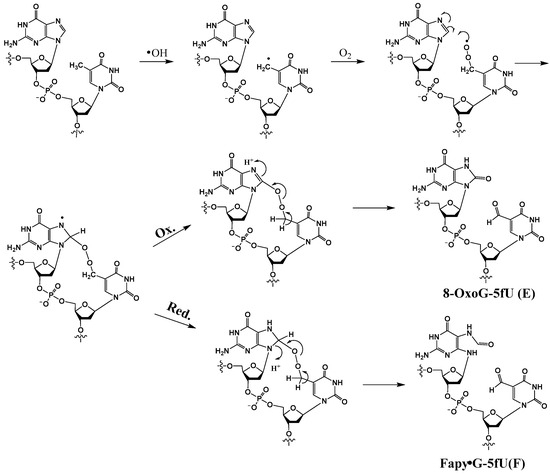
Scheme 5.
Postulated mechanism of formation of 8-OxoG-5fU (E) and Fapy•G-5fU (F).
3. Two Bases or a Base and 2-Deoxyribose Linked by One or More Covalent Bonds
Examples of the other type of tandem lesions with a covalent bond between the two neighboring bases or a base and a 2-deoxyribose are as follows (Figure 2).
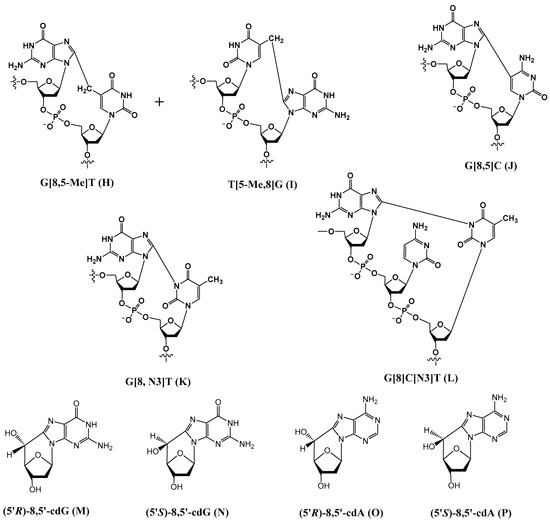
Figure 2.
Structures of tandem lesions, in which two neighboring bases (or a base and the 2-deoxyribose) are covalently linked.
3.1. G[8,5-Me]T (H), T[5-Me,8]G (I), and G[8-5]C (J)
In addition to 8-OxoG-F and F-8-OxoG, Box and coworkers also discovered tandem lesions with a covalent bond between two neighboring bases in DNA exposed to either ionizing radiation or metal-catalyzed H2O2 reactions [10,24,35]. In anoxic conditions, the predominant lesion is a cross-linked product in which the C8 of guanine is linked to the 5-methyl group of an adjacent thymine (G[8,5-Me]T) (Scheme 6).
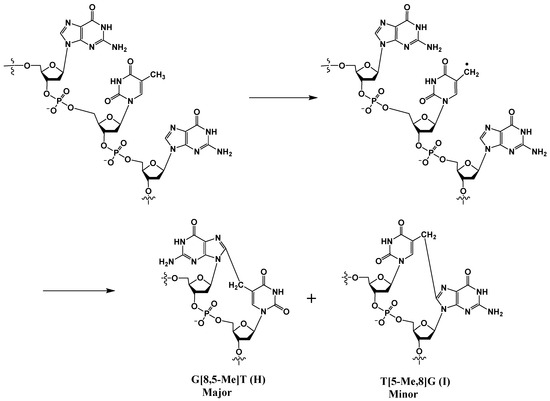
Scheme 6.
Radical-induced formation of cross-linked G[8,5-Me]T (H) and T[5-Me,8]G (I).
Although both G[8,5-Me]T (H) and T[5-Me,8]G (I) are formed, the former cross-link is formed at a much higher rate. This is likely due to the shorter distances between the pyrimidine radical and the 5′ purine base involved in the addition reaction. A computational study rooted in the density functional theory of analogous lesions A[8,5-Me]T and T[5-Me,8]A showed that in addition to steric accessibility, stereo-electronic effects play a major role in determining the reaction mechanism and the observed predominance of the A[8,5-Me]T lesion over T[5-Me,8]A [43]. Additional thymine-purine cross-links have been isolated and characterized from γ–irradiated DNA in an oxygen-free aqueous solution. Later, Wang et al. discovered the corresponding G[8-5]C lesion containing a covalent bond between guanine-C8 and the C5 position of an adjacent cytosine (J in Figure 2) [44], although the mechanism of its formation is unclear. Molecular dynamics simulations indicated that the methylene-bridged cross-links, such as G[8,5-Me]T or G[8,5-Me]mC lesions, are better accommodated in B-DNA than those that lack the methylene bridge, such as the G[8-5]C lesion, which suggests that the methylene group acts as a spacer, allowing the former cross-links more flexibility in DNA than the latter [45].
3.2. G[8,N3]T (K) and G[8]C[N3]T (L)
Another type of cross-linked DNA lesion is formed by one-electron oxidation of guanine under oxidative stress following inflammation in tissues, which results in the formation of G[8,N3]T (K) (Scheme 7) and G[8]C[N3]T (L) by carbonate radical anions [16,25,26,46]. In contrast to the G[8-5Me]T, T[5-Me,8]G, and G[8-5]C, which are formed in anaerobic conditions, these cross-links are formed in the presence of oxygen.
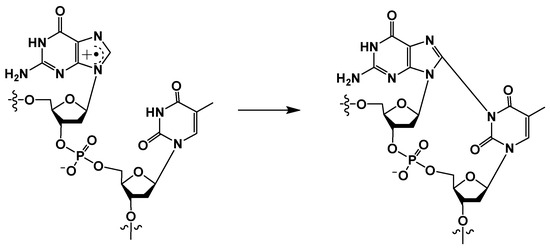
Scheme 7.
