Resource Recovery from Green Tide Biomass: Sustainable Cascading Biorefinery Strategies for Ulva spp.
Abstract
1. Addressing Green Tides and Valorizing Biomass
2. Sustainable Biorefinery Strategies
2.1. Cascade Strategy
2.2. Full Exploitation of the Biomass
3. Ulva Biomass Components and Extraction
3.1. Salt Recovery
3.2. Lipids Recovery
3.3. Protein Recovery
| Species | Protein Content [%DW] | Total Protein Yield [%] | Extraction Methodology | Protein Quantification Methodology | Reference |
|---|---|---|---|---|---|
| Ulva spp. | 9.5–11.2 | 3–10 | Acid precipitation pH 2, homogenization for 1 h with tap water at pH 8.5, and then screw-pressed | N to protein conversion factor of total amino acid content after protein hydrolysis | [55] |
| 16.0 | 5.5 | Acid precipitation pH 2 | Bradford assay | [53] | |
| 4.1 | Heat denaturation at 90 °C | ||||
| 18.0 | 6.8 | Alkaline extraction pH 8.5 + 12, acid precipitation pH 2 | Nitrogen analyzer for solids and modified Lowry assay for liquid | [58] | |
| 5.0 | Mechanical pressing, acid precipitation, pH 2 | ||||
| 23.9 | Mechanical pressing, acid precipitation pH 2 + alkaline extraction pH 8.5 + 12, acid precipitation pH 2 | ||||
| 6.9 | 84.9 | Subcritical water hydrolysis at 180 °C, 10.5 bar for 40 min, 8% w/w solid load | N to protein ratio for solids and Lowry assay for liquid | [56] | |
| U. lactuca | 12.3–19.8 | 19.5 | Osmotic shock at 30 °C for 24 h | Commercial Lowry assay of protein hydrolysate | [39] |
| 26.1 | Enzymatic incubation at 30 °C for 4 h with 2% PMC-R10 | ||||
| 15.1 | PEF 1 at 7.5 kv cm−1, 2 pulses of 0.05 ms | ||||
| 39.0 | HSH 2 2 phase set-up “21 → 16” m s−1 | ||||
| 14.4 | 84.0 | Alkaline extraction at 80 °C, then neutralization | Amino acid PITC 3 derivatization after protein hydrolysis and HPLC analysis | [36] | |
| Ulva fenestrata | 18.0 | 9.0 | Alkaline extraction pH 8.5 | Nitrogen analyzer for solids and modified Lowry assay for liquid | [54] |
| 5.3 | Alkaline extraction pH 8.5 + 12 | ||||
| 6.9 | Mechanical pressing |
3.4. Cellulose Recovery
3.5. Ulvan Recovery
4. Life Cycle Assessment
5. Chemical Modifications of Ulvan
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye, N.; Zhang, X.; Mao, Y.; Liang, C.; Xu, D.; Zou, J.; Zhuang, Z.; Wang, Q. ‘Green Tides’ Are Overwhelming the Coastline of Our Blue Planet: Taking the World’s Largest Example. Ecol. Res. 2011, 26, 477. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and Golden Seaweed Tides on the Rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=33 (accessed on 13 June 2025).
