Abstract
Sunflower seed hulls and meal are among the most abundant by-products of the food industry. They are an example of waste and, at the same time, a plentiful biomass that cannot be utilized directly in human and animal diets due to their hard digestibility and low nutritional value. Besides their main compounds—carbohydrates, lipids, and proteins—they possess valuable constituents such as vitamins, minerals, and especially phenolics that contribute to their antioxidant capacity. Numerous benefits can be retrieved from such by-products. Since sunflower meal and seed hulls are cheap renewable sources of beneficial substances, their potential in relation to the improvement in our daily life needs to be studied. This is the reason why, in recent years, there has been such a serious interest in their utilization and valorization towards the concept of a circular bio-based economy and process sustainability. This review aims to trace the potential applications and implementation of sunflower meal and hulls in the different fields of industry and environmental protection strategies.
1. Introduction
One of the major challenges in recent years is the generation of huge amounts of waste. According to recent reports by the Food and Agriculture Organization of the United Nations (FAO), about 30–40% of total food production is lost before it reaches the market. This loss is a result of the significant amount of industrial waste generated in production and supply chains. When mismanaged, this waste poses a potential hazard on a global scale, as its risk factors are derived from the areas of ecology, economy, and social activities, impacting climate change, soil and water quality, and public health. In this regard, there is an urgent need to establish sustainable management practices that will mitigate environmental impacts and improve resource efficiency in the long term [1]. In recent years, because of the diminishing resources and the growing population, and with the aim of implementing a circular economy based on organic sources, there has been a rising interest in the utilization of by-products from the food industry, including edible oil production. It is known that the most consumed oil product in the world is sunflower oil [2]. The by-products obtained from its production contain numerous valuable components that can be used in energy production (biofuels), agriculture (animal feed additives, organic fertilizers), food industry (production of food fibers), pharmaceutical industry (production of activated carbon and bioactive substances), construction (insulation materials), etc. [3]. The examples of such by-products are sunflower seed hulls and meal.
The sunflower (Helianthus annuus L.) comes from the Asteraceae family and represents an annual crop with more than 70 species characterized by a large yellow inflorescence oriented towards the sun. According to the FAO (2000–2022), the global cultivated area of sunflower exceeds 100 million acres, making it the third most important oilseed crop after soybean and rapeseed. World sunflower seed production was estimated to be 58 million metric tons in 2022, and the sunflower husk generated was about 14 million cubic meters. This production capacity reflects the contribution of the main sunflower-producing countries, mainly Ukraine, Russia, and Argentina [4].
Sunflower oil production generates various by-products such as hulls, flakes, expelled cakes, or extracted meal. These waste products are reported to contain non-lipophilic antioxidants [5]. After oil extraction from sunflower seeds, a protein-rich solid residue is left behind, known as the sunflower meal (SM) or cake. The procedure of crushing the sunflower seed into oil transforms about 25–33% of the initial seed into oil [6]. Then, it is followed by the extraction of the meal, which is usually performed with hexane or methanol, and oil filtration or decantation. This step turns SM into a by-product mainly used as animal feed or soil fertilizer [7].
Sunflower seed hulls (SSHs) account for about 20–30% of sunflower seeds and are usually removed before oil extraction. They are an abundant and inexpensive by-product that is subsequently stored outdoors (occupying considerable space), landfilled, or incinerated. Oil refineries burn sunflower hulls in furnaces, accompanied by various technical and economic problems, which in turn make their industrial use labor-intensive and challenging. The transportation of the biomass is expensive because of its low density. Furthermore, storage is associated with problems such as dry matter losses, compositional changes, and fire risks. On the other hand, sunflower hulls have an extremely low nutritional value for humans and animals owing to their difficult digestibility. However, this lignocellulosic agricultural waste is a promising source of renewable energy and chemical components [3].
2. Sunflower Meal and Hull Composition
SM is characterized by high protein content (about 30% to 50%) consisting mainly of albumins (17% to 30%), globulins (mainly helianthin protein, 300 to 350 kDa), and other minor proteins (including oleosins) [8]. SM protein isolates do not contain toxic substances and have fewer antinutritional components than other protein-rich sources [9]. These proteins are known to possess a well-balanced amino acid profile and include an abundant quantity of sulfur-containing amino acids in comparison with other plant proteins [10]. The amino acid profile of SM is demonstrated in Table 1.

Table 1.
Amino acid profile of SM [11].
SM proteins can exhibit satisfactory techno-functional properties such as foaming, gelling, emulsifying, and binding. These qualities make them appropriate to be incorporated in foods, including next-generation plant-based foods such as egg, meat, seafood, and dairy substitutes. However, SM protein incorporation in food still faces several drawbacks like the brown color of the meal, antinutritional agents (as they may reduce the nutrient intake, utilization, and absorption) [12], and low digestibility [13].
Other valuable components in SM are vitamins and minerals, fibers, and polyphenols (like chlorogenic acid) [14]. It is rich in calcium, phosphorus, and water-soluble B-group vitamins (nicotinic acid, pantothenic acid, thiamine, riboflavin, and biotin) [15] (Figure 1). All these compounds make SM an excellent product for the food industry.
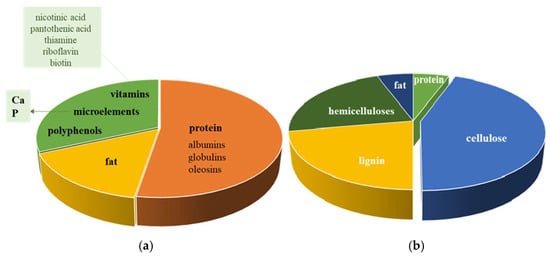
Figure 1.
Sunflower meal (a) and seed hull (b) content.
Many studies have reported the high antioxidant capacity of SM owing to the phenolic substances such as caffeic, chlorogenic, and ferulic acids that are the main contributors. Concerning chlorogenic acid, it plays important roles in the human body owing to its antioxidant, anti-inflammatory, anti-diabetic, antihypertensive chemo-preventive [16], and antimicrobial activities [17]. Therefore, these substances can be extracted and used as antioxidants from cheap food-related waste of natural origin [18]. Whole SM can be used either for recovery by the extraction of antioxidants or can be directly added to vegetable oil. For instance, Kreps and co-workers demonstrated that the addition of 20 g·kg−1 whole sunflower meal increased its protective effect by 1.35 times before sunflower oil oxidation [19].
As a by-product of sunflower oil production, sunflower hulls have been the subject of various studies and scientific publications. They are reported to contain carbohydrates (31–51% cellulose, 13–28% hemicelluloses, 20% lignin, xylose, furfural), 4–6% proteins and amino acids, 5% lipids (containing long-chain fatty acids), fatty alcohols, terpene alcohols, sterols, and flavonoids (such as anthocyanin) and phenolic acids [20]. These compounds turn SSHs into a highly suitable raw material for the purposes of the biorefinery and the circular economy. Phenolic compounds possess a wide range of physiological activities, such as antioxidant, antiallergic, antiatherogenic, anti-inflammatory, antimicrobial, and antithrombotic. In addition, they possess a vasodilating effect and have an overall beneficial effect on the cardiovascular system. Approximately 0.7–5.4% of the phenolic compounds of the sunflower seeds are concentrated in the hulls, which turns this waste product into a potential source for the extraction of valuable biologically active components [20]. The major phenolic compounds in the hulls include caffeic, cinnamic, ferulic, coumaric, hydroxycinnamic, and chlorogenic acids, the latter accounting for about 80% of the phenolic compound content [21].
