Cloud Point Extraction as a Green Method for the Extraction of Antioxidant Compounds from the Juice of Second-Grade Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Fruit Juice Analysis
2.4. Polyphenol Recovery Through CPE
2.4.1. CPE Procedure
2.4.2. Polyphenol Recovery Calculation
2.5. Bioactive Compound Determination
2.5.1. Total Polyphenol Content (TPC)
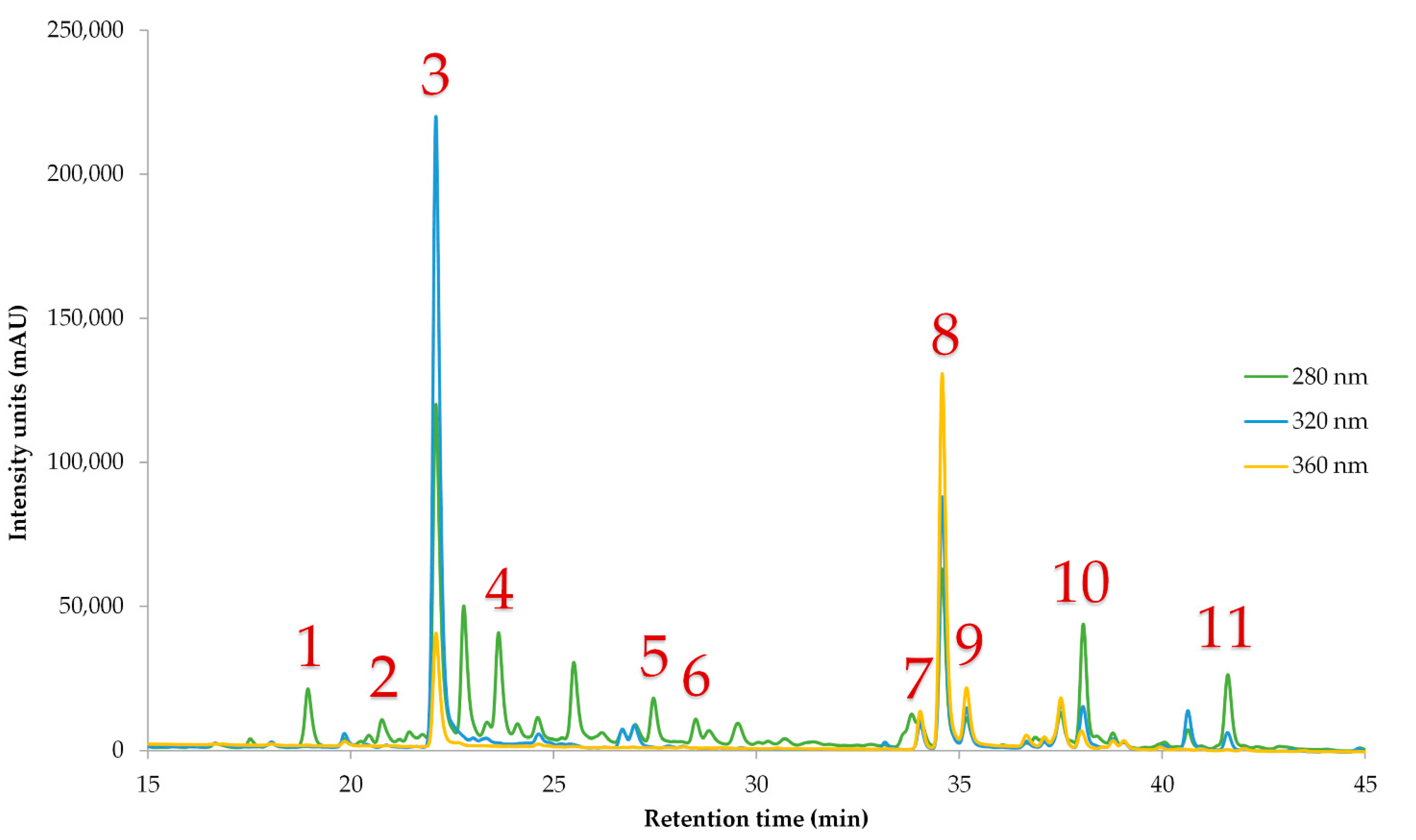
2.5.2. Individual Polyphenol Quantification
2.5.3. Ascorbic Acid Content (AAC)
2.6. In Vitro Antioxidant Capacity Evaluation
2.6.1. Ferric-Reducing Antioxidant Power (FRAP)
2.6.2. DPPH Radical Scavenging Activity
2.7. Statistical Processing
3. Results and Discussion
3.1. Physicochemical Characteristics of Apple Juice
3.2. Optimizing CPE Procedure
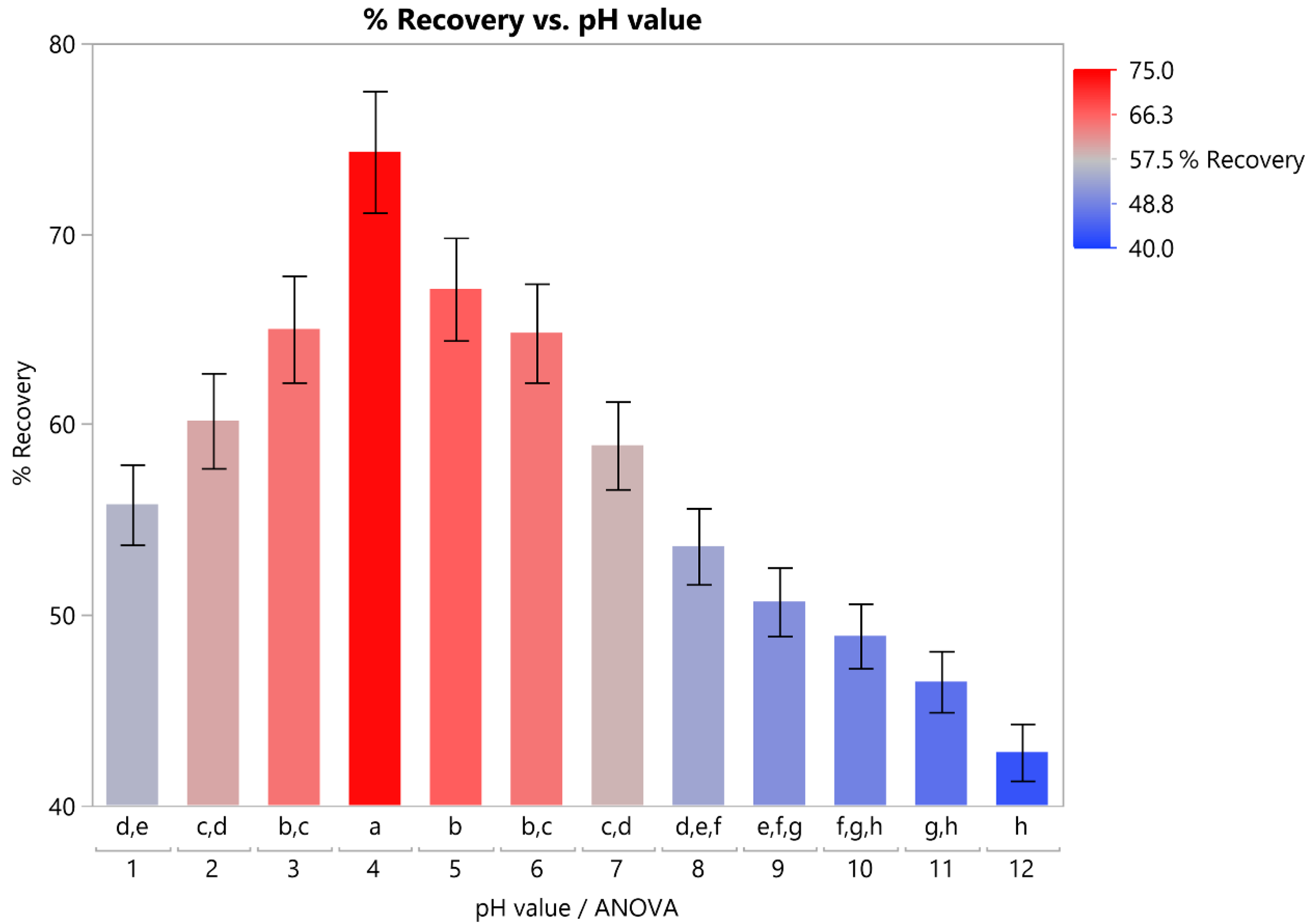
3.2.1. Optimal pH Level
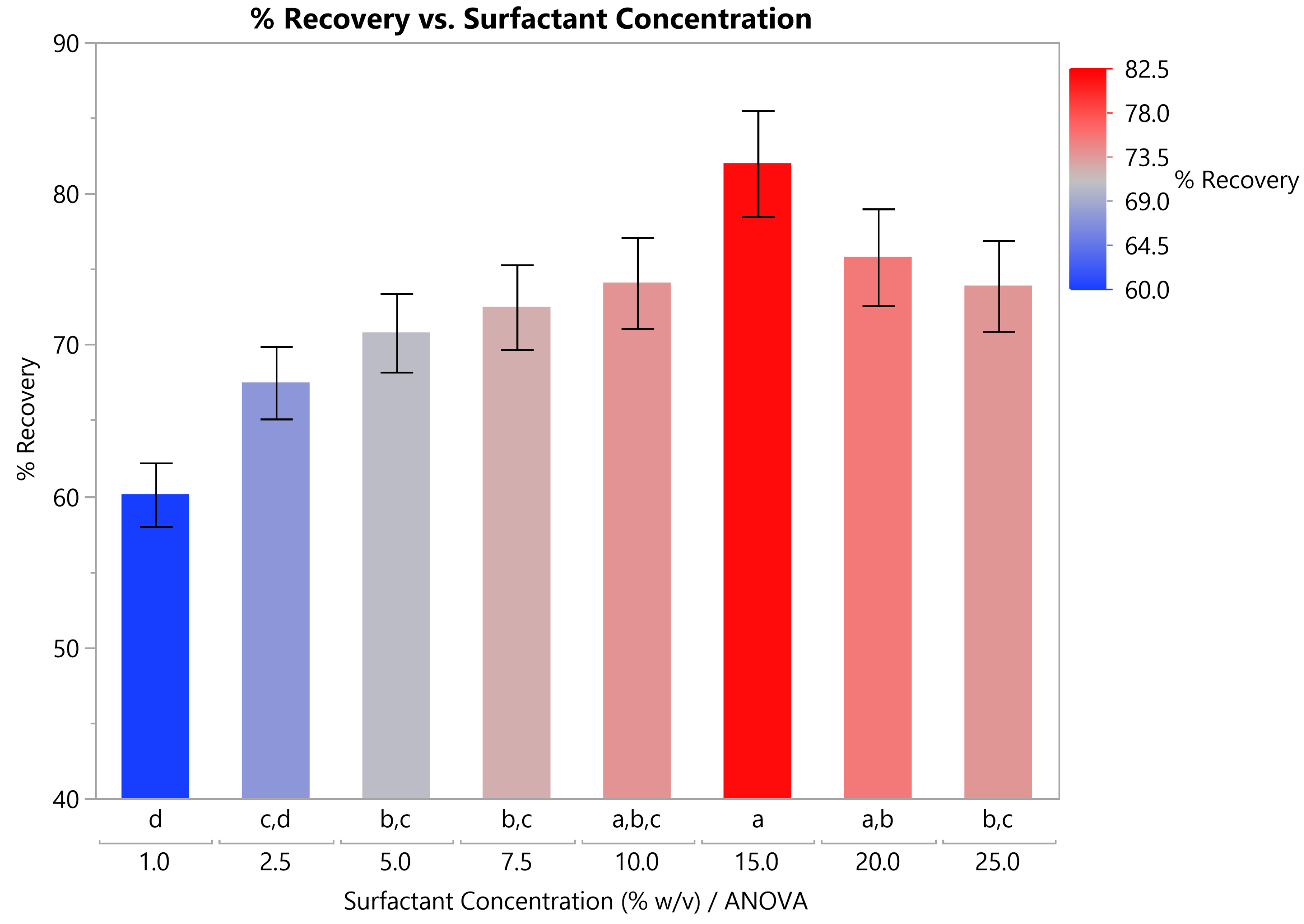
3.2.2. Optimal Surfactant Concentration
3.2.3. Optimal Salt Concentration
3.3. Optimal Sample Analysis
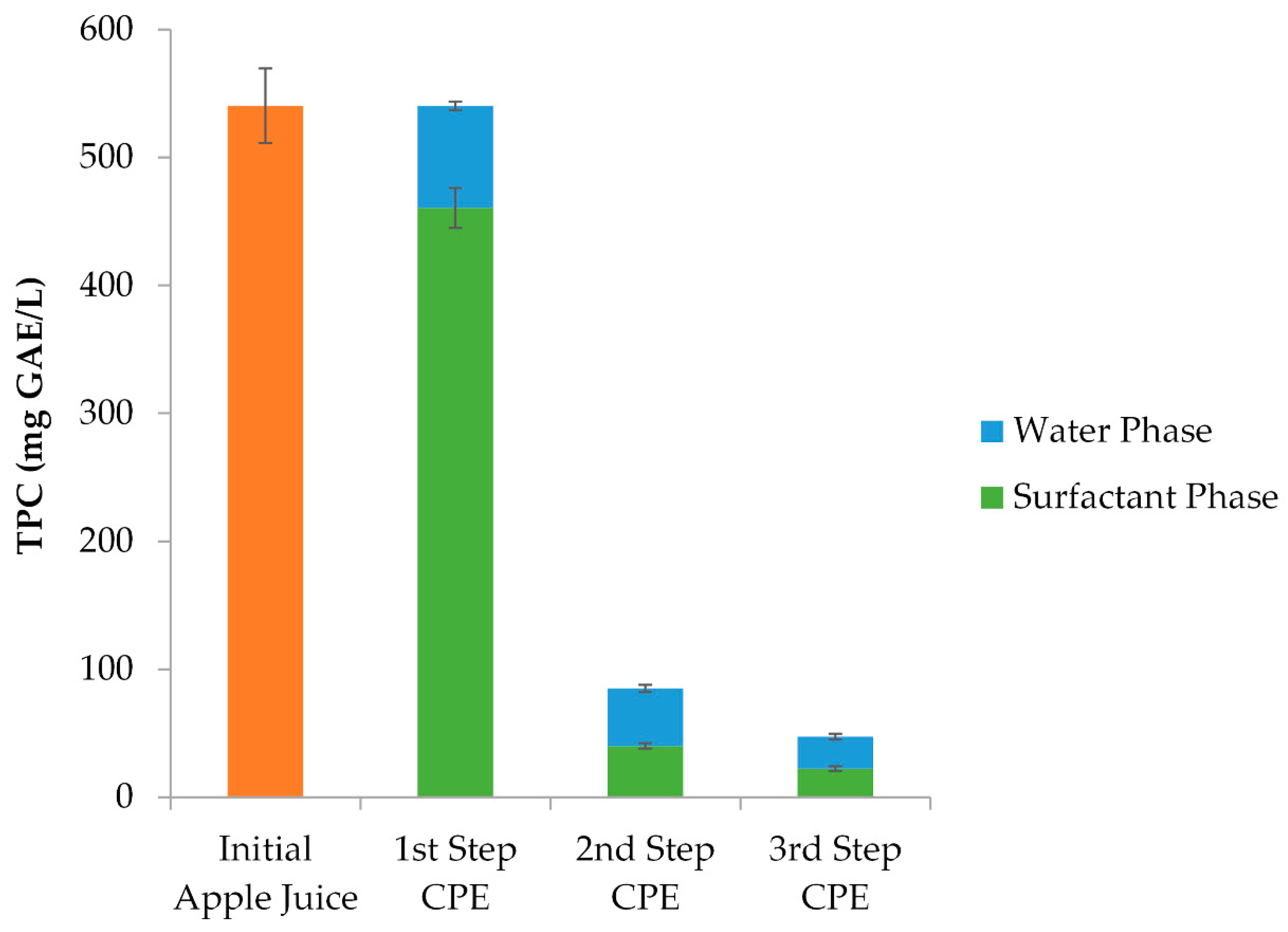
3.3.1. Optimized Polyphenol Recovery Through CPE
3.3.2. Comparison Between Apple Juice and Surfactant Phase
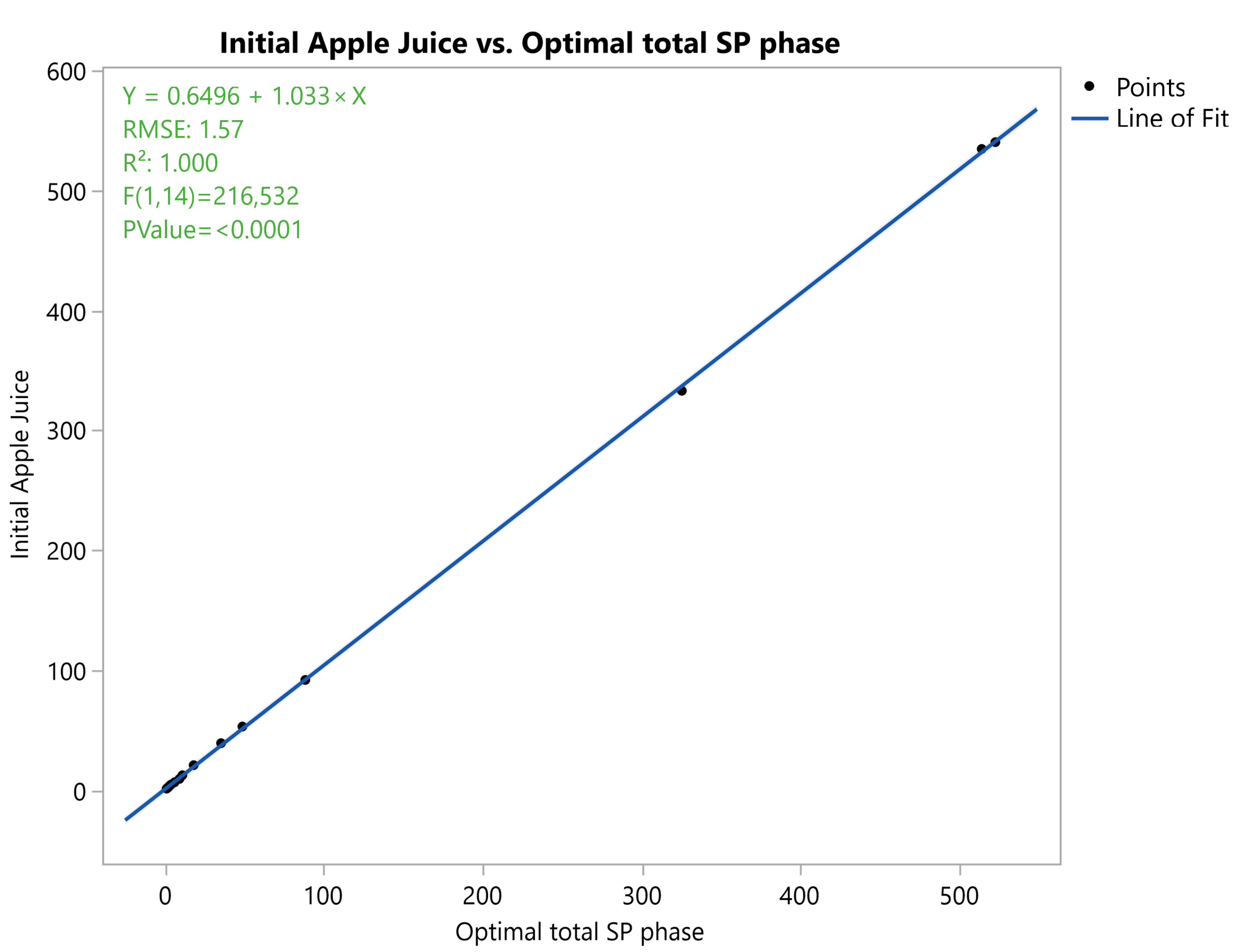
3.3.3. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Polyphenolic Compound | Equation | R2 | RT (min) | λmax | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|---|
| Pelargonin chloride | y = 1610.01x − 2626.92 | 0.997 | 18.900 | 275 | 2.84 | 8.61 |
| Catechin | y = 11,920.79x − 128.19 | 0.997 | 20.933 | 278 | 2.54 | 7.71 |