Reaction of guanine radical cation with a 3′ thymine.
3.3. 8,5′-Cyclopurine-2′-Deoxyribonucleosides
A different type of double lesion called 8,5′-cyclopurine-2′-deoxyribonucleosides, i.e., 8,5′-cyclo-2′-deoxyguanosine (cdG) (M and N in Figure 2) and 8,5′-cyclo-2′-deoxyadenosine (cdA) (O and P in Figure 2), has been known since the 1960s [22]. These DNA damages are not tandem lesion, as they do not contain damage to two contiguous nucleosides. However, as they contain concomitant damages to both the base and sugar moieties of the same nucleoside, we decided to include them in this paper. The mechanism of their formation by hydroxyl radicals has been postulated to be as follows (Scheme 8) [23,47].
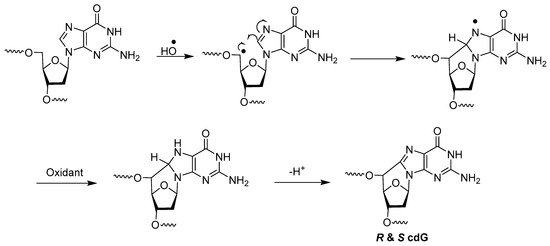
Scheme 8.
Mechanism of formation of cdG by hydroxyl radicals.
It is noteworthy that, in addition to 8,5′-cyclopurine-2′-deoxyribonucleoside formation by hydroxyl radicals (as shown in Scheme 8), these lesions are also derived directly from high-energy radiation. Direct generation of C5′-sugar radicals by γ- and Ar ion-beam-irradiated hydrated DNA samples has been reported, which leads to the formation of the cyclopurine lesions [48]. Indeed, in addition to single-nucleobase damage, these lesions were detected following Ne-22 ion-beam irradiation of hydrated DNA [49]. However, formation of these lesions is inhibited when O2 is present at high concentrations, due to its ease of reaction with the C5′-centered radical [50,51]. Additionally, it was shown that purine oxidation in dsDNA is highly dependent on DNA secondary structure and that greater damage occurs toward the extended B-DNA topology for both isolated (e.g., 8-OxoG) and tandem (e.g., cdG) DNA damage [52].
4. Biological Effects of the Tandem Lesions
4.1. 8-OxoG-F
8-OxoG is less efficiently excised from the 8-OxoG-F tandem lesion by human OGG1 (hOGG1) and Escherichia coli Fpg DNA glycosylases than when it is present as an isolated lesion [38]. As a general rule, tandem lesions are refractory to BER [53], which is thought to be due to the structural perturbation caused by the adjacent lesion [54,55]. They are also stronger blocks of DNA replication than the isolated lesions. For example, in simian COS-7 cells, polymerase bypasses of 8-OxoG and F are 70% and 45%, respectively, compared to 17% for the tandem 8-oxoG-F. In terms of mutagenic effects, 8-OxoG is only weakly mutagenic (mutation frequency (MF) 2–4%) both as an isolated lesion and as part of a tandem lesion, but adenine incorporation opposite F increases from a high of 71% as an isolated lesion to 94% as part of the tandem lesion [56].
4.2. Tg-8-OxoG
The BER enzyme hOGG1 cleaves 8-OxoG with reduced ability in Tg-8-OxoG, whereas it exhibits enhanced ability to cleave 8-OxoG in 8-OxoG-Tg [57]. In terms of replication, the bypass efficiencies for Tg-8-OxoG and 8-OxoG-Tg are approximately one half of those for the two isolated single-nucleobase lesions in wild-type and polymerase-deficient E. coli strains [40]. The presence of an adjacent Tg leads to significant increases in G→T transversions at the 8-OxoG site relative to an isolated 8-OxoG lesion [40]. In this study, while 18% of G→T mutations occur for an isolated 8-OxoG, the percentage increases to 32% and 28% in 8-OxoG-Tg and in Tg-8-OxoG, respectively. Experiments in pol IV- and pol V- backgrounds indicate that both pol IV and pol V are involved, in part, in translesion synthesis (TLS) of Tg, either as an isolated DNA damage incident or as part of the tandem lesion.
4.3. dL-Tg
While endonuclease III (Nth) and endonuclease IV (Nfo) excise isolated Tg and dL, respectively, they are unable to excise dL-Tg [58]. However, long-patch BER (LP-BER) repairs the tandem lesion by APE1 cleavage followed by a strand-displacement synthesis carried out by pol β, which adds 2–10 nucleotides; subsequent removal of the overhang by FEN1 and a final ligation of the two ends by ligase completes this process [58]. UvrABC, the E. coli NER enzymes, can also repair dL-Tg.
dL-Tg is a replication-blocking lesion in E. coli, which is bypassed only under SOS-induced conditions [21]. While Tg does not influence nucleotide incorporation opposite dL in wild-type cells, MF of Tg, negligible as an isolated lesion, increases to 10% in wild-type E. coli cells when dL-Tg is flanked by a 3′-guanine. A misalignment–realignment mechanism appears to be operating, and pol II and pol IV are responsible for misalignment-induced mutations and compete with the pol V bypass. Tg in a tandem lesion, therefore, increases mutagenesis by blocking replication, allowing the misalignment–realignment mechanism to compete with the direct bypass by pol V. Though many of the BER enzymes that repair an isolated Tg cannot repair dL-Tg, it is repaired by UvrABC with nearly the same efficiency as an isolated Tg [58]. In mammalian cells, pol β is involved in strand-displacement synthesis, which is increased by flap endonuclease (FEN1), as it cleaves the flap generated by this mechanism [58].
