A Review of Chemical and Physical Analysis, Processing, and Repurposing of Brewers’ Spent Grain †
Abstract
1. Introduction
1.1. Bachround
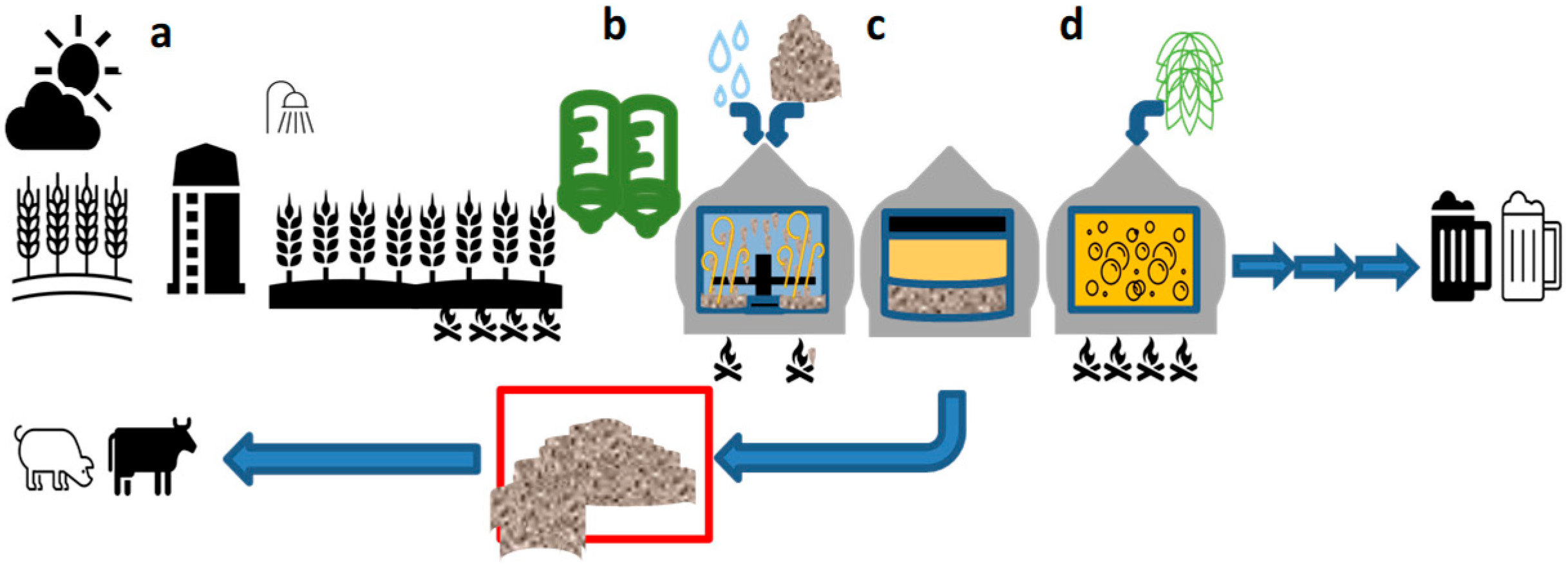
1.2. Origin and Composition of Brewers’ Spent Grains
2. Analyzing the Physical and Chemical Composition of Brewers’ Spent Grain
2.1. Nutritional Contents
2.2. Brewers Spent Grain: Composition, Stability Challenges
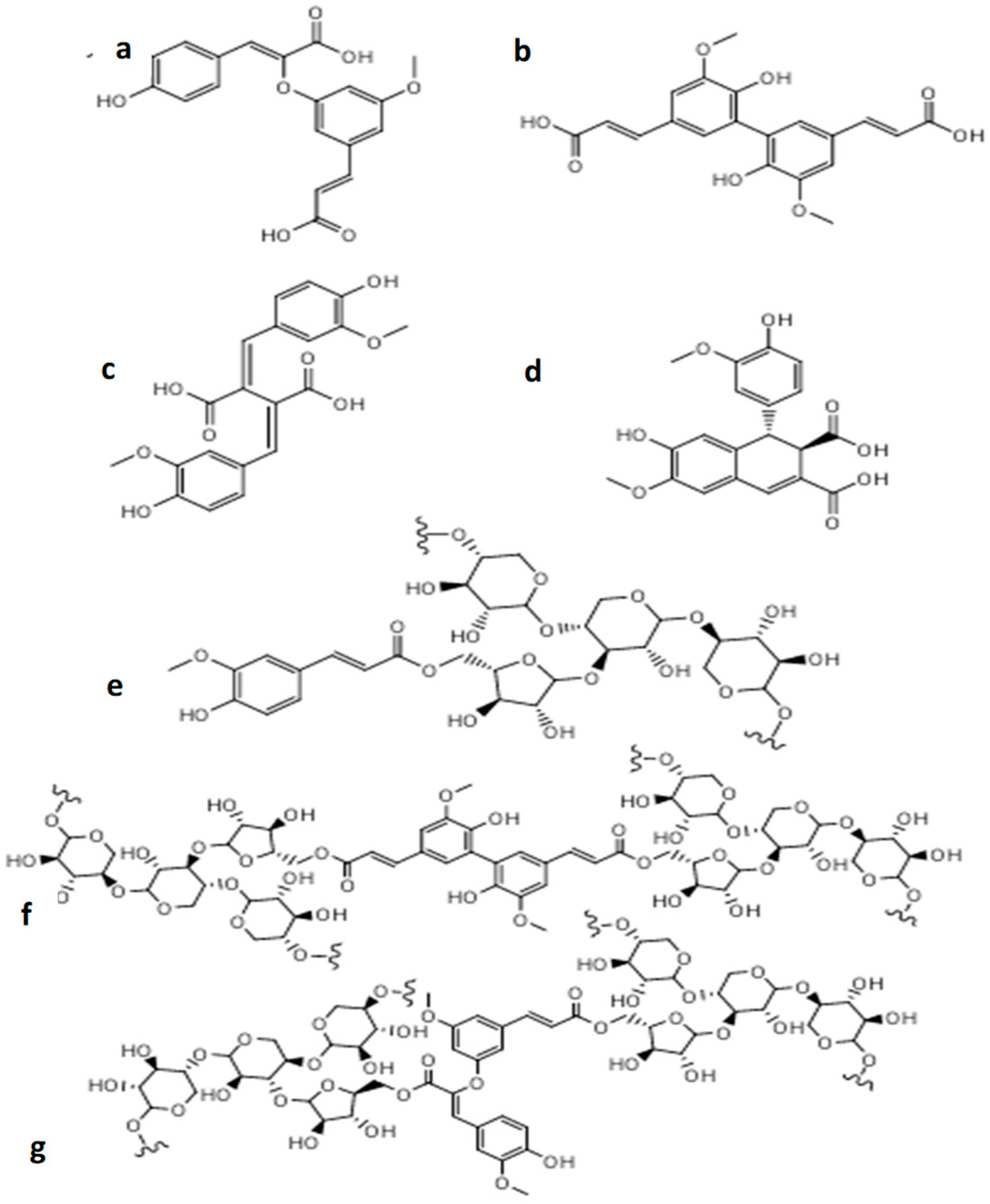
2.3. Unlocking the Biochemical Potential of Brewers’ Spent Grains
2.4. Yeast, Lipids, and Beer Chemistry: Insights from Brewers Spent Grain
3. Drying and Processing Brewers’ Spent Grain and Other Brewery Wastes to Produce Isolates and Other Value-Added Products
3.1. Drying Challengesof BSG
3.2. Microbial Activities
3.3. Overall Challenges and Limitations
4. Monitoring and Studying Grain Precursor Materials and Brewers’ Spent Grain
4.1. Non-Destructive Phenotyping of Barley Husk Adhesion via Raman Spectroscopy
4.2. Lipid Complexity and Emerging High-Resolution Technologies
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aluko, R.E.; Yada, R.Y. Structure-function relationships of cowpea (Vigna unguiculata) globulin isolate: Influence of pH and NaCl on physicochemical and functional properties. Food Chem. 1995, 53, 259–265. [Google Scholar] [CrossRef]
- Mainardis, M.; Hickey, M.; Dereli, R.K. Lifting craft breweries sustainability through spent grain valorisation and renewable energy integration: A critical review in the circular economy framework. J. Clean. Prod. 2024, 447, 141527. [Google Scholar] [CrossRef]
- Palmer, J.J. How to Brew: Everything You Need to Know to Brew Great Beer Every Time; Brewers Publications: Boulder, CO, USA, 2017. [Google Scholar]
- van Tongeren, F. Standards and International Trade Integration: A Historical Review of the German ‘Reinheitsgebot’. In The Economics of Beer; Swinnen, J.F.M., Ed.; OUP: Oxford, UK, 2011. [Google Scholar]
- Naibaho, J.; Korzeniowska, M. Brewers’ spent grain in food systems: Processing and final products quality as a function of fiber modification treatment. J. Food Sci. 2021, 86, 1532–1551. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M. The variability of physico-chemical properties of brewery spent grain from 8 different breweries. Heliyon 2021, 7, e06583. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; I’Anson, K.J.A.; Brocklehurst, T.F.; Faulds, C.B.; Waldron, K.W. Effect of storage conditions on the microbial ecology and biochemical stability of cell wall components in brewers’ spent grain. J. Agric. Food Chem. 2010, 58, 7266–7272. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; Del Nozal, M.J. Variability of brewer’s spent grain within a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s Spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar] [CrossRef]
- Durán-Sánchez, A.; del Río-Rama, M.d.l.C.; Álvarez-García, J.; Oliveira, C. Analysis of worldwide research on craft beer. Sage Open 2022, 12, 21582440221108154. [Google Scholar] [CrossRef]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, wastewater and waste management in brewing industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Tang, Z.; Cenkowski, S.; Izydorczyk, M. Thin-layer drying of spent grains in superheated steam. J. Food Eng. 2005, 67, 457–465. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Chemical characterization and liberation of pentose sugars from brewer’s spent grain. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Jay, A.J.; Parker, M.L.; Faulks, R.; Husband, F.; Wilde, P.; Smith, A.C.; Faulds, C.B.; Waldron, K.W. A systematic micro-dissection of brewers’ spent grain. J. Cereal Sci. 2008, 47, 357–364. [Google Scholar] [CrossRef]