| Chlorogenic acid | y = 50,320.40x − 23,038.36 | 0.994 | 21.947 | 325 | 3.67 | 11.11 |
| Vanillic acid | y = 28,178.39x + 15,571.9 | 0.999 | 23.900 | 270 | 2.31 | 6.99 |
| Syringic acid | y = 24,093.04x + 6513.28 | 0.999 | 27.410 | 360 | 3.17 | 9.59 |
| Carnosol | y = 2553.28x − 17,572.60 | 0.959 | 28.531 | 271 | 18.55 | 56.21 |
| Rutin | y = 46,365.62x − 31,562.74 | 0.997 | 34.107 | 254 | 2.65 | 8.03 |
| Quercetin 3-D-galactoside | y = 41,489.69x − 35,577.55 | 0.993 | 34.598 | 257 | 3.96 | 12.00 |
| Quercetin 3-b-D-glucoside | y = 45,580.75x + 94,644.94 | 0.990 | 35.166 | 256 | 4.83 | 14.65 |
| Narirutin | y = 48,756.23x + 20,853.70 | 0.998 | 38.023 | 282 | 1.98 | 6.00 |
| Hesperidin | y = 33,528.61x − 30,502.75 | 0.995 | 41.649 | 283 | 3.59 | 10.87 |
References
- Vasile, M.; Bunea, A.; Ioan, C.R.; Ioan, B.C.; Socaci, S.; Viorel, M. Phytochemical Content and Antioxidant Activity of Malus domestica Borkh Peel Extracts. Molecules 2021, 26, 7636. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Foti, L.; Carlucci, G.; Scrano, L.; Martelli, G.; Brienza, M.; Coviello, D.; Bianco, G.; Lelario, F. Analytical Methods for Extraction and Identification of Primary and Secondary Metabolites of Apple (Malus domestica) Fruits: A Review. Separations 2021, 8, 91. [Google Scholar] [CrossRef]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef]
- Perdana, T.; Kusnandar, K.; Perdana, H.H.; Hermiatin, F.R. Circular Supply Chain Governance for Sustainable Fresh Agricultural Products: Minimizing Food Loss and Utilizing Agricultural Waste. Sustain. Prod. Consum. 2023, 41, 391–403. [Google Scholar] [CrossRef]