Replication of dL-Tg was also studied in human cells. Although nearly 100% of Tg is bypassed in HEK 293T cells, dL constitutes a major replication block [59]. dL-Tg is an even stronger replication block with only 5% bypass efficiency [59]. The MF of Tg as a tandem lesion is 3.4%, which increased to 3.9% and 4.8% in cells deficient in pol ι and pol κ, respectively. A greater increase in the MF of Tg (to ∼5.5%) in cells that lack both pol κ and pol ζ suggests that they work together for error-free TLS of Tg. Bypass of a solitary dL results in 12–18% one-base deletions, which increases to as much as 60% in TLS polymerase-deficient cells. For dL-Tg also, the fraction of deletion products also increases in TLS polymerase-deficient cells. In full-length products and in all cell types, adenine is preferentially incorporated opposite an isolated dL as well as when it is part of a tandem lesion, whereas substantial misincorporation opposite Tg occurs only in dL-Tg. In wild-type cells, targeted mutations increase to 9.7% and to 17.4, 15.9, and 28.8% in cells deficient in pol κ, pol ζ, and pol ι, respectively. Consequently, Tg is much more mutagenic as part of a tandem lesion, and the TLS polymerases are involved in the error-free Tg replication in HEK 293T cells.
4.4. 8-OxoG-fU and Fapy•G-fU
The replications of 8-OxoG-5fU and Fapy•G-5fU tandem lesions in HEK 293T cells with or without deficiency of bypass polymerases were examined [60]. The local sequence of the tandem lesions encompasses the 273 codon of the p53 gene, a mutational hot-spot in human cancer. Replication of weakly mutagenic 5-fU remains the same in the 8-OxoG-5fU tandem lesion. But G→T transversions by 8-OxoG increase >10-fold. In contrast to 8-OxoG-5fU, Fapy•G-5fU exhibits significant error-prone bypass of both lesions. The MF of Fapy•G in Fapy•G-5fU increases 3-fold compared to isolated Fapy•G. In addition, MF of 5fU significantly increases with a 5′-adjacent Fapy•G. But G→T transversions in Fapy•G-5fU decrease by almost a third in hPol κ-deficient cells, in contrast to isolated Fapy•G in the same sequence context, which showed an increase in this mutation.
4.5. G[8,5-Me]T, T[5-Me,8]G, and G[8-5]C
The tandem intra-strand cross-links are substrates for NER by the UvrABC proteins of E. coli [61]. However, even though G[8,5-Me]T and the UV-derived T[6,4]T cross-links are recognized well by the UvrA protein, the UvrABC incision of the G[8,5-Me]T cross-link is far less efficient than that of either the T[6,4]T cross-link or the bulky C8-AAF-dG adduct, owing to poor recognition by the UvrB protein [61].
Replication of the tandem lesions in mammalian cells revealed that both G[8,5-Me]T and T[5-Me,8]G cross-links cause targeted and semi-targeted mutations in simian (COS-7) and human embryonic kidney (HEK 293T) cells, and each lesion displays a unique mutational pattern [62]. While targeted base substitutions occur at a frequency of 5.8% for G[8,5-Me]T in HEK 293T cells, 11.0% of semi-targeted single base substitutions near the lesion have been detected. The semi-targeted mutations include up to two bases 5′ and three bases 3′ to the cross-link. The dominant semi-targeted mutation is a C→T transition immediately 5′ to the G[8,5-Me]T cross-link. For the T[5-Me,8]G cross-link, the MF is higher but a similar pattern of mutations is noted. The targeted mutation frequency is 16.3%, with G→T as the dominant mutation. The semi-targeted mutations occur at 15.8% frequency, of which the G immediately 5′ to the cross-link gives the highest frequency of G→T transversions.
4.6. G[8,N3]T and G[8]C[N3]T
Both G[8,N3]T and G[8]C[N3]T lesions are removed by NER mechanisms [63]. But unlike the G[8,5-Me]T and T[5-Me,8]G cross-links and the cyclopurine lesions (described in the next section) that are only repaired by the NER mechanism, BER is active at the sites of the G[8,N3]T and G[8]C[N3]T cross-links in double-stranded DNA [64]. A number of eukaryotic and prokaryotic bifunctional DNA glycosylases/lyases (NEIL1, Nei, Fpg, Nth, and NTH1) and apurinic/apyrimidinic (AP) endonucleases (Apn1, APE1, and Nfo) incise G[8,N3]T and G[8]C[N3]T cross-links on either side of the cross-linked G. Additionally, the higher yield of NER products in the case of G[8]C[N3]T accompanies a lower yield of BER products. Likewise, the NER product yield is about five times smaller for G[8,N3]T, while the BER product yield is much higher. This is an indication that BER and NER compete with one another. This interesting observation deserves further examination.
Replication by the A-family polymerase BF (bacillus fragment) from Bacillus stearothermophilus is strongly blocked by G[8,N3]T and G[8]C[N3]T, but weak TLS occurs by the Y-family polymerases Dpo4 and pol κ [17]. Primer extension by pol η is also partially stalled at several sites at or near the G[8,N3]T cross-link; even so, a substantial and distributive primer extension occurs beyond the sites of the lesions. It is worth noting that the efficiency of primer extension is always greater on templates with G[8]C[N3]T relative to G[8,N3]T [17]. The investigators suggest that in comparison to the rigid configuration of G[8,N3]T, the unmodified cytosine in G[8]C[N3]T allows enough flexibility for it to adopt multiple configurations, of which some are favorable for polymerase bypass.
4.7. 8,5′-Cyclopurine-2′-Deoxyribonucleosides
The 5′R- and 5′S-diastereomers of cdA and cdG have been detected in vitro and in vivo from a number of cells and organisms [23,65,66]. Their yield decreases steadily with the increase in the oxygen concentration [67]. It has been suggested that these lesions play roles in neurologic disease in xeroderma pigmentosum complementation group C patients as well as in Cockayne syndrome, cancer, and familial Mediterranean fever [47].