- Cook, A.H. (Ed.) Barley and Malt: Biology, Biochemistry, Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current extraction techniques towards bioactive compounds from brewer’s spent grain–A review. In Critical Reviews in Food Science and Nutrition; Taylor and Francis, Inc.: Abingdon, UK, 2020; Volume 60, pp. 2730–2741. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Linko, M.; Haikara, A.; Ritala, A.; Penttilä, M. Recent advances in the malting and brewing industry. J. Biotechnol. 1998, 65, 85–98. [Google Scholar] [CrossRef]
- Panjičko, M.; Zupančič, G.D.; Fanedl, L.; Logar, R.M.; Tišma, M.; Zelić, B. Biogas production from brewery spent grain as a mono-substrate in a two-stage process composed of solid-state anaerobic digestion and granular biomass reactors. J. Clean. Prod. 2017, 166, 519–529. [Google Scholar] [CrossRef]
- Silva, J.P.; Sousa, S.; Rodrigues, J.; Antunes, H.; Porter, J.J.; Gonçalves, I.; Ferreira-Dias, S. Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains. Sep. Purif. Technol. 2004, 40, 309–315. [Google Scholar] [CrossRef]
- Devolli, A.; Shahinasi, E.; Stafasani, M.; Feta, D.; Dara, F. Evaluation of brewery waste and its reduction methods. Yeast 2018, 210, 506–513. [Google Scholar]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley Protein Properties, Extraction and Applications, with a Focus on Brewers’ Spent Grain Protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef]
- Mandalari, G.; Faulds, C.B.; Sancho, A.I.; Saija, A.; Bisignano, G.; Locurto, R.; Waldron, K.W. Fractionation and characterisation of arabinoxylans from brewers’ spent grain and wheat bran. J. Cereal Sci. 2005, 42, 205–212. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of brewers’ spent grain for the production of lipids by oleaginous yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef]
- Yalçın, E.; Çelik, S.; İbanoğlu, E. Foaming properties of barley protein isolates and hydrolysates. Eur. Food Res. Technol. 2008, 226, 967–974. [Google Scholar] [CrossRef]
- Hang, Y.D.; Splittstoesser, D.F.; Woodams, E.E.; Sherman, R.M. Citric acid fermentation of brewery waste. J. Food Sci. 1977, 42, 383–384. [Google Scholar] [CrossRef]
- Arranz, J.I.; Miranda, M.T.; Sepúlveda, F.J.; Montero, I.; Rojas, C.V. Analysis of Drying of Brewers’ Spent Grain. Proceedings 2018, 2, 1467. [Google Scholar] [CrossRef]
- Mallen, E.; Najdanovic-Visak, V. Brewers’ spent grains: Drying kinetics and biodiesel production. Bioresour. Technol. Rep. 2018, 1, 16–23. [Google Scholar] [CrossRef]
- Di Mario, J.; Bertoldi, A.; Priolo, D.; Calzoni, E.; Gambelli, A.M.; Dominici, F.; Rallini, M.; Del Buono, D.; Puglia, D.; Emiliani, C.; et al. Characterization of Processes Aimed at Maximizing the Reuse of Brewery’s Spent Grain: Novel Biocomposite Materials, High-Added-Value Molecule Extraction, Codigestion and Composting. Recycling 2025, 10, 124. [Google Scholar] [CrossRef]