- Kechagias, E.P.; Gayialis, S.P.; Panayiotou, N.; Papadopoulos, G.A. A Holistic Framework for Evaluating Food Loss and Waste Due to Marketing Standards across the Entire Food Supply Chain. Foods 2024, 13, 3273. [Google Scholar] [CrossRef]
- Said, Z.; Vigneshwaran, P.; Shaik, S.; Rauf, A.; Ahmad, Z. Climate and Carbon Policy Pathways for Sustainable Food Systems. Environ. Sustain. Indic. 2025, 27, 100730. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Selmi, H.; Presutto, E.; Totaro, M.; Spano, G.; Capozzi, V.; Fragasso, M. Apple Waste/By-Products and Microbial Resources to Promote the Design of Added-Value Foods: A Review. Foods 2025, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Witrowa-Rajchert, D.; Nowacka, M. Turning Apple Pomace into Value: Sustainable Recycling in Food Production—A Narrative Review. Sustainability 2024, 16, 7001. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. [Google Scholar] [CrossRef] [PubMed]
- Bhadange, Y.A.; Carpenter, J.; Saharan, V.K. A Comprehensive Review on Advanced Extraction Techniques for Retrieving Bioactive Components from Natural Sources. ACS Omega 2024, 9, 31274–31297. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Kurup, S.S.; Maqsood, S. Comparative Evaluation of Ultrasound-Assisted Extraction with Other Green Extraction Methods for Sustainable Recycling and Processing of Date Palm Bioresources and by-Products: A Review of Recent Research. Ultrason. Sonochem. 2025, 114, 107252. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; da Fonseca Machado, A.P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Chen, Y.; Du, K.; Li, J.; Bai, Y.; An, M.; Tan, Z.; Chang, Y. A Green and Efficient Method for the Preconcentration and Determination of Gallic Acid, Bergenin, Quercitrin, and Embelin from Ardisia japonica Using Nononic Surfactant Genapol X-080 as the Extraction Solvent. Int. J. Anal. Chem. 2018, 2018, e1707853. [Google Scholar] [CrossRef]
- Sznek, B.; Kupczyk, O.; Czyrski, A. Cloud Point Extraction as an Environmentally Friendly Technique for Sample Preparation. Processes 2025, 13, 430. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Travičić, V.; Cvanić, T.; Vidović, S.; Pezo, L.; Hidalgo, A.; Šovljanski, O.; Ćetković, G. Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction. Foods 2024, 13, 2863. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction. Eng 2023, 4, 3026–3038. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Implementation of Cloud Point Extraction Using Surfactants in the Recovery of Polyphenols from Apricot Cannery Waste. Eng 2023, 4, 1225–1235. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Soto-Ángeles, A.G.; Rodríguez-Hidalgo, M.d.R.; Soto-Figueroa, C.; Vicente, L. Complementary Experimental-Simulational Study of Surfactant Micellar Phase in the Extraction Process of Metallic Ions: Effects of Temperature and Salt Concentration. Chem. Phys. 2018, 501, 15–25. [Google Scholar] [CrossRef]