The levels of 8-oxopurines are typically 40-fold higher relative to the cyclopurine lesions in reactions of hydroxyl radicals (generated by γ-radiolysis or Fenton reaction) with calf thymus DNA [50,68,69]. The yields of the R-diastereomers are greater than those of the S-diastereomers for both cdA and cdG in γ-irradiated calf thymus DNA and Ne-22 ion-irradiated hydrated DNA [49,50]. However, there have been controversies over the detection of these lesions in human cells and animal tissues. Cadet and colleagues were unsuccessful in detecting them in the brains of NER knockout mice and γ-irradiated human cells (1 kGy) [67,70]. On the other hand, the Chatgilialoglu, Dizdaroglu, and Wang laboratories independently detected these lesions in cultured cell lines and in human and animal tissues [71,72,73,74]. These cyclopurine lesions accumulate in wild-type mice with age, and in progeroid mice, which are deficient in DNA repair, they accumulate rapidly with aging in a tissue-specific manner [75].
Unlike most other oxidatively generated damage, 5′S- and 5′R-cdA and 5′S-cdG are not repaired by BER [76,77]. Additionally, cdA impedes gene expression and is poorly repaired in NER-deficient cells, suggesting that the cyclopurine lesions may accumulate in NER-deficient cells. Both 5′R-cdA and 5′S-cdA also accumulate in Neil1-/- mice, indicating a role for this BER protein in NER of these lesions. A comprehensive review of these lesions encapsulates the present state of knowledge [47], and therefore, we will only summarize the site-specific in vitro and cellular studies here.
Geacintov, Chatgilialoglu and coworkers have reported the relative NER efficiencies of all four cyclopurine lesions in the same sequence context in human cell extracts [78]. Although NER excision of the cdA and cdG lesions occurs with comparable efficiencies, both R diastereoisomers are excised twice as efficiently as the S lesions. Molecular modeling and molecular dynamics simulations indicate that the C5′–C8 bond causes a significant local distortion of the DNA backbone as well as a major disruption of the van der Waals stacking interactions with the neighboring bases in the R diastereoisomers of cdA and cdG lesions than the S forms. In another study, the NER efficiency of 5′S-cdG with different complementary bases shows that excision of the wobble pair 5′S-cdG·dT is more efficient than the 5′S-cdG·dC pair, even though the latter maintains a nearly perfect Watson–Crick hydrogen bonding [77]. It is also puzzling that excision of the 5′S-cdG·dC pair is more efficient than that of the 5′S-cdG·dA mispair that has no hydrogen bonds. In experiments with 5′S-cdA, the excision of the 5′S-cdA·dC mispair was found to be much more efficient than that of the 5′S-cdA·dT pair, while the 5′S-cdA·dA pair excision is the least efficient. These results underscore the complexity of human NER, which probably uses multiple criteria to discriminate among base pairs containing a DNA lesion.
5′S-cdG blocks replication strongly in E. coli with a viability <1%, which increases to 5.5% with SOS [79]. In a pol II-deficient strain, viability further decreases, whereas it increases substantially in a strain lacking pol IV. But no progeny is recovered from a pol V-deficient strain, suggesting an absolute requirement of pol V for bypassing 5′S-cdG. This lesion induces ∼34% mutation with SOS. 5′S-cdG→A dominates the mutational spectrum, though 5′S-cdG → T mutation and 5′C deletion also occur.
In contrast to 5′S-cdG, 5′S-cdA→T and 5′S-cdA→G substitutions occur in comparable frequency in wild-type E. coli [80]. However, the frequency of 5′S-cdA→G transitions reduce in a pol IV-deficient strain, particularly upon induction of SOS, suggesting pol IV’s participation in this mutation. In a pol II-deficient strain, MF is increased dramatically, which suggests that pol II is likely involved in its error-free bypass. In vitro studies using pol IV, exo-free Klenow fragment (KF (exo-)), and Dpo4 on 5′S-cdA and 5′S-cdG templates provided additional insight. Primer extension by pol IV was quite inefficient, as it stalls before the lesion, and nucleotide incorporation opposite the lesion occurred only very sluggishly. Kinetic studies show that pol IV incorporates dCMP and dTMP opposite 5′S-cdA with nearly equal efficiency, whereas incorporation of the correct nucleotide dCMP opposite 5′S-cdG by pol IV occurred 10-fold more efficiently than any other dNMP. However, pol IV is quite inefficient in further extension of each cyclopurine-containing pair. While these results suggest a possible involvement of pol IV in 5′S-cdA→G mutations in E. coli, they also imply the lack of its role in 5′S-cdG mutagenesis. Unlike pol IV, KF (exo -), though hindered by both cyclopurines, can slowly incorporate the correct nucleotide opposite them. In contrast, Dpo4 can carry out a more facile bypass of both 5′S-cdG and 5′S-cdA, incorporating the correct nucleotide dTMP opposite 5′S-cdA, but it is only 2–8-fold more proficient relative to the other dNMPs, and it cannot extend the 5′S-cdA:T and 5′S-cdA:C pair. However, Dpo4 incorporates dTMP opposite 5′S-cdG preferentially relative to dCMP, even though extension of the 5′S-cdG:T pair is less efficient than the 5′S-cdG:C pair. In summary, the in vitro and cellular studies suggest that both 5′S-cdA and 5′S-cdG are replication-blocking lesions and 5′S-cdG, in addition, can induce G→A transition in E. coli. Furthermore, the Y-family DNA polymerases can initiate error-prone synthesis of these lesions, albeit inefficiently.
For the roles of mammalian DNA polymerases on these cyclopurines, Wang and coworkers determined that pol η is involved in the insertion step of TLS and showed that the human pol ι and yeast pol ζ complexes (REV3/REV7) efficiently carry out the insertion and extension steps, respectively, of the replicative bypass of 5′S-cdA and 5′S-cdG [81]. Human pol κ and pol η also bypass these lesions but less efficiently. In human embryonic kidney epithelial cells, in addition to major inhibition of DNA replication, 5′S-cdA and 5′S-cdG exhibit potent mutagenicity. While pol κ does not appear to be involved in TLS of 5′S-cdA and 5′S-cdG in human cells, pol η, pol ι, and pol ζ play crucial roles. Based on these results, the investigators propose that pol η and/or pol ι carry out nucleotide insertion opposite 5′S-cdA and 5′S-cdG, whereas pol ζ executes the extension step.