- Chu, H.-Y.I.; Miri, T.; Onyeaka, H. Valorization of Bioactive Compounds Extracted from Brewer’s Spent Grain (BSG) for Sustainable Food Waste Recycling. Sustainability 2025, 17, 2477. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.Y.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Mainali, K.; Yadav, M.P.; Sharma, B.K.; Sarker, M.I.; Ngo, H.; Hotchkiss, A.; Simon, S. Isolation and Characterization of the Physiochemical Properties of Brewer’s Spent Grain. Agriculture 2024, 15, 47. [Google Scholar] [CrossRef]
- Macleod, A.M. The Physiology of Malting. In Brewing Science; Pollock, J.R.A., Ed.; Academic Press: New York, NY, USA, 1979; Volume 1, pp. 145–232. [Google Scholar]
- Zupančič, G.D.; Škrjanec, I.; Logar, R.M. Anaerobic co-digestion of excess brewery yeast in a granular biomass reactor to enhance the production of biomethane. Bioresour. Technol. 2012, 124, 328–337. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The potential of brewer’s spent grain in the circular bioeconomy: State of the art and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y. Development of a functional germinated barley foodstuff from brewer’s spent grain for the treatment of ulcerative colitis. J. Am. Soc. Brew. Chem. 2001, 59, 59–62. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Esteves, M.P.; Parajó, J.C.; Pereira, H.; Gırio, F.M. Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour. Technol. 2008, 99, 5427–5435. [Google Scholar] [CrossRef]
- Niemi, P.; Faulds, C.B.; Sibakov, J.; Holopainen, U.; Poutanen, K.; Buchert, J. Effect of a milling pre-treatment on the enzymatic hydrolysis of carbohydrates in brewer’s spent grain. Bioresour. Technol. 2012, 116, 155–160. [Google Scholar] [CrossRef]
- Sobukola, O.P.; Babajide, J.M.; Ogunsade, O. Effect of brewers spent grain addition and extrusion parameters on some properties of extruded yam starch-based pasta. J. Food Process. Preserv. 2013, 37, 734–743. [Google Scholar] [CrossRef]
- Kemppainen, K.; Rommi, K.; Holopainen, U.; Kruus, K. Steam explosion of Brewer’s spent grain improves enzymatic digestibility of carbohydrates and affects solubility and stability of proteins. Appl. Biochem. Biotechnol. 2016, 180, 94–108. [Google Scholar] [CrossRef]
- Yu, D.; Sun, Y.; Wang, W.; O’Keefe, S.F.; Neilson, A.P.; Feng, H.; Wang, Z.; Huang, H. Recovery of protein hydrolysates from brewer’s spent grain using enzyme and ultrasonication. Int. J. Food Sci. Technol. 2020, 55, 357–368. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s spent grains—Valuable beer industry by-product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- Taylor, G.T.; Thurston, P.A.; Kirsop, B.H. The influence of lipids derived from malt spent grains on yeast metabolism and fermentation. J. Inst. Brew. 1979, 85, 219–227. [Google Scholar] [CrossRef]
- Thibault, J.; Micard, V.; Renard, C.; Asther, M.; Delattre, M.; Lesage-Meessen, L.; Faulds, C.; Kroon, P.; Williamson, G.; Duarte, J.; et al. Fungal bioconversion of agricultural by-products to vanillin. LWT Food Sci. Technol. 1998, 31, 530–536. [Google Scholar] [CrossRef][Green Version]
- Thompson, J.M.; Dodd, C.E.; Waites, W.M. Spoilage of bread by Bacillus. Int. Biodeterior. Biodegrad. 1993, 32, 55–66. [Google Scholar] [CrossRef]
- Kirssel, L.; Prentice, M. Protein and fibre enrichment of cookie flour with brewer’s spent grains. Cereal Chem. 1979, 50, 261–265. [Google Scholar]
- Niemi, P.; Tamminen, T.; Smeds, A.; Viljanen, K.; Ohra-Aho, T.; Holopainen-Mantila, U.; Faulds, C.B.; Poutanen, K.; Buchert, J. Characterization of lipids and lignans in brewer’s spent grain and its enzymatically extracted fraction. J. Agric. Food Chem. 2012, 60, 9910–9917. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing and Malting—International Edition; Mieth, H.O., Ed.; VLB: Berlin, Germany, 1996. [Google Scholar]