3. Valorization and Potential Applications of SM and SSHs in Industry
3.1. Corrosion Inhibitor
Despite the fact that organic inhibitors are conveniently used for the protection of corrosion, most of them are synthesized by chemical means. Chemical synthesis is an expensive and environmentally hazardous process, so its applications are limited. Hence, implementing low-cost “green” compounds is a very important strategy [21].
SSH extract was tested for an environmentally friendly corrosion inhibitor for carbon steel. Analysis has shown that SSHs contain oxygen and nitrogen heteroatoms and aromatic loops, which are suitable for corrosion inhibitors in acidic media (1 M HCl). Inhibition efficiency was 98% in the presence of 400 ppm of inhibitor. The inhibition occurred through absorption on the metal surface, and its adsorption was followed by the Langmuir adsorption isotherm. Deeper analysis revealed that a surface complex between the functional groups of the inhibitor and ions of the metal was formed. Therefore, this large complex blocked the metal surface for the corrosion agents, thus reducing the charge and the ion transfer. Consequently, the corrosion resistance was improved [21].
3.2. Concrete Stabilizer
It was reported that the SSH ash influences the structure formation and properties of cement concrete. The increase in SSH content was found to be in correlation with the decrease in concrete mixture workability. Concrete mixtures with SSHs possess reduced density in comparison with those without the admixture. The density gradually decreases with the percentage increase in the substitution rate from 2 to 16%. When compared to traditional concrete, the reduction in durability was insignificant. The addition of SSH ash to the concrete mixture had a negative effect on the workability characterized by the cone slump. When SSH ash dosage increased above 8%, the workability reduction became more significant. The reason why SSH ash influences the slump of concrete mixtures is that its particles possess a high dispersion and water requirement.
It was also observed that the substitution of cement with SSH ash in the range between 2 and 12% enhances the physical and mechanical properties of concrete. The most visible effect was demonstrated at 8%, with a 14.89% increase in strength and a 15.78% reduction in the absorption of water in comparison with the control mixture. This is a result of the fact that the reactive oxides SiO2 and CaO of the added microsilica and SSH additives led to the formation of additional crystallization centers of calcium hydrosilicates, which creates a more stable structure. The addition of more than 12% SSHs is irrational and results in the deterioration of concrete properties. The observations of this study demonstrate that concrete with ash from SSHs is a possible option in cases when construction with additional requirements for durability and quality is needed [22]. These discoveries are important as they can eventually solve the problems with plant waste recycling worldwide, which is an urgent problem [23].
3.3. A Source of Antioxidants
Phenolics are known for their bioactive properties, such as antioxidant, antimicrobial, antihypertensive, and inhibition of carcinogenesis [24]. The antioxidant action of phenolic compounds is correlated with the neutralization of free radicals, which are responsible for oxidative stress and are a prerequisite for many ailments and diseases. These qualities make them particularly important for maintaining health. A number of scientific papers are focused on the study of phenolic compounds about their incorporation into functional foods in the pharmaceutical and food industries [25]. In this regard, ground dehulled seed shells were employed in a procedure for antioxidant production. The first step was the removal of the phenol fraction with the following solvents: water, methanol/water (60:40), ethanol/water (60:40), and acetone/water (60:40). Both ethanol/water and acetone/water mixtures were shown to be suitable for the dephenolization process. The next step was the hydrolysis of sunflower seed phenols with NaOH and, finally, the recovery of caffeic acid derived from chlorogenic acid with ethyl acetate. These steps resulted in 90 mg of powdery antioxidant product from 25 g of partially defatted shells. Additional tests estimated that the effectiveness of the sunflower antioxidant was very similar to that of caffeic acid standard and a little lower than that of propyl gallate [26]. Kachrimanidou obtained 214.3 mg/100 g (dry basis) of chlorogenic acid from SM through liquid fractionation, ultrasound, and 50% ethanol solution [27]. Laguna et al. achieved 100% extracted phenolics in a single step of simultaneous extraction of proteins and phenolic compounds. Caffeic, 3-caffeoylquinic, and mono- and di-caffeoylquinic acids were among the extracted compounds [28].
Menzel and co-workers incorporated the antioxidant properties of SSHs in starch films for food packing [3]. They extracted valuable phenolic compounds from SSHs with 80% methanol and embedded them into starch films. Chlorogenic acid was defined as the main active component with antioxidant activity in SSHs. The highest antioxidant capacity of the films was reached with 4–6% extracts, which led to the lowest oxygen permeability, high stiffness, and low extensibility. Phenolic extracts predominantly affected the mechanical properties of the starch films [3].
Antioxidant films produced by a cheap by-product of the food industry, like SSHs, would greatly contribute to the material and process sustainability towards a circular bio-based economy. Incorporation of antioxidants or antimicrobials into the packaging materials is an intelligent concept for shelf-life extension, thus improving food safety or sensory properties [29]. Active packaging systems are designed to either deliver a compound to the packed food or to remove an undesired substance from the product and its environment. In the food industry, edible packaging represents a thin layer formed on the food surface before the outermost package of the product. These layers could be consumed directly with the food, and if not, they will degrade more easily in the environment in comparison to synthetic packaging materials. Another advantage of edible packaging is that it will not contribute to health problems if contact occurs between the packaging layer and the food, which means that they are safe for human consumption [30]. Moreover, they may even promote beneficial effects on human health. In addition, natural films are far more cost-effective [10].
Salgado et al. achieved 28.4% antioxidant capacity for films from SM protein and bovine plasma protein hydrolysates [31]. Adding bovine plasma hydrolysates improves the antioxidant properties of soybean and sunflower protein-based films. In another investigation, clove essential oil was incorporated into formulations from SM protein, which led to biodegradable edible films with antioxidant activities [32]. Moreover, the films exhibited antimicrobial properties. The interchange between the proteins and essential oil caused diminished water solubility and glass transition temperature, and at the same time, it did not affect the moisture content, opacity, color, thickness, water vapor permeability, or film mechanical properties. The films achieved delayed oxidation and reduced growth of mesophilic microbes. Moreover, the team developed a film with SM protein, red seaweed, and grapefruit extract meant for duck meat preservation. The result was that, after 12 days of preservation, the film could inhibit Listeria monocytogenes growth [31].
Natural products with antioxidant characteristics like SSHs are capable of potentially displacing synthetic ones as they are biologically biodegradable and are usually considered safe [33]. Phenolics are suitable food additives as they control lipid oxidation and microbial growth [34,35]. They can be employed in the cosmetic industry as additives in eye creams, mouthwashes, and herbal mixtures [36,37].