- Hagarová, I.; Urík, M. Cloud Point Extraction in Beverage Analysis: Innovations and Applications for Trace Elements. Beverages 2024, 10, 67. [Google Scholar] [CrossRef]
- Kiai, H.; Raiti, J.; El-Abbassi, A.; Hafidi, A. Recovery of Phenolic Compounds from Table Olive Processing Wastewaters Using Cloud Point Extraction Method. J. Environ. Chem. Eng. 2018, 6, 1569–1575. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Mantiniotou, M.; Bujor, B.-C.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Methodology-Aided Optimization of Bioactive Compound Extraction from Apple Peels Through Pulsed Electric Field Pretreatment and Ultrasonication. Eng 2024, 5, 2886–2901. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A New Colorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Cendrowski, A.; Przybył, J.L.; Studnicki, M. Physicochemical Characteristics, Vitamin C, Total Polyphenols, Antioxidant Capacity, and Sensory Preference of Mixed Juices Prepared with Rose Fruits (Rosa rugosa) and Apple or Strawberry. Appl. Sci. 2024, 14, 113. [Google Scholar] [CrossRef]
- Will, F.; Roth, M.; Olk, M.; Ludwig, M.; Dietrich, H. Processing and Analytical Characterisation of Pulp-Enriched Cloudy Apple Juices. LWT-Food Sci. Technol. 2008, 41, 2057–2063. [Google Scholar] [CrossRef]
- Hodala, A.J.; Wood, I.G.; Carbone, P. Understanding the Influence of Electrostatic Interactions on Observed pKa Shifts in Surfactant Aggregates Using Classical Simulations. J. Mol. Liq. 2025, 427, 127410. [Google Scholar] [CrossRef]