- Mulholland, S.C.; Rapp, G., Jr. (Eds.) Phytolith Systematics: Emerging Issues; Springer Science & Business Media: Berlin, Germany, 1992; Volume 1. [Google Scholar]
- Huige, N.J. Barley and malt. In Handbook of Brewing; Hardwick, W.A., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 501–550. [Google Scholar]
- Sganzerla, W.G.; Ampese, L.C.; Mussatto, S.I.; Forster-Carneiro, T. A bibliometric analysis on potential uses of brewer’s spent grains in a biorefinery for the circular economy transition of the beer industry. Biofuels Bioprod. Biorefin. 2021, 15, 1965–1988. [Google Scholar] [CrossRef]
- Viëtor, R.J.; Angelino, S.A.G.F.; Voragen, A.G.J. Structural features of arabinoxylans from barley and malt cell wall material. J. Cereal Sci. 1992, 15, 213–222. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Gruppen, H.; Verbruggen, M.A.; Vietor, R.J. Characterization of cereal arabinoxylans. In Progress in Biotechnology 7: Xylans and Xylanases; Visser, J., Beldman, G., Kusters-van Someren, M.A., Voragen, A.G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 51–67. [Google Scholar]
- Vitanza, R.; Cortesi, A.; Gallo, V.; Colussi, I.; de Arana-Sarabia, M.E. Biovalorization of brewery waste by applying anaerobic digestion. Chem. Biochem. Eng. Q. 2016, 30, 351–357. [Google Scholar] [CrossRef]
- Brillouet, J.M.; Mercier, C. Fractionation of wheat bran carbohydrates. J. Sci. Food Agric. 1981, 32, 243–251. [Google Scholar] [CrossRef]
- Brillouet, J.M.; Joseleau, J.P.; Utille, J.P.; Lelievre, D. Isolation, purification and characterization of a complex heteroxylan from industrial wheat bran. J. Agric. Food Chem. 1982, 30, 488–495. [Google Scholar] [CrossRef]
- DuPont, M.S.; Selvendran, R.R. Hemicellulosic polymers from the cell walls of beeswing wheat bran: Part I, polymers solubilised by alkali at 2°. Carbohydr. Res. 1987, 163, 99–113. [Google Scholar] [CrossRef]
- Olšovská, K.; Sytar, O.; Kováčik, P. Optimizing nitrogen application for enhanced barley resilience: A comprehensive study on drought stress and nitrogen supply for sustainable agriculture. Sustainability 2024, 16, 2016. [Google Scholar] [CrossRef]
- Nordkvist, E.; Salomonsson, A.C.; Åman, P. Distribution of insoluble bound phenolic acids in barley grain. J. Sci. Food Agric. 1984, 35, 657–661. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Methacanon, P.; Lloyd, L.L. The identification and quantitation of the hydroxycinnamic acid substituents of a polysaccharide extracted from maize bran. J. Sci. Food Agric. 1999, 79, 464–470. [Google Scholar] [CrossRef]
- Chanliaud, E.; Saulnier, L.; Thibault, J.F. Alkaline extraction and characterisation of heteroxylans from maize bran. J. Cereal Sci. 1995, 21, 195–203. [Google Scholar] [CrossRef]
- Colquhoun, I.J.; Ralet, M.C.; Thibault, J.F.; Faulds, C.B.; Williamson, G. Structure identification of feruloylated oligosaccharides from sugar-beet pulp by NMR spectroscopy. Carbohydr. Res. 1994, 263, 243–256. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Ralph, J.; Quideau, S.; Grabber, J.H.; Hatfield, R.D. Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Soc. Perkin Trans. 1994, 1, 3485–3498. [Google Scholar] [CrossRef]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Renger, A.; Steinhart, H. Ferulic acid dehydrodimers as structural elements in cereal dietary fibre. Eur. Food Res. Technol. 2000, 211, 422–428. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Biotechnological potential of brewers spent grain and its recent applications. Waste Biomass Valorization 2012, 3, 213–232. [Google Scholar] [CrossRef]