3.4. Protein Source for the Food Industry
The by-products from sunflower processing could be a source of protein isolates for dietary purposes. In this regard, their advantages are their high biological value and good protein functional properties, as well as their lack of antinutrients (and toxic substances) [9]. Plant proteins are a good alternative to animal-origin proteins due to their lower price and the fact that plants are in sufficient quantity [38]. Currently, despite these qualities, sunflower seed hulls and meal are used for animal food [39]. One of the bottlenecks for their conversion to protein isolates is the presence of phenols. During the sunflower processing, these phenols are readily oxidized and interact with proteins. As a result, substances with an unacceptable dark color are produced, which are hardly metabolized, and possess low biological value and decreased functional properties [40]. For this reason, research has been directed towards the implementation of methods for protein extraction with negligible protein/phenol interaction, where light-colored protein isolates are obtained. One of the possibilities is salt extraction in a weakly acidic medium. According to Pickard et al., the most proper conditions are a pH of about 6 and NaCl at a concentration of 1.6–2.1 mol·L−1. This high salt concentration, on the one hand, inhibits the interaction of proteins with polyphenols and, on the other hand, increases their extractability, which allows the production of protein isolates with a high yield and a light color [5].
SM was reported to be an additive in the preparation of bread, pretzels, pasta, tortillas, brownies, pancakes, cookies, protein bars, and cereal [41]. It was demonstrated that bread prepared with 5–20% SM ingredient improves its protein content [42]. Muffins with 15% and 30% of the defatted SM ingredient showed an increase in the protein (19.4% and 37.3%, respectively) and mineral content (23.9% and 47.0%, respectively) and reduced carbohydrate content (1.7% and 4.8%, respectively) [43]. Added to cookies, 18% and 36% of SM ingredients increased the protein content (35.3% and 50.0%, respectively) and total phenolics (~42.8% and 85.7%, respectively), as well as antioxidant activity (166.7% and 350.0%, respectively) [43].
Zaky and colleagues added sunflower meal protein isolate (SMPI) to wheat flour pasta, targeting enhanced protein content. Generally, the protein in the wheat flour is about 10.90%, while in SM, it is 87.12%. The wheat flour was supplemented with SMPI in three different concentrations—3.0, 6.0, and 9.0% w/w, respectively. It was established that water absorption, the time required for dough development processes, stability, and mixing tolerance index are augmented with the increase in SMPI concentration. The color of the pasta became darker with the increase in the SM protein concentration. Although the mouth feel and overall acceptability of pasta were enhanced by 3.0% and 6.0%, SMPI had no noticeable effect on the pasta compared to the control sample, while the taste was not significantly affected by the pasta with 3.0% SMPI [38]. SMPI proteins were also reported to be used as matrices loaded with antioxidants and antimicrobial agents like essential oils, grapefruit seed extract for the food industry, and protein hydrolysates [31,32,44].
Recently, a team of scientists developed a meat alternative mix with textured sunflower protein consisting of 36% sunflower protein meal, tomato powder, spices, and meat-flavored yeast. The final product contained 20.10% proteins, 9.86% carbohydrates, 38.15% lipids, and 14.76% fibers. The managed amino acid profile of the developed product is shown in Table 2. The product met 67.1% approval for appearance and 65.9% for aroma among consumers. Although its overall acceptability was 57.4%, the texture and flavor of the product still need to be improved [45].

Table 2.
Amino acid profile of the meat alternative mix with textured sunflower protein.
The challenges in the development of alternative meal mixes are related to the harmonization of the nutritional, technological, and sensory aspects. Regarding the nutritional factor, it is of particular significance to achieve the necessary amino acid profile and nutritional density, which is an obstacle when using plant-based ingredients with possible limiting amino acids [46]. Sensory challenges correlate with the replication of meat flavor and taste. For the incorporation of sunflower products as a protein source, the identification of off-flavor compounds and pleasant-smelling and pleasant-tasting substances is of basic importance [47].
3.5. Additive to Cultivation Media
Recycling secondary raw materials is one of the principal sectors of biotechnology. Different by-products from the wood-processing industry and agriculture, like hulls, sawdust, and bran, are being utilized as an additive to the nutrient media for higher mushroom cultivation. SSHs are an appropriate additive because of their rich content of lignin, cellulose, carbohydrates, and nitrogen [48].
Milled sunflower seed hulls were used for fungi cultivation, aiming at the production of composite biodegradable materials. Basidial fungi are well known to produce lignolytic enzymes, which are capable of degrading plant waste. These properties are an attractive strategy in the creation of composite materials that can be employed in bioplastics, bio-packing, and building materials. Sysoeva et al. cultivated Trametes polyzona (Pers.) Justo (2011) and Trichaptum abietinum (Dicks) Ryvarden (1972) on different solid nutrient media consisting of SSHs. The most durable composite structure was established in the case of nutrient media consisting of SSHs and oat bran [49]. SSHs are not sufficient on their own for the fungi as these organisms require Mn (II), Zn (II), and Cu (II) salts, glucose, urea, and ammonium sulfate for their growth [50,51,52].
Table 3 demonstrates the different studies addressing the possible alternative applications of SM and hulls in various industrial fields and in environmental protection.

Table 3.
Alternative applications of sunflower by-products.
4. Environmental Benefits of the Sunflower By-Products
Environmental issues are a topic of primary significance. Thus, more natural treatments and approaches should be sought. Biodegradable and renewable sources are of environmental interest.
4.1. Bio-Oil Production
Pyrolysis is a method that allows the storage and densification of huge amounts of SSHs. In this regard, it is a thermochemical conversion process that produces energy-dense bio-oils from biomass. Because of their low pH, these liquids are corrosive [66], and the spontaneous condensation of lignin compounds results in tar precipitation. In their work, Casoni and co-workers performed pyrolysis at 400 °C under N2 flow with the aim of obtaining valuable chemicals. Two types of pretreatments were performed for the production of bio-oils—washing with dilute mineral acid and a biotransformation by the enzymatic lignin-attacking system of the mushroom Ganoderma lucidum. The obtained liquid corresponding to untreated SSHs was characterized by high acetic acid concentration and low storage stability, while the bio-oil from the acid-pretreated SSHs contained high amounts of furfural and levoglucosenone. As the mushroom enzymatic complex degraded lignin, this method of pretreatment resulted in the highest bio-oil yield with relatively high acetic acid concentration and good storage stability [53].
In another study of Casoni, the authors compare the composition of bio-oils obtained from raw and pretreated sunflower seed hulls. The preliminary treatment of sunflower hulls consisted of washing with solutions of phosphoric and sulfuric acids and sodium hydroxide solution, followed by hydrothermal treatment at 180 °C. Then, the raw and pretreated hulls underwent pyrolysis at 450 °C under 200 mL·min−1 N2. As a result of the different conditions, serious differences in the bio-oils’ characteristics were observed. The hydrothermal pretreatment achieved a bio-oil highly rich in levoglucosan, while the oil from the phosphoric acid pretreatment yielded high furfural content. The bio-oil from the NaOH pretreatment was found to be very unstable [54]. Acid pretreatment has advantages for its simplicity and cost-effectiveness, making it the closest to commercialization pretreatment [67]. Diluted acids are known to hydrolyze hemicellulose while cellulose remains mainly unaffected. Lignin can undergo solubilization and re-condensation [68]. On the other hand, hydrothermal treatment is an environmentally friendly method as it uses just water and chemicals [69]. Lignin solubilization is the aim of the alkaline pretreatment while cellulose and hemicellulose usually stay unchanged [70]. Reports show that pyrolysis results in bio-oils with absolutely different content from those produced from raw materials [54].