5. Concluding Comments
Tandem lesions comprise a diverse group of DNA lesions. They present a greater challenge for the DNA repair systems than the isolated lesions. In addition, as enumerated in this minireview, they also are stronger replication blocks compared to the isolated DNA lesions. In many cases, these DNA lesions show a synergistic effect in mutagenesis, or at least one of the lesions is more mutagenic than when it is replicated as a solitary lesion. Site-specific incorporations of some of these lesions now allow the investigators to critically examine their repair and replication properties in great detail. As yet, only a limited number of studies have been carried out. Additional systematic studies in the future will provide a better understanding of the biological roles of these tandem lesions. Moreover, as the levels of the tandem lesions are usually lower than some of the isolated lesions, such as the 8-oxopurines, in human cells and tissues, more efforts in their detection are warranted.
Author Contributions
Conceptualization, A.K.B.; writing—original draft preparation, A.K.B.; writing—review and editing, A.K.B., L.C.C. and J.H.T.B.; funding acquisition, A.K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institute of Environmental Health Sciences, NIH (R01 ES-027558 and ES-023350).
Conflicts of Interest
The authors declare no conflicts of interest. Author L.C.C. and author J.H.T.B. are affiliated with Spark Therapeutics, Inc., Philadelphia, PA, USA and Alexion Pharmaceuticals, Lexington, MA, USA, respectively. These companies have no conflicts of interest in this work.
Abbreviations
| NER | nucleotide excision repair |
| MDS | multiply damaged sites |
| DSB | Double-strand breaks |
| 8-OxodGuo | 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-OxoG, 8-oxo-7,8-dihydroguanine |
| dF | N-(2-deoxy-β-D-erythro-pentofuranosyl)formylamine |
| Tg | thymine glycol |
| dL | 2-deoxyribonolactone |
| 5fU | 5-formyluracil |
| Fapy•G | 2,6-diamino-4-hydroxy-5-formamidopyrimidine |
| cdG | 8,5′-cyclo-2′-deoxyguanosine; cdA, 8,5′-cyclo-2′-deoxyadenosine |
| HEK | human embryonic kidney |
| TLS | translesion synthesis |
| MF | mutation frequency |
| pol | DNA polymerase |
| KF (exo-) | exo-free Klenow fragment |
| BER | base excision repair |
References
- Lomax, M.E.; Gulston, M.K.; O‘Neill, P. Chemical aspects of clustered DNA damage induction by ionising radiation. Radiat. Prot. Dosim. 2002, 99, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Bellon, S.; Douki, T.; Frelon, S.; Gasparutto, D.; Muller, E.; Pouget, J.P.; Ravanat, J.L.; Romieu, A.; Sauvaigo, S. Radiation-induced DNA damage: Formation, measurement, and biochemical features. J. Environ. Pathol. Toxicol. Oncol. 2004, 23, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.M.; Bennett, P.V.; Sidorkina, O.; Laval, J. Clustered damages and total lesions induced in DNA by ionizing radiation: Oxidized bases and strand breaks. Biochemistry 2000, 39, 8026–8031. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.M.; Bennett, P.V.; Sidorkina, O.; Laval, J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 103–108. [Google Scholar] [CrossRef]
- Sage, E.; Shikazono, N. Radiation-induced clustered DNA lesions: Repair and mutagenesis. Free Radic. Biol. Med. 2017, 107, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Adhikary, A. The Effects of Particle LET and Fluence on the Complexity and Frequency of Clustered DNA Damage. DNA 2024, 4, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Ravanat, J.L.; TavernaPorro, M.; Menoni, H.; Angelov, D. Oxidatively generated complex DNA damage: Tandem and clustered lesions. Cancer Lett. 2012, 327, 5–15. [Google Scholar] [CrossRef]
- Becker, D.; Kumar, A.; Adhikary, A.; Sevilla, M.D. Gamma- and Ion-beam DNA radiation damage: Theory and experiment. In DNA Damage, DNA Repair and Disease; Dizdaroglu, M., Lloyd, R.S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; Volume 2, pp. 426–452. [Google Scholar]
- Patrzyc, H.B.; Dawidzik, J.B.; Budzinski, E.E.; Iijima, H.; Box, H.C. Double lesions are produced in DNA oligomer by ionizing radiation and by metal-catalyzed H2O2 reactions. Radiat. Res. 2001, 155, 634–636. [Google Scholar] [CrossRef]
- Cadet, J.; Berger, M.; Douki, T.; Ravanat, J.L. Oxidative damage to DNA: Formation, measurement, and biological significance. Rev. Physiol. Biochem. Pharmacol. 1997, 131, 1–87. [Google Scholar]
- Sage, E.; Harrison, L. Clustered DNA lesion repair in eukaryotes: Relevance to mutagenesis and cell survival. Mutat. Res. 2011, 711, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.R.; Phillips, D.H. Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) fenton reactions: Evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links. Mutat. Res. 1999, 424, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Bulky DNA lesions induced by reactive oxygen species. Chem. Res. Toxicol. 2008, 21, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Shafirovich, V.; Dourandin, A.; Huang, W.; Geacintov, N.E. The carbonate radical is a site-selective oxidizing agent of guanine in double-stranded oligonucleotides. J. Biol. Chem. 2001, 276, 24621–24626. [Google Scholar] [CrossRef] [PubMed]