- del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical composition of lipids in brewer’s spent grain: A promising source of valuable phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Poerschmann, J.; Górecki, T. Characterization of the Lipid Soluble Fraction of Brewer’s Spent Grain. Curr. Chromatogr. 2016, 3, 86–98. [Google Scholar] [CrossRef]
- Correa-Ascencio, M.; Robertson, I.G.; Cabrera-Cortés, O.; Cabrera-Castro, R.; Evershed, R.P. Pulque production from fermented agave sap as a dietary supplement in Prehispanic Mesoamerica. Proc. Natl. Acad. Sci. USA 2014, 111, 14223–14228. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Borisjuk, L.; Junker, B.H.; Mock, H.P.; Rolletschek, H.; Seiffert, U.; Weschke, W.; Wobus, U. Barley grain development: Toward an integrative view. Int. Rev. Cell Mol. Biol. 2010, 281, 49–89. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; Ibanoglu, S. The recycling of brewer’s processing by-product into ready-to-eat snacks using extrusion technology. J. Cereal Sci. 2008, 47, 469–479. [Google Scholar] [CrossRef]
- Erkinbaev, C.; Ramachandran, R.P.; Cenkowski, S.; Paliwal, J. A comparative study on the effect of superheated steam and hot air drying on microstructure of distillers’ spent grain pellets using X-ray micro-computed tomography. J. Food Eng. 2019, 241, 127–135. [Google Scholar] [CrossRef]
- Mathmann, K.; Kuhn, M.; Briesen, H. Application of micro-computed tomography in food and beverage technology using the examples of textured vegetable protein and filtration steps in the brewing process. In Proceedings of the Conference on Industrial Computed Tomography (iCT2014), Wels, Austria, 25–28 February 2014. [Google Scholar]
- Forssell, P.; Kontkanen, H.; Schols, H.A.; Hinz, S.; Eijsink, V.G.H.; Treimo, J.; Robertson, J.A.; Waldron, K.W.; Faulds, C.B.; Buchert, J. Hydrolysis of brewers’ spent grain by carbohydrate degrading enzymes. J. Inst. Brew. 2008, 114, 306–314. [Google Scholar] [CrossRef]
- Fooks, L.J.; Fuller, R.; Gibson, G.R. Prebiotics, probiotics and human gut microbiology. Int. Dairy J. 1999, 9, 53–61. [Google Scholar] [CrossRef]
- Bedő, S.; Rozbach, M.; Nagy, L.; Fehér, A.; Fehér, C. Optimised fractionation of brewer’s spent grain for a biorefinery producing sugars, oligosaccharides, and bioethanol. Processes 2021, 9, 366. [Google Scholar] [CrossRef]
- Ghajavand, B.; Avesani, C.; Stenvinkel, P.; Bruchfeld, A. Unlocking the potential of brewers’ spent grain: A sustainable model to use beer for better outcome in chronic kidney disease. J. Ren. Nutr. 2024, 34, 482–492. [Google Scholar] [CrossRef]
- Dervilly, G.; Saulnier, L.; Roger, P.; Thibault, J.F. Isolation of homogeneous fractions from wheat water-soluble arabinoxylans. Influence of the structure on their macromolecular characteristics. J. Agric. Food Chem. 2000, 48, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Winkelhausen, E.; Kuzmanova, S. Microbial conversion of D-xylose to xylitol. J. Ferment. Bioeng. 1998, 86, 1–14. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of brewers’ spent grains: Pretreatments and fermentation, a review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Pepper, T.; Olinger, P.M. Xylitol in sugar-free confections. Food Technol. 1988, 42, 98–106. [Google Scholar]