A technology like pyrolysis could successfully address environmental issues concerning waste biomass treatment. In this regard, the thermal conversion of waste biomass into valuable compounds would pose an economically profitable strategy.
A schematic diagram of the valorization of SM and SSHs is demonstrated in Figure 2.
4.2. Bio-Ethanol Production
A wide variety of biomass has been tested for biofuel production, such as cotton, corn stalks and cobs, wheat and rice straw, groundnut shells, etc. [71]. Lignocellulosic waste products are considered an attractive feedstock for fuel ethanol production as they are widely available at low cost; however, little attention has been paid to SSHs. Sharma and co-authors examined their potential to be used as a biomass for ethanol production. The crop was first pretreated with Trichoderma reesei Rut C30 cellulase, which resulted in 59.8% saccharification. Then, the concentrated hydrolysate was fermented with Saccharomyces cerevisiae var. ellipsoideus at 30 °C. The yielded ethanol was calculated to be 0.454 g/g. A scale-up was performed in 1 L and 15 L fermenters under optimal conditions, which revealed maximum ethanol yields of 0.449 and 0.446 g/g, respectively, under optimal conditions [55].
Pichia stripitis is another documented microorganism used for ethanol production from SSHs. In this study, SSHs were first pretreated with sulfuric acid at 90 °C. The resulting hydrolysate was employed as a substrate for Pichia stripitis fermentation. The hydrolysate with 48 g·L−1 total reducing sugars yielded 0.32 g/g ethanol with a volumetric productivity of 0.065 g/g [56].
The application of bio-ethanol in fuel blends would contribute to reduced CO2 emissions. Contrary to fossil fuels, ethanol is a renewable energy source and can be produced in a sugar fermentation process [56].
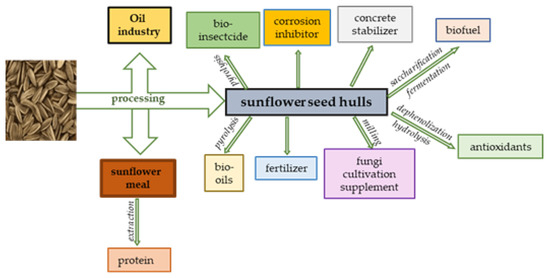
Figure 2.
Valorization of sunflower meal and sunflower seed hulls.
4.3. Bioinsecticide and Fertilizer
SSHs were reported to be used as a starting material for the co-production of bioinsecticides and biochar. The biomass residues were first pretreated with H3PO4; then, a two-bench scale pyrolysis (Py1 and Py2) method was conducted. In Py1, the hulls were pyrolyzed at 450 °C with 20 mL·min−1 N2, while in the Py2 methodology, they were pyrolyzed from room temperature to 450 °C with 50 mL·min−1 N2. Acid pretreatment is a necessary step for alkali metal reduction and the removal of inorganic salts. This pretreatment proved to be beneficial for the bio-oil production during pyrolysis. The produced bio-oil was rich in furfural, which is responsible for insecticidal activity and could be used as a bioinsecticide. The authors established that biochar also fulfills the prerequisites for soil amendment. Biochar enriches soil organic matter, microbial activity, nutrient availability, and water capacity retention [72,73]. They even supposed that about 23,158 kg SSH per year can be processed into bioinsecticides with a minimal selling price of USD 11/kg [57].
Within the framework of the circular economy, Ninkov and team proposed that the seed husks resulting from the processing of sunflowers be buried and used as fertilizing agents. The analysis of the samples demonstrated no heavy metals, low content of soluble phosphorus and microelements, and completely water-soluble potassium. The content of Ca, Na, and partially Mg is considered a limiting factor because of the possible degradation in soil, alkalization, and salinization in the case of higher concentrations and excessive application. Additionally, the content of water-soluble microelements with a favorable effect on plant growth and development (Fe, Zn, Cu, and Mn) is low [58].
4.4. Adsorbent
Besides being incorporated in edible packing, sunflower by-products could address environmental issues as an adsorbent of unwanted hazardous substances in water. Overproduction and overconsumption of pharmaceuticals pose an environmental risk to water [74]. For instance, chloroquine and its derivatives (which are products from the pharmaceutical industry) are used for the treatment of malaria and autoimmune and rheumatoid diseases. Ferreira and team proposed SSHs as a potential cost-effective bio-adsorbent for chloroquine removal from water. They used natural and chemically treated SSHs. The maximal adsorption capacities were 168.09 ± 22.98 mg/g for SSHs and 185.91 ± 27.23 mg/g for the chemically treated SSHs. The contact times varied between 2.5 and 1440 min, and the contaminant solution concentration was 20 mg·L−1. Chemical treatment resulted in larger and more open pores, which were favorable for the adsorption process [65]. This could be a consequence of the oxidative lignin depolymerization by methanol [59] used for the chemical treatment. The experiments demonstrated that both natural and treated SSHs are feasible chloroquine adsorbents as they showed high maximum adsorption capacities and efficient removal at the lowest adsorbent concentrations tested. The literature review unveils that SSHs have the capacity to adsorb not only chloroquine but also other pharmaceutical contaminants such as tetracycline, ciprofloxacin, ibuprofen, and sulfamethoxazole [75,76].
Phenol and naphthenic acids present in petroleum-produced water are other dangerous compounds of environmental threat. Their effective removal requires specially designed adsorbents due to their diverse structure, competitive adsorption, and non-polarity. An alternative green approach was proposed by an international team of scientists. They developed a reusable cross-linked adsorbent from SSHs and β-cyclodextran (SFSH-β-CD) for the removal of aromatics at a dosage of 1.0 g·L−1. Removal efficiencies over 90% for a wide pH range for phenol (1–8) and cyclohexane carboxylic acid (1–3) were reported. A rapid initial uptake for the adsorption of both phenol and cyclohexane carboxylic acid within the first 50 min was observed. The equilibrium was reached after about 180 min for both adsorbents. Moreover, the new adsorbent maintained recovery efficiencies between 96 and 98%. Additionally, it achieved 94% removal for both compounds in real petroleum-produced water [60].
There are also reports on the efficiency of SSHs in the removal of metal ions, such as Cu(II) [61] and Ni(II) [62], pesticides, simazine, chlorpyrifos, trifluralin, chlorfenvinphos [63], and dyes, such as astrazon red [64], reactive red 195, and reactive blue 49 [77].
Sunflower by-products are a promising alternative in environmental protection because they are low-cost adsorbents, are available, require low processing, and yield favorable results [65]. Consequently, they could play a pivotal role in environmental protection. Moreover, according to some authors, conventional wastewater treatment cannot completely remove the pharmaceuticals from water [76,78,79].