- Madugundu, G.S.; Wagner, J.R.; Cadet, J.; Kropachev, K.; Yun, B.H.; Geacintov, N.E.; Shafirovich, V. Generation of guanine-thymine cross-links in human cells by one-electron oxidation mechanisms. Chem. Res. Toxicol. 2013, 26, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Lee, Y.C.; Geacintov, N.E.; Shafirovich, V. Translesion synthesis past guanine(C8)-thymine(N3) intrastrand cross-links catalyzed by selected A- and Y-family polymerases. Mol. Biosyst. 2016, 12, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, S.; O‘Neill, P.; Greenberg, M.M.; Lomax, M.E. Reduced repair capacity of a DNA clustered damage site comprised of 8-oxo-7,8-dihydro-2′-deoxyguanosine and 2-deoxyribonolactone results in an increased mutagenic potential of these lesions. Mutat. Res. 2014, 762, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, S.; Walker, A.; Stabler, R.; O‘Neill, P.; Lomax, M.E. Increased mutability and decreased repairability of a three-lesion clustered DNA-damaged site comprised of an AP site and bi-stranded 8-oxoG lesions. Int. J. Radiat. Biol. 2014, 90, 468–479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greenberg, M.M. Tandem and Clustered Lesions from Radicals in Nucleic Acids from a Single Initial Chemical Event. In DNA Damage, DNA Repair and Disease; Dizdaroglu, M., Lloyd, R.S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2021; Volume 1, pp. 27–60. [Google Scholar]
- Huang, H.; Imoto, S.; Greenberg, M.M. The mutagenicity of thymidine glycol in Escherichia coli is increased when it is part of a tandem lesion. Biochemistry 2009, 48, 7833–7841. [Google Scholar] [CrossRef][Green Version]
- Keck, K. Formation of cyclonucleotides during irradiation of aqueous solutions of purine nucleotides. Z. Naturforsch. B 1968, 23, 1034–1043. [Google Scholar] [CrossRef]
- Jaruga, P.; Dizdaroglu, M. 8,5′-Cyclopurine-2′-deoxynucleosides in DNA: Mechanisms of formation, measurement, repair and biological effects. DNA Repair 2008, 7, 1413–1425. [Google Scholar] [CrossRef]
- Box, H.C.; Patrzyc, H.B.; Dawidzik, J.B.; Wallace, J.C.; Freund, H.G.; Iijima, H.; Budzinski, E.E. Double base lesions in DNA X-irradiated in the presence or absence of oxygen. Radiat. Res. 2000, 153, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Crean, C.; Geacintov, N.E.; Shafirovich, V. Intrastrand G-U cross-links generated by the oxidation of guanine in 5′-d(GCU) and 5′-r(GCU). Free Radic. Biol. Med. 2008, 45, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Crean, C.; Uvaydov, Y.; Geacintov, N.E.; Shafirovich, V. Oxidation of single-stranded oligonucleotides by carbonate radical anions: Generating intrastrand cross-links between guanine and thymine bases separated by cytosines. Nucleic Acids Res. 2008, 36, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.H.; Geacintov, N.E.; Shafirovich, V. Generation of guanine-thymidine cross-links in DNA by peroxynitrite/carbon dioxide. Chem. Res. Toxicol. 2011, 24, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R.; Shafirovich, V.; Geacintov, N.E. One-electron oxidation reactions of purine and pyrimidine bases in cellular DNA. Int. J. Radiat. Biol. 2014, 90, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Yamaizumi, Z.; Yamamoto, F.; Bessho, T.; Nishimura, S.; Berger, M.; Cadet, J. Photosensitized formation of 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) in DNA by riboflavin. Nucleic Acids Symp. Ser. 1992, 27, 181–182. [Google Scholar]
- Shukla, L.I.; Adhikary, A.; Pazdro, R.; Becker, D.; Sevilla, M.D. Formation of 8-oxo-7,8-dihydroguanine-radicals in gamma-irradiated DNA by multiple one-electron oxidations. Nucleic Acids Res. 2004, 32, 6565–6574. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. Oxidatively generated base damage to cellular DNA by hydroxyl radical and one-electron oxidants: Similarities and differences. Arch. Biochem. Biophys. 2014, 557, 47–54. [Google Scholar] [CrossRef]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef]
- Hung, K.F.; Sidorova, J.M.; Nghiem, P.; Kawasumi, M. The 6-4 photoproduct is the trigger of UV-induced replication blockage and ATR activation. Proc. Natl. Acad. Sci. USA 2020, 117, 12806–12816. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Wagner, J.R.; Cadet, J. Oxidatively generated tandem DNA modifications by pyrimidinyl and 2-deoxyribosyl peroxyl radicals. Free Radic. Biol. Med. 2023, 196, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Box, H.C.; Dawidzik, J.B.; Budzinski, E.E. Free radical-induced double lesions in DNA. Free Radic. Biol. Med. 2001, 31, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Douki, T.; Riviere, J.; Cadet, J. DNA tandem lesions containing 8-oxo-7,8-dihydroguanine and formamido residues arise from intramolecular addition of thymine peroxyl radical to guanine. Chem. Res. Toxicol. 2002, 15, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Bourdat, A.-G.; Douki, T.; Frelon, S.; Gasparutto, D.; Cadet, J. Tandem base lesions are generated by hydroxyl radical within isolated DNA in aerated aqueous solution. J. Am. Chem. Soc. 2000, 122, 4549–4556. [Google Scholar] [CrossRef]
- Bergeron, F.; Auvre, F.; Radicella, J.P.; Ravanat, J.L. HO* radicals induce an unexpected high proportion of tandem base lesions refractory to repair by DNA glycosylases. Proc. Natl. Acad. Sci. USA 2010, 107, 5528–5533. [Google Scholar] [CrossRef]