- Kojro, G.; Wroczyński, P. Cloud Point Extraction in the Determination of Drugs in Biological Matrices. J. Chromatogr. Sci. 2020, 58, 151–162. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method. AgriEngineering 2023, 5, 2139–2154. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Exploring the Antioxidant Properties of Citrus limon (Lemon) Peel Ultrasound Extract after the Cloud Point Extraction Method. Biomass 2024, 4, 202–216. [Google Scholar] [CrossRef]
- Wang, M.; Yan, W.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Progress in the Application of Lecithins in Water-in-Oil Emulsions. Trends Food Sci. Technol. 2021, 118, 388–398. [Google Scholar] [CrossRef]
- Mortada, W.I. Recent Developments and Applications of Cloud Point Extraction: A Critical Review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- Hyde, A.M.; Zultanski, S.L.; Waldman, J.H.; Zhong, Y.-L.; Shevlin, M.; Peng, F. General Principles and Strategies for Salting-Out Informed by the Hofmeister Series. Org. Process Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef]
- Dewulf, B.; Cool, V.; Li, Z.; Binnemans, K. Effect of Polar Molecular Organic Solvents on Non-Aqueous Solvent Extraction of Rare-Earth Elements. Sep. Purif. Technol. 2022, 294, 121197. [Google Scholar] [CrossRef]
- Motikar, P.D.; More, P.R.; Arya, S.S. A Novel, Green Environment-Friendly Cloud Point Extraction of Polyphenols from Pomegranate Peels: A Comparative Assessment with Ultrasound and Microwave-Assisted Extraction. Sep. Sci. Technol. 2021, 56, 1014–1025. [Google Scholar] [CrossRef]