4.5. Pelletization
One of the modern approaches for the consolidation of this voluminous biomass is pellet production. Pelletization is a solid fuel densification process aiming at the increase in biomass density and the reduction in its volume by 8–10 times [80,81,82]. It is a very attractive innovative methodology that has been applied to various biomass residues—canola, hull mixed meal, beach sawdust, corncobs, wheat straw, Scenedesmus microalgae, and corn fibers [83]. Cui and colleagues applied response surface methodology for the optimization of pelletization parameters—energy consumption, bulk density, and mechanical durability. They established that the three variables (temperature, pressure, and raw material moisture content) are positively correlated with bulk density and the mechanical durability of pellets, while energy consumption is inversely correlated with the moisture content of raw biomass and positively correlated with pressure and temperature [83].
5. Green Methods for Extraction of Valuable Substances from Sunflower By-Products
Some of the requirements for “green” solvents in the extraction of natural products are to be non-toxic, biodegradable, non-explosive, non-flammable, non-polluting, reusable, and recyclable [77]. Contemporary green solvents are water, aqueous two-phase systems, ionic liquids, deep eutectic solvents, and bio-based solvents [84]. The main natural compounds of interest in sunflower by-products are phenolics and proteins.
As the chemical structure of phenolics is quite diverse, there is no uniform methodology for the complete extraction of all their modifications from plants. The method employed should be able to retrieve the compounds of interest without degradation or structural modification of the target compounds.
Conventional extraction techniques like liquid/liquid and solid/liquid, assisted with external factors (agitation, pressing, heating), are characterized by low efficiency, high solvent consumption, and long processing times. Therefore, more practical and environmentally focused techniques should be used for the extraction of valuable substances [20].
One of the contemporary green extraction methods proposed for the extraction of phenolics is microwave-assisted extraction (MAE) [85]. MAE is a non-destructive method that fulfills the economic and environmental demands to obtain high-quality extracts. In this technique, microwave energy is used to heat the sample, leading to an increased mass transfer rate of the solute from the sample matrix to the solvent. The advantages of MAE are efficacy and promptness, thus resulting in lower energy and solvent consumption. Therefore, the extracts produced are of higher quality compared to the extracts obtained from other conventional extraction methods [85,86,87]. Generally, MAE efficacy depends on diverse process variables such as type and solvent composition, particle size, extraction temperature, sample-to-solvent ratio, microwave power, and irradiation time. MAE with water at 600 W was applied for the extraction of phenolic compounds from SSHs at 70 °C and 90 °C, respectively. The higher temperature resulted in significantly higher total phenol content, total flavonoid content, and antioxidant activity [20].
Another green technique that can be employed for the extraction of valuable substances is ultrasonication (US). It is a physical pretreatment method aiming at reduced power consumption, a shorter extraction time, and improved protein extractability and functionality [88,89]. Ali and co-workers compared conventional and US extraction methods for the extraction of polyphenols from SM. The conventional extraction was conducted using methanol/distilled water/acetic acid (80/19/1, v/v/v) with shaking (500 rpm) for 1 h at 30 °C. For the US extraction, the defatted meal was dispersed in the above-mentioned solvent and sonicated for an hour at 30 °C. The results demonstrated that US extracts achieved higher total polyphenolic content and higher antioxidant capacity values [90].
An innovative alternative to organic solvents is deep eutectic solvents (DESs). They are formed by mixing at least two components—a hydrogen donor and an acceptor. They are blended under specific conditions, forming a eutectic mixture that is a liquid at a low temperature, and its melting point is lower than the melting points of its constituents [91]. DESs are non-flammable, non-volatile, and are characterized by low toxicity. These modern solvents are suitable for phenolic extraction as they are viscous, polar, and, when optimized, are more efficient than the conventional organic solvents [92]. In addition, the results show that DES plant and seaweed extracts are safe to be ingested or topically applied [93,94]. Two different formulations of DESs were tested by Bezzera and co-authors for the extraction of phenolic compounds from SM at 45 °C with the assistance of ultrasound for one minute at a maximal power of 99%. In addition, the samples were heated in order to obtain more stable extracts over time. The team demonstrated that the lactic acid/glucose (5:1) solvent managed to extract 1786 mg chlorogenic acid per liter of the extract while ethanol (40%) extracted 1305 mg·L−1 and choline chloride/glycerol extracted 807 mg·L−1 [95].
A recent study compared three extraction techniques—ultrasound-assisted (59 kHz for 20 min and temperature controlled at 40 °C), CO2-assisted extraction (pressure between 55.5 and 57.8 bar, temperature from 20.7 to 22.3 °C for 20 min), and mechanical stirring (20 min at 400 rpm, 40 °C)—aiming at the optimization of alkaline extraction of proteins from SM. Among the three tested extraction methods, CO2-assisted extraction resulted in the highest protein content, namely, 44.23 ± 4.1 on % dry basis. This superior performance could be attributed to the enhanced mass transfer and cell disruption facilitated by the effects of agitation, pressure, and CO2 solubilization [96].
6. Conclusions and Future Perspectives
Sunflower by-products are wastes of underutilized residues for the circular bio-based economy. Thanks to their protein content and low antinutrient amount, they provide an important opportunity for the conception of a zero-hunger world. The protein in SM may be transformed into a protein hydrolysate and bioactive peptides that can be incorporated into foods or edible films. These residues are viable sources of widely available, cheap natural antioxidants necessary for maintaining human health. Once isolated, the phenolic compounds extracted from SM can be applied to food products instead of the chemically synthesized ones. Therefore, these valuable substances can be incorporated in functional foods and biodegradable edible materials.
On the other hand, the development of adsorbents composed of sunflower by-products can potentially play a crucial role in environmental protection and remediation. In addition, they are promising sources of renewable energy.
For the recycling of biogenic waste, it is necessary to assemble a multidisciplinary team of specialists such as process industry experts, agrochemists, and material technologists. Also, there still exist diverse limitations like the lack of infrastructure for material manipulation, material granularity, and the high heterogenicity of waste biomass. Nevertheless, the qualities and potential of such voluminous waste make it an exceptionally suitable choice for future investigations and valorization towards improving the nutritional and environmental sustainability.
Author Contributions
Conceptualization, F.T.; writing—original draft preparation, F.T., G.N., and S.B.; writing—review and editing, F.T. and S.B.; project administration, F.T., G.N., and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank to The Bulgarian National Science Fund, Grant number KП-06-M87/3 BG-175467353-2024-10-0021 “Innovative approaches for the utilization of sunflower husks by “green” extraction methods with supercritical CO2 and newly synthesized deep eutectic solvents.” for the support. During the preparation of this manuscript, the authors used ChatGPT, version: 1.2025.153 for the purposes of sunflower image creation. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SM | Sunflower meal |
| SSHs | Sunflower seed hulls |
| SMPI | Sunflower meal protein isolate |
| MAE | Microwave-assisted extraction |
| US | Ultrasonication |
| DESs | Deep eutectic solvents |
References
- Jaski, J.; Abrantes, K.; Zanqui, A.; Stevanto, N.; Da Silva, C.; Barao, C.; Bonfim-Rocha, L.; Cardozo-Filho, L. Simultaneous extraction of sunflower oil and active compounds from olive leaves using pressurized propane. Curr. Res. Food Sci. 2022, 5, 531–544. [Google Scholar] [CrossRef]
- Zardo, I.; Sobczyk, D.; Marczak, L.; Sarkis, J. Optimization of ultrasound-assisted extraction of phenolic compounds from sunflower seed cake using response surface methodology. Waste Biomass Valorization 2019, 10, 33–44. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Chiralt, A.; Chiralt, V. Antioxidant starch films containing sunflower hull extracts. Carbohydr. Polym. 2019, 214, 142–151. [Google Scholar] [CrossRef]
- Agricultural Production Statistics (2000–2022). Available online: https://www.fao.org/statistics/highlights-archive/highlights-detail/agricultural-production-statistics-(2000-2022) (accessed on 10 February 2025).