- Carter, K.N.; Greenberg, M.M. Tandem lesions are the major products resulting from a pyrimidine nucleobase radical. J. Am. Chem. Soc. 2003, 125, 13376–13378. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Jiang, Y.; Wang, Y.; Wang, Y. Efficient formation of the tandem thymine glycol/8-oxo-7,8-dihydroguanine lesion in isolated DNA and the mutagenic and cytotoxic properties of the tandem lesions in Escherichia coli cells. Chem. Res. Toxicol. 2010, 23, 11–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robert, G.; Wagner, J.R. Tandem Lesions Arising from 5-(Uracilyl)methyl Peroxyl Radical Addition to Guanine: Product Analysis and Mechanistic Studies. Chem. Res. Toxicol. 2020, 33, 565–575. [Google Scholar] [CrossRef]
- Hong, I.S.; Carter, K.N.; Sato, K.; Greenberg, M.M. Characterization and mechanism of formation of tandem lesions in DNA by a nucleobase peroxyl radical. J. Am. Chem. Soc. 2007, 129, 4089–4098. [Google Scholar] [CrossRef]
- Xerri, B.; Morell, C.; Grand, A.; Cadet, J.; Cimino, P.; Barone, V. Radiation-induced formation of DNA intrastrand crosslinks between thymine and adenine bases: A theoretical approach. Org. Biomol. Chem. 2006, 4, 3986–3992. [Google Scholar] [CrossRef]
- Gu, C.; Wang, Y. LC-MS/MS identification and yeast polymerase eta bypass of a novel gamma-irradiation-induced intrastrand cross-link lesion G[8-5]C. Biochemistry 2004, 43, 6745–6750. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Garrec, J.; Dupont, C.; Dumont, E. What singles out the G[8-5]C intrastrand DNA cross-link? Mechanistic and structural insights from quantum mechanics/molecular mechanics simulations. Biochemistry 2013, 52, 425–431. [Google Scholar] [CrossRef]
- Crean, C.; Lee, Y.A.; Yun, B.H.; Geacintov, N.E.; Shafirovich, V. Oxidation of guanine by carbonate radicals derived from photolysis of carbonatotetramminecobalt(III) complexes and the pH dependence of intrastrand DNA cross-links mediated by guanine radical reactions. Chembiochem 2008, 9, 1985–1991. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Geacintov, N.E.; Krokidis, M.G.; Liu, Y.; Masi, A.; Shafirovich, V.; Terzidis, M.A.; Tsegay, P.S. 5′,8-Cyclopurine Lesions in DNA Damage: Chemical, Analytical, Biological, and Diagnostic Significance. Cells 2019, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, A.; Becker, D.; Palmer, B.J.; Heizer, A.N.; Sevilla, M.D. Direct formation of the C5′-radical in the sugar-phosphate backbone of DNA by high-energy radiation. J. Phys. Chem. B 2012, 116, 5900–5906. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.; Jaruga, P.; Coskun, E.; Ward, S.; Stark, A.D.; Baumann, T.; Becker, D.; Adhikary, A.; Sevilla, M.D.; Dizdaroglu, M. Ne-22 Ion-Beam Radiation Damage to DNA: From Initial Free Radical Formation to Resulting DNA-Base Damage. ACS Omega 2021, 6, 16600–16611. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M.A. On the relevance of hydroxyl radical to purine DNA damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Eriksson, L.A.; Krokidis, M.G.; Masi, A.; Wang, S.; Zhang, R. Oxygen Dependent Purine Lesions in Double-Stranded Oligodeoxynucleotides: Kinetic and Computational Studies Highlight the Mechanism for 5′,8-Cyclopurine Formation. J. Am. Chem. Soc. 2020, 142, 5825–5833. [Google Scholar] [CrossRef]
- Terzidis, M.A.; Prisecaru, A.; Molphy, Z.; Barron, N.; Randazzo, A.; Dumont, E.; Krokidis, M.G.; Kellett, A.; Chatgilialoglu, C. Radical-induced purine lesion formation is dependent on DNA helical topology. Free Radic. Res. 2016, 50, S91–S101. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; O’Neill, P.; Stewart, R.D. Induction and repair of clustered DNA lesions: What do we know so far? Radiat. Res. 2013, 180, 100–109. [Google Scholar] [CrossRef]
- Bignon, E.; Gillet, N.; Chan, C.H.; Jiang, T.; Monari, A.; Dumont, E. Recognition of a tandem lesion by DNA bacterial formamidopyrimidine glycosylases explored combining molecular dynamics and machine learning. Comput. Struct. Biotechnol. J. 2021, 19, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Monari, A.; Dumont, E.; Bignon, E. Molecular Mechanisms Associated with Clustered Lesion-Induced Impairment of 8-oxoG Recognition by the Human Glycosylase OGG1. Molecules 2021, 26, 6465. [Google Scholar] [CrossRef] [PubMed]
- Gentil, A.; Le Page, F.; Cadet, J.; Sarasin, A. Mutation spectra induced by replication of two vicinal oxidative DNA lesions in mammalian cells. Mutat. Res. 2000, 452, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Y.; Wang, Y. In vitro replication and repair studies of tandem lesions containing neighboring thymidine glycol and 8-oxo-7,8-dihydro-2′-deoxyguanosine. Chem. Res. Toxicol. 2009, 22, 574–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imoto, S.; Bransfield, L.A.; Croteau, D.L.; Van Houten, B.; Greenberg, M.M. DNA tandem lesion repair by strand displacement synthesis and nucleotide excision repair. Biochemistry 2008, 47, 4306–4316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naldiga, S.; Huang, H.; Greenberg, M.M.; Basu, A.K. Mutagenic Effects of a 2-Deoxyribonolactone-Thymine Glycol Tandem DNA Lesion in Human Cells. Biochemistry 2020, 59, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Bacurio, J.H.T.; Gao, S.; Yang, H.; Basu, A.K.; Greenberg, M.M. Synergistic effects on mutagenicity of tandem lesions containing 8-oxo-7,8-dihydro-2′-deoxyguanosine or Fapy*dG flanked by a 3′5-formyl-2′-deoxyuridine in human cells. DNA Repair 2023, 129, 103527. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Colis, L.C.; Basu, A.K.; Zou, Y. Recognition and incision of gamma-radiation-induced cross-linked guanine-thymine tandem lesion G[8,5-Me]T by UvrABC nuclease. Chem. Res. Toxicol. 2005, 18, 1339–1346. [Google Scholar] [CrossRef]