- Baloch, M.I.; Akunna, J.C.; Collier, P.J. The performance of a phase separated granular bed bioreactor treating brewery wastewater. Bioresour. Technol. 2007, 98, 1849–1855. [Google Scholar] [CrossRef]
- Connaughton, S.; Collins, G.; O’Flaherty, V. Psychrophilic and mesophilic anaerobic digestion of brewery effluent: A comparative study. Water Res. 2006, 40, 2503–2510. [Google Scholar] [CrossRef]
- Zoutberg, G.R.; Frankin, R. Anaerobic treatment of chemical and brewery waste water with a new type of anaerobic reactor; The Biobed® EGSB reactor. Water Sci. Technol. 1996, 34, 375–381. [Google Scholar] [CrossRef]
- Zupančič, G.D.; Roš, M.; Klemenčič, M.; Oset, M.; Logar, R.M. Biogas production from brewery yeast in an EGSB reactor. Brauwelt Int. 2016, 34, 108–113, ISSN: 0934-9340. [Google Scholar]
- Kavalopoulos, M.; Stoumpou, V.; Christofi, A.; Mai, S.; Barampouti, E.M.; Moustakas, K.; Malamis, D.; Loizidou, M. Sustainable valorisation pathways mitigating environmental pollution from brewers’ spent grains. Environ. Pollut. 2021, 270, 116069. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Technofunctional properties of a brewers’ spent grain protein-enriched isolate and its associated enzymatic hydrolysates. LWT Food Sci. Technol. 2014, 59, 1061–1067. [Google Scholar] [CrossRef]
- Moshtagh, B.; Hawboldt, K.; Zhang, B. Optimization of biosurfactant production by Bacillus Subtilis N3-1P using the brewery waste as the carbon source. Environ. Technol. 2019, 40, 3371–3380. [Google Scholar] [CrossRef]
- Nazareth, T.C.; Zanutto, C.P.; Tripathi, L.; Juma, A.; Maass, D.; de Souza, A.A.U.; de Arruda Guelli Ulson de Souza, S.M.; Banat, I.M. The use of low-cost brewery waste product for the production of surfactin as a natural microbial biocide. Biotechnol. Rep. 2020, 28, e00537. [Google Scholar] [CrossRef] [PubMed]
- Pepe, O.; Blaiotta, G.; Moschetti, G.; Greco, T.; Villani, F. Rope-producing strains of Bacillus spp. from wheat bread and strategy for their control by lactic acid bacteria. Appl. Environ. Microbiol. 2003, 69, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Benigar, E.; Dogsa, I.; Stopar, D.; Jamnik, A.; Cigić, I.K.; Tomšič, M. Structure and dynamics of a polysaccharide matrix: Aqueous solutions of bacterial levan. Langmuir 2014, 30, 4172–4182. [Google Scholar] [CrossRef] [PubMed]
- Obire, O. Activity of Zymomonas species in palm-sap obtained from three areas in Edo State, Nigeria. J. Appl. Sci. Environ. Manag. 2005, 9, 25–30. [Google Scholar]
- Jackson, R.S. Innovations in winemaking. In Science and Technology of Fruit Wine Production; Academic Press: Cambridge, MA, USA, 2017; pp. 617–662. [Google Scholar] [CrossRef]
- Brennan, M.; McDonald, A.; Topp, C.F.E. Use of Raman microspectroscopy to predict malting barley husk adhesion quality. Plant Physiol. Biochem. 2019, 139, 587–590. [Google Scholar] [CrossRef]
- Grant, K.R.; Brennan, M.; Hoad, S.P. The structure of the barley husk influences its resistance to mechanical stress. Front. Plant Sci. 2021, 11, 614334. [Google Scholar] [CrossRef]
- Brennan, M.; Shepherd, T.; Mitchell, S.; Topp, C.F.E.; Hoad, S.P. Husk to caryopsis adhesion in barley is influenced by pre-and post-anthesis temperatures through changes in a cuticular cementing layer on the caryopsis. BMC Plant Biol. 2017, 17, 169. [Google Scholar] [CrossRef]