- Pickard, M.; da Silva Lima, R.; Shahidi, F. By-Product Utilization. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2020; pp. 1–10. [Google Scholar] [CrossRef]
- Kartika, I.; Pontalier, I.; Rigal, R. Twin-screw extruder for oil processing of sunflower seeds: Thermo-mechanical pressing and solvent extraction in a single step. Ind. Crops Prod. 2010, 32, 297–304. [Google Scholar] [CrossRef]
- Anal, A. Food Processing By-Products and Their Utilization; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 1–10. [Google Scholar] [CrossRef]
- González-Pérez, S.; Johan, M.V. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- González-Pérez, S. Sunflower proteins. In Sunflower; AOCS Press: Urbana, IL, USA, 2015; pp. 331–393. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Egea, M.B. Sunflower seed byproduct and its fractions for food application: An attempt to improve the sustainability of the oil process. J. Food Sci. 2021, 86, 1497–1510. [Google Scholar] [CrossRef]
- Georgieva, P.; Bozadzhiev, B.; Koleva, L.; Pishtiyski, I. Characterization of sunflower materials as sources for production of protein isolates. In Proceedings of the Scientific Works of UFT, Plovdiv, Bulgaria, 19–20 October 2012; pp. 128–132. [Google Scholar]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Subaşı, B.; Vahapoğlu, B.; Capanoglu, E.; Mohammadifar, M. A review on protein extracts from sunflower cake: Techno-functional properties and promising modification methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 6682–6697. [Google Scholar] [CrossRef] [PubMed]
- Wanjari, N.; Waghmare, J. Phenolic and antioxidant potential of sunflower meal. Adv. Appl. Sci. Res. 2015, 6, 221–229. [Google Scholar]
- Pickard, M.D.; da Silva Lima, R.S.; Shahidi, F. By-product utilization. In Bailey’s Industrial Oil and Fat Products, 7th volume, 7th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2005; Volume 4, pp. 391–416. [Google Scholar]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Regente, M.; Jacobi, S.; Rio, M.; Pinedo, M.; Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef]
- Weisz, G.; Rolf, D.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Kreps, F.; Vrbiková, L.; Schmidt, S. Industrial Rapeseed and Sunflower Meal as Source of Antioxidants. Int. J. Eng. Res. Appl. 2014, 4, 45–54. [Google Scholar]
- Rodríguez, M.; Nolasco, S.M.; Izquierdo, N.G.; Mascheroni, R.H.; Sanchez Madrigal, M.; Chávez Flores, D.; Quintero Ramos, A. Microwave-assisted extraction of antioxidant compounds from sunflower hulls. Heat Mass Transf. 2019, 55, 3017–3027. [Google Scholar] [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2018, 254, 377–382. [Google Scholar] [CrossRef]
- Shcherban’, E.; Stel’makh, S.; Beskopylny, A.; Mailyan, L.; Meskhi, B.; Chernil’nik, A.; El’shaeva, D.; Pogrebnyak, A.; Yaschenko, R. Influence of Sunflower Seed Husks Ash on the Structure Formation and Properties of Cement Concrete. Civ. Eng. J. 2024, 10, 1475–1493. [Google Scholar] [CrossRef]
- Stel’makh, S.; Beskopylny, A.; Shcherban, A.; Mailyan, E.; Meskhi, L.; Shilov, B.; El’shaeva, A.; Chernil’nik, D.; Kurilova, S. Alteration of Structure and Characteristics of Concrete with Coconut Shell as a Substitution of a Part of Coarse Aggregate. Materials 2023, 16, 4422. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Res. Signpost 2006, 661, 23–67. [Google Scholar]
- De Leonardis, A.; Macciola, V.; Di Domenico, N. A first pilot study to produce a food antioxidant from. Eur. J. Lipid Sci. Technol. 2005, 107, 220–227. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Alexandri, M.; Strati, A.; Gardeli, C.; Papanikolaou, S.; Komaitis, M.; Kookos, I.; Koutinas, A. Integrated sunflower-based biorefinery for the production of antioxidants, protein isolate and poly(3-hydroxybutyrate). Ind. Crops Prod. 2015, 71, 106–113. [Google Scholar] [CrossRef]
- Laguna, O.; Odinot, E.; Bissoto, A.; Barea, B.; Villeneuve, P.; Sigollot, J.-C.; Record, E.; Faulds, C.; Fine, F.; Lesage-Meesen, L.; et al. Release of phenolic acids from sunflower and rapeseed meals using different carboxylic esters hydrolases from Aspergillus niger. Ind. Crops Prod. 2019, 139, 111579. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.; Ramos, M.; Garrigós, М.; Alfonso, J. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.; Sriram, V.; Britto, J.; Mageshwaran, D.; Durairaj, R. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Salgado, P.; Fernández, G.; Silvina, R.; Mauri, A. Addition of bovine plasma hydrolysates improves the antioxidant properties of soybean and sunflower protein-based films. Food Hydrocoll. 2011, 25, 1433–1440. [Google Scholar] [CrossRef]
- Salgado, P.; López-Caballero, M.; Gómez-Guillén, M.; Mauri, A.; Montero, M. Sunflower protein films incorporated with clove essential oil have potential application for the preservation of fish patties. Food Hydrocoll. 2013, 33, 74–84. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Huang, M.; Wang, H.; Xu, X.; Lu, X.; Song, X.; Zhou, G. Effects of nanoemulsion-based edible coatings with composite mixture of rosemary extract and ε-poly-l-lysine on the shelf life of ready-to-eat carbonado chicken. Food Hydrocoll. 2020, 102, 105576. [Google Scholar] [CrossRef]
- Zhang, D.; Bi, W.; Kai, K.; Ye, Y.; Liu, J. Effect of chlorogenic acid on controlling kiwifruit postharvest decay caused by Diaporthe sp. LWT 2020, 132, 109805. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R. Green tea: A novel functional food for the oral health of older adults. Geriatr. Gerontol. Int. 2014, 14, 238–250. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.; Hussein, A.; Mostafa, S.; Abd El-Aty, A. Impact of Sunflower Meal Protein Isolate Supplementation. Separations 2022, 9, 429. [Google Scholar] [CrossRef]
- Meng, X.; Slominski, B.A. Affected by a Multicarbohydrase Preparation of Cell Wall Degrading Enzymes. Poult. Sci. 2005, 4, 1242–1251. [Google Scholar] [CrossRef]
- Saeed, M.; Cheryan, M. Sunflower Protein Concentrates and Isolates’ Low in Polyphenols and Phytate. J. Food Sci. 1988, 53, 1127–1131. [Google Scholar] [CrossRef]