- Nour, V. Increasing the Content of Bioactive Compounds in Apple Juice Through Direct Ultrasound-Assisted Extraction from Bilberry Pomace. Foods 2024, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Z.; Chang, C. Chlorogenic Acid Intake Guidance: Sources, Health Benefits, and Safety. Asia Pac. J. Clin. Nutr. 2022, 31, 602–610. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Apple Juice |
|---|---|
| Active acidity (pH) | 3.83 ± 0.13 |
| Titratable acidity (TA) (as % w/w malic acid) | 0.93 ± 0.06 |
| Total soluble solids (TSS) (°Brix) | 14.41 ± 0.79 |
| Sweetness index (TSS/TA ratio) | 15.5 ± 0.17 |
| Astrigency index (TA/TSS ratio) | 0.06 ± 0 |
| L* (lightness) | 75.1 ± 0.9 |
| a* (redness) | 3.2 ± 0.1 |
| b* (yellowness) | 20.3 ± 0.6 |
| HEX code | CFB594 |
| Color |
| Parameters | Initial Apple Juice | Optimal Total SP | p-Value |
|---|---|---|---|
| TPC (mg GAE/L) | 540 ± 29 | 523 ± 20 | 0.4377 |
| FRAP (mmol AAE/L) | 4.52 ± 0.29 * | 3.79 ± 0.21 | 0.0247 |
| DPPH (mmol AAE/L) | 3.13 ± 0.13 * | 2.68 ± 0.09 | 0.0075 |
| AAC (mg/L) | 39.1 ± 1.7 | 35.4 ± 1.9 | 0.0645 |
| Polyphenolic Compounds (mg/L) | |||
| Pelargonin chloride | 91.8 ± 3.6 | 88.4 ± 5 | 0.3952 |
| Catechin | 6.48 ± 0.45 | 6.11 ± 0.35 | 0.3278 |
| Chlorogenic acid | 333 ± 19 | 326 ± 15 | 0.6170 |
| Vanillic acid | 12.3 ± 0.9 | 11 ± 0.8 | 0.1324 |
| Syringic acid | 9.27 ± 0.43 | 9.15 ± 0.25 | 0.6883 |
| Carnosol | 52.9 ± 1.5 * | 48.8 ± 1 | 0.0165 |
| Rutin | 2.05 ± 0.04 | 1.92 ± 0.13 | 0.1922 |
| Quercetin 3-D-galactoside | 20.7 ± 0.7 * | 18.1 ± 1.3 | 0.0397 |