- Brennan, M.; Topp, C.F.E.; Hoad, S.P. Variation in grain skinning among spring barley varieties induced by a controlled environment misting screen. J. Agric. Sci. 2017, 155, 317–325. [Google Scholar] [CrossRef]
- Hoad, S.P.; Brennan, M.; Wilson, G.W.; Cochrane, P.M. Hull to caryopsis adhesion and grain skinning in malting barley: Identification of key growth stages in the adhesion process. J. Cereal Sci. 2016, 68, 8–15. [Google Scholar] [CrossRef]
- Greene, P.R.; Bain, C.D. Total internal reflection Raman spectroscopy of barley leaf epicuticular waxes in vivo. Colloids Surf. B Biointerfaces 2005, 45, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R. Lipids in cereal starches: A review. J. Cereal Sci. 1988, 8, 1–15. [Google Scholar] [CrossRef]
- Morrison, W.R.; Tester, R.F.; Gidley, M.J.; Karkalas, J. Resistance to acid hydrolysis of lipid-complexed amylose and lipid-free amylose in lintnerised waxy and non-waxy barley starches. Carbohydr. Res. 1993, 245, 289–302. [Google Scholar] [CrossRef]
- González-Thuillier, I.; Pellny, T.K.; Tosi, P.; Mitchell, R.A.C.; Haslam, R.; Shewry, P.R. Accumulation and deposition of triacylglycerols in the starchy endosperm of wheat grain. J. Cereal Sci. 2021, 98, 103167. [Google Scholar] [CrossRef]
- Cank, K.B.; Henkin, J.M.; Cook, A.G.; Oberlies, N.H. Droplet probe: A non-destructive residue analysis of Wari ceramics from the imperial heartland. J. Archaeol. Sci. 2021, 134, 105468. [Google Scholar] [CrossRef]
- Kao, D.; Henkin, J.M.; Soejarto, D.D.; Kinghorn, A.D.; Oberlies, N.H. Non-destructive chemical analysis of a Garcinia mangostana L. (Mangosteen) herbarium voucher specimen. Phytochem. Lett. 2018, 28, 124–129. [Google Scholar] [CrossRef]
- Coronado, M.A.; Montero, G.; Montes, D.G.; Valdez-Salas, B.; Ayala, J.R.; García, C.; Carrillo, M.; León, J.A.; Moreno, A. Physicochemical characterization and SEM-EDX analysis of brewer’s spent grain from the craft brewery industry. Sustainability 2020, 12, 7744. [Google Scholar] [CrossRef]
- Pestle, W.J.; Ahmad, F.; Vesper, B.J.; Cordell, G.A.; Colvard, M.D. Ancient bone collagen assessment by hand-held vibrational spectroscopy. J. Archaeol. Sci. 2014, 42, 381–389. [Google Scholar] [CrossRef]
- Pestle, W.J.; Brennan, V.; Sierra, R.L.; Smith, E.K.; Vesper, B.J.; Cordell, G.A.; Colvard, M.D. Hand-held Raman spectroscopy as a pre-screening tool for archaeological bone. J. Archaeol. Sci. 2015, 58, 113–120. [Google Scholar] [CrossRef]
- Neuberger, T.; Rolletschek, H.; Webb, A.; Borisjuk, L. Noninvasive mapping of lipids in plant tissue using magnetic resonance imaging. Methods Mol. Biol. 2009, 579, 485–496. [Google Scholar] [CrossRef]

| Paper | Lignin | Cellulose | Hemicellulose | Starch | Phenolics | Lipids | Protein | Ash |
|---|---|---|---|---|---|---|---|---|
| Kanauchi et al., 2001 [38] | 11.9 | 25.4 | 21.8 | — | — | 10.6 | 24.0 | 2.4 |
| Carvalheiro et al., 2004 [39] | 21.7 | 21.9 | 29.6 | — | — | — | 24.6 | 1.2 |
| Silva et al., 2004 [21] | 16.9 | 25.3 | 41.9 | — | — | — | — | 4.6 |
| Mussatto and Roberto, 2006 [25] | 27.8 | 16.8 | 28.4 | — | — | — | 15.2 | 4.6 |
| Jay et al., 2008 [15] | 20.0–22.0 | 31.0–33.0 | — | 10–12 | 1.0–1.5 | 6.0–8.0 | 15.0–17.0 | — |
| Xiros et al., 2008 [40] | 11.5 | 12.0 | 40.0 | 2.7 | 2.0 | 13.0 | 14.2 | 3.3 |
| Robertson et al., 2010b [7] | 13.0–17.0 | — | 22.0–29.0 | 2.0–8.0 | — | — | 20.0–24.0 | — |