- Manchuliantsau, A.; Tkacheva, A. Upcycling Solid Food Wastes and By-Products into Human Consumption Products. US Patent 20190223475A1, 25 July 2019. [Google Scholar]
- Gatta, C.; Piergiovanni, A. Technological and nutritional aspects in hyperproteic bread prepared with the addition of sunflower meal. Food Chem. 1996, 57, 493–496. [Google Scholar] [CrossRef]
- Grasso, S.; Liu, S.; Methven, L. Quality of muffins enriched with upcycled defatted sunflower seed flour. LWT 2020, 119, 108893. [Google Scholar] [CrossRef]
- Song, N.-B.; Song, H.-Y.; Jo, W.-S.; Song, K. Physical properties of a composite film containing sunflower seed meal protein and its application in packaging smoked duck meat. J. Food Eng. 2013, 116, 789–795. [Google Scholar] [CrossRef]
- Andrade, T.; Arbach, C.; Garcia, A.; Domingues, L.; Marinho, T.; Nabeshima, E.; Ramirez, B.; Pacheko, M. Exploring new plant-based products: Acceptance of sunflower meal as a protein source in meat alternative products. Food Res. Int. 2025, 209, 116158. [Google Scholar] [CrossRef] [PubMed]
- Harnack, L.; Mork, S.; Valluri, S.; Weber, C.; Schmitz, K.; Stevenson, J.; Pettit, J. Nutrient composition of a selection of plant-based ground beef alternative products available in the United States. J. Acad. Nutr. Diet. 2021, 121, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, D.; Clausen, M.; Jaeger, S. Understanding barriers to consumption of plant-based foods and beverages: Insights from sensory and consumer science. Curr. Opin. Food Sci. 2022, 48, 100919. [Google Scholar] [CrossRef]
- Minakov, D. Influence of Ecological and Biochemical Parameters of Bioconversion of Plant Raw Materials on the Yield of Biomass of Fruiting Bodies of Xylotrophic Basidiomycetes. Ph.D. Thesis, Tomsk State University, Tomsk, Russia, 2018. [Google Scholar]
- Sysoeva, M.; Prozoreva, I.; Sysoeva, E. Study of the process of solid-phase cultivation of higher fungi on milled sunflower seeds hulls for the obtaining of composite materials. Chem. Plant Raw Mater. 2024, 3, 313–319. [Google Scholar] [CrossRef]
- Gonzales-Matute, R.; Figlas, D.; Curvetto, N. Sunflower seed hull based compost for Agaricus blazei Murrill cultivation. Int. Biodeterior. Biodegrad. 2010, 64, 742–747. [Google Scholar] [CrossRef]
- Gonzales-Matute, R.; Figlas, D.; Devalis, R.; Delmastro, S.; Curvetto, N. Sunflower seed hulls as a main nutrient source for cultivating Ganoderma lucidum. Micol. Apl. Int. 2002, 14, 19–24. [Google Scholar]
- Figlas, N.; Gonzalez-Matute, R.; Curvetto, N. Sunflower seed hull: Its value as a broad mushroom substrate. J. Food Process. Preserv. 2016, 8, 1–7. [Google Scholar]
- Casoni, A.; Bidegain, M.; Cubitto, M.; Curvetto, N.; Volpe, M. Pyrolysis of sunflower seed hulls for obtaining bio-oils. Bioresour. Technol. 2015, 177, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Casoni, A.; Gutierrez, V.; Volpe, M. Conversion of sunflower seed hulls, waste from edible oil production, into valuable products. J. Environ. Chem. Eng. 2019, 7, 102893. [Google Scholar] [CrossRef]
- Sharma, S.; Kalra, K.; Kocher, G. Fermentation of enzymatic hydrolysate of sunflower hulls for ethanol production and its scale-up. Biomass Bioenergy 2004, 27, 399–402. [Google Scholar] [CrossRef]
- Telli-Okur, M.; Eken-Saraçoğlu, N. Fermentation of sunflower seed hull hydrolysate to ethanol by Pichia stipitis. Bioresour. Technol. 2008, 99, 2162–2169. [Google Scholar] [CrossRef]
- Urrutia, R.; Aagaard, T.; Gutierrez, V.; Gonz’alez, J.; Frechero, M.; Volpe, M. Co-production of bioinsecticide and biochar from sunflower edible oil waste: A preliminary feasibility study. Bioresour. Technol. Rep. 2024, 26, 101836. [Google Scholar] [CrossRef]
- Ninkov, J.; Jakšić, S.; Nenin, P.; Gvozdenović, M.; Mijić, B.; Radović, B.; Milić, S. Waste ashes from burned sunflower hulls as new fertilising materials. Environ. Eng. 2024, 10, 19–23. [Google Scholar] [CrossRef]
- Sang, Y.; Chen, H.; Khalifeh, M.; Li, Y. Catalysis and chemistry of lignin depolymerization in alcohol solvents—A review. Catal. Today 2023, 408, 168–181. [Google Scholar] [CrossRef]
- Jemli, S.; Vieira, Y.; Chamtouri, F.; Silva, L.; Oliveira, M.; Amara, F.; Bejar, S.; Vizzotto, B.; Wahab, R.; Irshad, S.; et al. Development of sunflower seed hulls crosslinked β-cyclodextrin (SFSH-β-CD) composite materials for green adsorption of phenol and naphthenic acid. J. Environ. Chem. Eng. 2025, 13, 115419. [Google Scholar] [CrossRef]
- Stanković, S.; Šoštarić, T.; Bugarčić, M.; Janićijević, A.; Pantović-Spajić, K.; Lopičić, Z. Adsorption of Cu(II) ions from synthetic solution by sunflower seed husks. Acta Period. Technol. 2019, 50, 268–277. [Google Scholar] [CrossRef]
- Tadayon, Y.; Bahrololoom, M.; Javadpour, S. An experimental study of sunflower seed husk and zeolite as adsorbents of Ni(II) ion from industrial wastewater. Water Resour. Ind. 2023, 30, 100214. [Google Scholar] [CrossRef]
- Rojas, R.; Morillo, J.; Usero, J.; Vanderlinden, E.; Bakouri, H. Adsorption study of low-cost and locally available organic substances and a soil to remove pesticides from aqueous solutions. J. Hydrol. 2015, 520, 461–472. [Google Scholar] [CrossRef]
- Kocadagistan, B.; Kocadagistan, E. The effects of sunflower seed shell modifying process on textile dye adsorption: Kinetic, thermodynamic and equilibrium study. Desalination Water Treat. 2016, 57, 3168–3178. [Google Scholar] [CrossRef]
- Ferreira, I.; Wernke, J.; Diório, A.; Bergamasco, R.; Vieira, M. Sunflower seed husks as a cost-effective adsorbent for chloroquine removal from water. Braz. J. Environ. Sci. 2024, 59, 1907. [Google Scholar] [CrossRef]
- Bridgewater, T. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, K. Acidic Pretreatment. In Pretreatment of Biomass: Processes and Technologies; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 27–50. [Google Scholar] [CrossRef]
- Nitsos, C.; Mihailof, C.; Matis, K.; Lappas, A.; Triantafyllidis, K. Chapter 7—The Role of Catalytic Pretreatment in Biomass Valorization Toward Fuels and Chemicals. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; Volume 223, pp. 217–260. [Google Scholar] [CrossRef]