| Quercetin 3-β-D-glucoside | 3.11 ± 0.12 * | 2.65 ± 0.19 | 0.0227 |
| Narirutin | 1.86 ± 0.07 * | 1.55 ± 0.07 | 0.0056 |
| Hesperidin | 1.17 ± 0.05 | 1.09 ± 0.04 | 0.1213 |
| Total identified | 535 ± 27 | 514 ± 24 | 0.3804 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togantzi, M.-I.; Mantiniotou, M.; Kalompatsios, D.; Athanasiadis, V.; Giovanoudis, I.; Lalas, S.I. Cloud Point Extraction as a Green Method for the Extraction of Antioxidant Compounds from the Juice of Second-Grade Apples. Biomass 2025, 5, 48. https://doi.org/10.3390/biomass5030048
Togantzi M-I, Mantiniotou M, Kalompatsios D, Athanasiadis V, Giovanoudis I, Lalas SI. Cloud Point Extraction as a Green Method for the Extraction of Antioxidant Compounds from the Juice of Second-Grade Apples. Biomass. 2025; 5(3):48. https://doi.org/10.3390/biomass5030048
Chicago/Turabian StyleTogantzi, Maria-Ioanna, Martha Mantiniotou, Dimitrios Kalompatsios, Vassilis Athanasiadis, Ioannis Giovanoudis, and Stavros I. Lalas. 2025. "Cloud Point Extraction as a Green Method for the Extraction of Antioxidant Compounds from the Juice of Second-Grade Apples" Biomass 5, no. 3: 48. https://doi.org/10.3390/biomass5030048
APA StyleTogantzi, M.-I., Mantiniotou, M., Kalompatsios, D., Athanasiadis, V., Giovanoudis, I., & Lalas, S. I. (2025). Cloud Point Extraction as a Green Method for the Extraction of Antioxidant Compounds from the Juice of Second-Grade Apples. Biomass, 5(3), 48. https://doi.org/10.3390/biomass5030048