| Niemi et al., 2012a [41] | 19.4 | 46.7 * | 2.8 | — | 7.8 | 23.3 | 4.9 | |
| Sobukola et al., 2013 [42] | 9.2 ± 0.1 | ±0.3 | — | — | 6.2 ± 0.1 | 24.4 ± 0.5 | 2.5 ± 0.1 | |
| Kemppai-nen et al., 2016 [43] | 19.6 | 45.0 * | — | — | — | 20.3 | 4.1 | |
| Yu et al., 2020 [44] | — | 51.0 ± 0.7 * | — | — | 9.4 ± 0.1 | 23.4 ± 0.2 | 4.1 ± 0.1 | |
| Naibaho and Korzeniowska, 2021a [5] | — | 50.7 ± 0.4–60.2 ± 1.7 * | — | — | 9.5 ± 0.5–13.1 ± 0.3 | 22.2 ± 0.1–30.2 ± 0.1 | 3.3 ± 0.1–4.3 ± 0.1 | |
| Compound | Molecular Formula | Molecular Mass, Parent ion (Da) | Solvent Concentration (µg/g) | Saponifiable Extract Concentration (µg/g) |
|---|---|---|---|---|
| Fatty Acids | ||||
| Arachidic acid | C20H40O2 | 312 | 65 | 330 |
| Behenic acid | C22H44O2 | 340 | 90 | 400 |
| Dimorphecolic acid | C18H32O3 | 296 | 115 | 91 |
| 2-Hydroxyarachidic acid | C20H40O3 | 328 | 65 | 180 |
| Lignoceric acid | C20H48O2 | 368 | 105 | 440 |
| Linoleic acid | C18H32O2 | 280 | 2450 | 12,200 |
| Linolenic acid | C18H30O2 | 278 | 330 | 2900 |
| Myristic acid | C14H28O2 | 228 | 310 | 1350 |
| Oleic acid | C18H34O2 | 282 | 970 | 5150 |
| Palmitic acid | C16H32O2 | 256 | 2850 | 20,300 |
| Pentadecylic acid | C15H30O2 | 242 | 105 | 450 |
| Phloionic acid | C18H34O6 | 346 | 90 | 310 |
| Stearic acid | C18H36O2 | 284 | 455 | 2100 |
| Monoacyl Glycerols and Diacyl Glycerols | ||||
| 1,3-Dipalmitoyl glycerol | C35H68O5 | 568 | 370 | — |
| 1-Linoleoyl-3-palmitoyl-rac-glycerol | C37H68O5 | 592 | 150 | — |
| 1-Monopalmitoyl glycerol | C19H38O4 | 330 | 1390 | — |
| 2-Monopalmitoyl glycerol | C19H38O4 | 330 | 110 | — |
| 1-Monolinoleoyl glycerol | C21H38O4 | 354 | 240 | — |
| 1-Monooleoyl glycerol | C21H40O4 | 356 | 710 | — |
| 1-Monostearoyl glycerol | C21H42O4 | 358 | 85 | — |
| 1-Palmitoyl-3-linoleoyl-rac-glycerol | C37H68O5 | 592 | 850 | — |
| Sterols and Tocopherols | ||||
| Δ5-Avenasterol | C29H48O | 412 | 35 | 120 |
| Campesterol | C28H48O | 400 | 95 | 330 |
| β-Sitosterol | C29H50O | 414 | 205 | 710 |
| α-Tocotrienol | C29H44O2 | 424 | 28 | — |
| β-Tocotrienol | C28H42O2 | 410 | 12 | — |
| Alkylresorcinol Derivatives | ||||
| 5-(2,3-Dihydroxypropyl)-2-methoxy-benzene-1,3-diol | C10H14O5 | 214 | 105 | — |
| 5-(2-Hydroxyethyl)-2-methoxy-benzene-1,3-diol | C9H12O4 | 184 | 160 | — |
| 5-(2,3,4-Trihydroxy-n-butyl)-2-methoxy-benzene-1,3-diol | C11H16O6 | 244 | 270 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henkin, J.M.; Mainali, K.; Sharma, B.K.; Yadav, M.P.; Ngo, H.; Sarker, M.I. A Review of Chemical and Physical Analysis, Processing, and Repurposing of Brewers’ Spent Grain. Biomass 2025, 5, 42. https://doi.org/10.3390/biomass5030042
Henkin JM, Mainali K, Sharma BK, Yadav MP, Ngo H, Sarker MI. A Review of Chemical and Physical Analysis, Processing, and Repurposing of Brewers’ Spent Grain. Biomass. 2025; 5(3):42. https://doi.org/10.3390/biomass5030042
Chicago/Turabian StyleHenkin, Joshua M., Kalidas Mainali, Brajendra K. Sharma, Madhav P. Yadav, Helen Ngo, and Majher I. Sarker. 2025. "A Review of Chemical and Physical Analysis, Processing, and Repurposing of Brewers’ Spent Grain" Biomass 5, no. 3: 42. https://doi.org/10.3390/biomass5030042
APA StyleHenkin, J. M., Mainali, K., Sharma, B. K., Yadav, M. P., Ngo, H., & Sarker, M. I. (2025). A Review of Chemical and Physical Analysis, Processing, and Repurposing of Brewers’ Spent Grain. Biomass, 5(3), 42. https://doi.org/10.3390/biomass5030042