- Himmel, M.; Ding, S.; Johnson, D.; Adney, W.; Nimlos, M.; Brady, J. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, Y.; Liu, T.; Zhao, J.; Huang, J.; Fang, Y. Investigations on interactions between sodium species and coal char by thermogravimetric analysis. Fuel 2018, 214, 561–568. [Google Scholar] [CrossRef]
- Olsson, L.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzym. Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Li, L.; Yao, Z.; You, S.; Wang, C.; Chong, C.; Wang, X. Optimal design of negative emission hybrid renewable energy systems with biochar production. Appl. Energy 2019, 243, 233–249. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef]
- Midassi, S.; Bedoui, A.; Bensalah, N. Efficient degradation of chloroquine drug by electro-Fenton oxidation: Effects of operating conditions and degradation mechanism. Chemosphere 2020, 260, 127558. [Google Scholar] [CrossRef]
- Nguyen, T.; Nguyen, T.; Chen, W.; Chen, C.; Patel, A.; Bui, X.; Chen, L.; Singhania, R.; Dong, C. Phosphoric acid-activated biochar derived from sunflower seed husk: Selective antibiotic adsorption behavior and mechanism. Bioresour. Technol. 2023, 371, 128593. [Google Scholar] [CrossRef]
- Nippes, R.; Macruz, P.; Silva, G.; Scaliante, M. A critical review on environmental presence of pharmaceutical drugs tested for the covid-19 treatment. Process Saf. Environ. Prot. 2021, 152, 568–582. [Google Scholar] [CrossRef]
- Alhares, H.; Shaban, M.; Salman, M.; Ridha, M.M.; Mohammed, S.; Abed, K.; Ibrahim, M.; Al-Banaa, A.; Hasan, H. Sunflower Husks Coated with Copper Oxide Nanoparticles for Reactive Blue 49 and Reactive Red 195 Removals: Adsorption Mechanisms, Thermodynamic, Kinetic, and Isotherm Studies. Water Air Soil Pollut. 2023, 234, 35. [Google Scholar] [CrossRef]
- Delgado, N.; Capparelli, A.; Navarro, A.; Marino, D. Pharmaceutical emerging pollutants removal from water using powdered activated carbon: Study of kinetics and adsorption equilibrium. J. Environ. Manag. 2019, 236, 301–308. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Janani, F.; Elhalil, A.; Mahjoubi, F.; Barka, N. Removal of emerging pharmaceutical pollutants: A systematic mapping study review. J. Environ. Chem. Eng. 2020, 8, 104251. [Google Scholar] [CrossRef]
- Azargohar, R.; Soleimani, M.; Nosran, S.; Bond, T.; Karunakaran, C.; Dalai, A.; Tabil, L. Thermo-physical characterization of torrefied fuel pellet from co-pelletization of canola hulls and meal. Ind. Crops Prod. 2018, 128, 424–435. [Google Scholar] [CrossRef]
- Mostafa, M.; Hu, S.; Wang, Y.; Su, S.; Hu, X.; Elsayed, S.; Xiang, J. The significance of pelletization operating conditions: An analysis of physical and mechanical characteristics as well as energy consumption of biomass pellets. Renew. Sustain. Energy Rev. 2019, 105, 332–348. [Google Scholar] [CrossRef]
- Dai, X.; Theppitak, S.; Yoshikawa, K. Pelletization of Carbonized Wood Using Organic Binders with Biomass Gasification Residue as an Additive. Energy Fuels 2018, 33, 323–329. [Google Scholar] [CrossRef]
- Cui, X.; Yang, J.; Shi, X.; Lei, W.; Huang, T.; Bai, C. Pelletization of Sunflower Seed Husks: Evaluating and Optimizing Energy Consumption and Physical Properties by Response Surface Methodology (RSM). Processes 2019, 7, 591. [Google Scholar] [CrossRef]
- Shuur, B.; Brouwer, T.; Smink, D.; Sprakel, L. Green solvents for sustainable separation processes. Curr. Opin. Green Sustain. Chem. 2019, 18, 67–75. [Google Scholar] [CrossRef]
- Nkhili, E.; Tomao, V.; Hajji, H.; Boustani, E.-S.; Chemat, F.; Dangles, O. Microwave-assisted water extraction of green tea polyphenols. Phytochem. Anal. 2009, 20, 408–415. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Sahak, S.; Sairam, K. Valorisation of black carrot pomace: Microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Elhag, H.; Naila, A.; Nour, A.; Ajit, A.; Sulaiman, A.; Abd Aziz, B. Optimization of protein yields by ultrasound assisted extraction from Eurycoma longifolia roots and effect of agitation speed. J. King Saud. Univ.—Sci. 2019, 31, 913–930. [Google Scholar] [CrossRef]
- Mintah, B.; He, R.; Dabbour, M.; Xiang, J.; Akomeah, A.; Ma, H. Techno-functional attribute and antioxidative capacity of edible insect protein preparations and hydrolysates thereof: Effect of multiple mode sonochemical action. Ultrason. Sonochemistry 2019, 58, 104676. [Google Scholar] [CrossRef]
- Ali, M.; Khalil, M.; Badawy, W.; Hellwig, M. Ultrasonic treatment as a modern technique to facilitate the extraction of phenolic compounds from organic sunflower seed cakes. J. Sci. Food Agric. 2023, 104, 2245–2251. [Google Scholar] [CrossRef]
- Bashir, I.; Dar, A.; Dash, K.; Pandey, V.; Fayaz, U.; Shams, R.; Srivastava, R.; Singh, R. Deep eutectic solvents for extraction of functional components from plant-based products: A promising approach. Sustain. Chem. Pharm. 2023, 33, 101102. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.; Duarte, A. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 10663–11071. [Google Scholar] [CrossRef]
- Shikov, A.; Obluchinskaya, E.; Flisyuk, E.; Terninko, I.; Generalova, Y.; Pozharitskaya, O. The impact of natural deep eutectic solvents and extraction method on the co-extraction of trace metals from Fucus vesiculosus. Mar. Drugs 2022, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.; Shikova, V.; Whaley, A.; Burakova, M.; Flisyuk, E.; Whaley, A.; Terninko, I.; Generalova, Y.; Gravel, I.; Pozharitskaya, O. The Ability of Acid-Based Natural Deep Eutectic Solvents to Co-Extract Elements from the Roots of Glycyrrhiza glabra L. and Associated Health Risks. Molecules 2022, 27, 7690. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.; Ramos, G.; Carvalho, M.; Koblitz, M. Natural deep eutectic solvents characteristics determine their extracting and protective power on chlorogenic acids from sunflower meal. Sustain. Chem. Pharm. 2024, 37, 101430. [Google Scholar] [CrossRef]
- Becze, A.; Senila, M.; Senila, L.; Dordai, L.; Cadar, O.; Fuss-Babalau, V.; Roman, M.; Levei, L.; Uiuiu, P.; Naghiu, M. Optimization of protein extraction from sunflower meal using taguchi design and regression modeling for human nutrition applications. Foods 2025, 14, 2415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).