- Colis, L.C.; Raychaudhury, P.; Basu, A.K. Mutational specificity of gamma-radiation-induced guanine-thymine and thymine-guanine intrastrand cross-links in mammalian cells and translesion synthesis past the guanine-thymine lesion by human DNA polymerase eta. Biochemistry 2008, 47, 8070–8079. [Google Scholar] [CrossRef]
- Ding, S.; Kropachev, K.; Cai, Y.; Kolbanovskiy, M.; Durandina, S.A.; Liu, Z.; Shafirovich, V.; Broyde, S.; Geacintov, N.E. Structural, energetic and dynamic properties of guanine(C8)-thymine(N3) cross-links in DNA provide insights on susceptibility to nucleotide excision repair. Nucleic Acids Res. 2012, 40, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, I.; Shafirovich, V.; Liu, Z.; Saint-Pierre, C.; Akishev, Z.; Matkarimov, B.T.; Gasparutto, D.; Geacintov, N.E.; Saparbaev, M. Oxidatively Generated Guanine(C8)-Thymine(N3) Intrastrand Cross-links in Double-stranded DNA Are Repaired by Base Excision Repair Pathways. J. Biol. Chem. 2015, 290, 14610–14617. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Terzidis, M.A. Purine 5′,8-cyclonucleoside lesions: Chemistry and biology. Chem. Soc. Rev. 2011, 40, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Merecz, A.; Karwowski, B.T. DNA tandem lesion: 5′,8-cyclo-2′-deoxyadenosine. The influence on human health. Mol. Biol. 2016, 50, 899–905. [Google Scholar] [CrossRef]
- Belmadoui, N.; Boussicault, F.; Guerra, M.; Ravanat, J.L.; Chatgilialoglu, C.; Cadet, J. Radiation-induced formation of purine 5′,8-cyclonucleosides in isolated and cellular DNA: High stereospecificity and modulating effect of oxygen. Org. Biomol. Chem. 2010, 8, 3211–3219. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Krokidis, M.G.; Masi, A.; Barata-Vallejo, S.; Ferreri, C.; Terzidis, M.A.; Szreder, T.; Bobrowski, K. New Insights into the Reaction Paths of Hydroxyl Radicals with Purine Moieties in DNA and Double-Stranded Oligodeoxynucleotides. Molecules 2019, 24, 3860. [Google Scholar] [CrossRef] [PubMed]
- Terzidis, M.A.; Chatgilialoglu, C. An ameliorative protocol for the quantification of purine 5′,8-cyclo-2′-deoxynucleosides in oxidized DNA. Front. Chem. 2015, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Di Mascio, P.; Wagner, J.R. (5′R)-and (5′S)-purine 5′,8-cyclo-2′-deoxyribonucleosides: Reality or artifactual measurements? A reply to Chatgilialoglu’s comments (this issue). Free Radic. Res. 2019, 53, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C. Cyclopurine (cPu) lesions: What, how, and why? Free Radic. Res. 2019, 53, 941–943. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Coskun, E.; Jaruga, P. Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques. Free Radic. Res. 2015, 49, 525–548. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, P.; Cui, Y.; Wang, Y. Chemical Analysis of DNA Damage. Anal. Chem. 2018, 90, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, B.; Guerrero, C.; Bahde, R.; Gupta, S.; Wang, Y. Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope-dilution method. Anal. Chem. 2011, 83, 2201–2209. [Google Scholar] [CrossRef]
- Wang, J.; Clauson, C.L.; Robbins, P.D.; Niedernhofer, L.J.; Wang, Y. The oxidative DNA lesions 8,5′-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell 2012, 11, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, I.; Bender, C.; Romieu, A.; Cadet, J.; Wood, R.D.; Lindahl, T. Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc. Natl. Acad. Sci. USA 2000, 97, 3832–3837. [Google Scholar] [CrossRef] [PubMed]
- Pande, P.; Das, R.S.; Sheppard, C.; Kow, Y.W.; Basu, A.K. Repair efficiency of (5′S)-8,5′-cyclo-2′-deoxyguanosine and (5′S)-8,5′-cyclo-2′-deoxyadenosine depends on the complementary base. DNA Repair 2012, 11, 926–931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kropachev, K.; Ding, S.; Terzidis, M.A.; Masi, A.; Liu, Z.; Cai, Y.; Kolbanovskiy, M.; Chatgilialoglu, C.; Broyde, S.; Geacintov, N.E.; et al. Structural basis for the recognition of diastereomeric 5′,8-cyclo-2′-deoxypurine lesions by the human nucleotide excision repair system. Nucleic Acids Res. 2014, 42, 5020–5032. [Google Scholar] [CrossRef] [PubMed]
- Jasti, V.P.; Das, R.S.; Hilton, B.A.; Weerasooriya, S.; Zou, Y.; Basu, A.K. (5′S)-8,5′-cyclo-2′-deoxyguanosine is a strong block to replication, a potent pol V-dependent mutagenic lesion, and is inefficiently repaired in Escherichia coli. Biochemistry 2011, 50, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Pednekar, V.; Weerasooriya, S.; Jasti, V.P.; Basu, A.K. Mutagenicity and genotoxicity of (5′S)-8,5′-cyclo-2′-deoxyadenosine in Escherichia coli and replication of (5′S)-8,5′-cyclopurine-2′-deoxynucleosides in vitro by DNA polymerase IV, exo-free Klenow fragment, and Dpo4. Chem. Res. Toxicol. 2014, 27, 200–210. [Google Scholar] [CrossRef]
- You, C.; Swanson, A.L.; Dai, X.; Yuan, B.; Wang, J.; Wang, Y. Translesion synthesis of 8,5′-cyclopurine-2′-deoxynucleosides by DNA polymerases eta, iota, and zeta. J. Biol. Chem. 2013, 288, 28548–28556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).