- Dominguez, H.; Loret, E.P. Ulva Lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Rakhasiya, B.; Somya, A.; Alichen, A.; Yadav, D.S.; Bhagiya, B.K.; Rajai, J.V.; Yannam, S.K.; Seth, A.; Singh, J.K.; Seth, T.; et al. Potential Utilization of Seaweed-Derived Fusion Salt in Human Diet. Appl. Food Res. 2025, 5, 100806. [Google Scholar] [CrossRef]
- Rani, R.; Karmakar, P.; Singh, B. Potential of Seaweeds as Antioxidants and Their Role in Animal Health and Nutrition. In Multidisciplinary Applications of Marine Resources: A Step Towards Green and Sustainable Future; Rafatullah, M., Siddiqui, M.R., Khan, M.A., Kapoor, R.T., Eds.; Springer Nature: Singapore, 2024; pp. 243–264. ISBN 978-981-97-5057-3. [Google Scholar]
- Milledge, J.J.; Harvey, P.J. Golden Tides: Problem or Golden Opportunity? The Valorisation of Sargassum from Beach Inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Israel, A.; Palatnik, R.R.; Zilberman, D.; Golberg, A. Integrated Biorefinery Process for Sustainable Fractionation of Ulva ohnoi (Chlorophyta): Process Optimization and Revenue Analysis. J. Appl. Phycol. 2020, 32, 2271–2282. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; De Saeger, J.; Depuydt, S.; Asselman, J.; Janssen, C.; Heynderickx, P.M.; Wu, D.; Ronsse, F.; Tack, F.M.G.; et al. Harnessing Green Tide Ulva Biomass for Carbon Dioxide Sequestration. Rev. Environ. Sci. Biotechnol. 2024, 23, 1041–1061. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, M.; Yang, Y.; Bi, M.; Li, M.; Liu, W. Environmental and Economic Impacts of Different Disposal Options for Ulva Prolifera Green Tide in the Yellow Sea, China. ACS Sustain. Chem. Eng. 2022, 10, 11483–11492. [Google Scholar] [CrossRef]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pacheco, D.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L.; Figueirinha, A.; Dias, J.M. Valorisation of Marine Macroalgae Waste Using a Cascade Biorefinery Approach: Exploratory Study. J. Clean. Prod. 2023, 385, 135672. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, P.; Baltrusch, K.L.; Díaz-Reinoso, B.; López-Álvarez, M.; Novoa-Carballal, R.; González, P.; González-Novoa, A.; Rodríguez-Montes, A.; Kennes, C.; Veiga, M.C.; et al. Hydrothermal Extraction of Ulvans from Ulva spp. in a Biorefinery Approach. Sci. Total Environ. 2024, 951, 175654. [Google Scholar] [CrossRef] [PubMed]
- Kostas, E.T.; White, D.A.; Cook, D.J. Bioethanol Production from UK Seaweeds: Investigating Variable Pre-Treatment and Enzyme Hydrolysis Parameters. Bioenerg. Res. 2020, 13, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Immanuel Suresh, J.; Divyeswari, S. Seaweeds Are Potential Source for Production of Sustainable Bioethanol for the Imminent Future. In Multidisciplinary Applications of Marine Resources: A Step Towards Green and Sustainable Future; Rafatullah, M., Siddiqui, M.R., Khan, M.A., Kapoor, R.T., Eds.; Springer Nature: Singapore, 2024; pp. 141–160. ISBN 978-981-97-5057-3. [Google Scholar]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy Potential of Ulva lactuca: Biomass Yield, Methane Production and Combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Thien, V.Y.; Misson, M.; Chin, G.J.W.L.; Said Hussin, S.N.I.; Chong, H.L.H.; Yusof, N.A.; Ma, N.L.; Rodrigues, K.F. Seaweed: A Bioindustrial Game-Changer for the Green Revolution. Biomass Bioenergy 2024, 183, 107122. [Google Scholar] [CrossRef]
- Tong, K.T.X.; Tan, I.S.; Foo, H.C.Y.; Lam, M.K.; Lim, S.; Lee, K.T. Advancement of Biorefinery-Derived Platform Chemicals from Macroalgae: A Perspective for Bioethanol and Lactic Acid. Biomass Conv. Bioref. 2024, 14, 1443–1479. [Google Scholar] [CrossRef]
- Amalapridman, V.; Ofori, P.A.; Abbey, L. Valorization of Algal Biomass to Biofuel: A Review. Biomass 2025, 5, 26. [Google Scholar] [CrossRef]
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Livney, Y.D.; Golberg, A. Starch from the Sea: The Green Macroalga Ulva ohnoi as a Potential Source for Sustainable Starch Production in the Marine Biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Magnusson, M.; Carl, C.; Mata, L.; de Nys, R.; Paul, N.A. Seaweed Salt from Ulva: A Novel First Step in a Cascading Biorefinery Model. Algal Res. 2016, 16, 308–316. [Google Scholar] [CrossRef]
- van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of Acetone, Butanol, and Ethanol from Biomass of the Green Seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical Composition and Functional Properties of Ulva lactuca Seaweed Collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Cañedo-Castro, B.; Piñón-Gimate, A.; Carrillo, S.; Ramos, D.; Casas-Valdez, M. Prebiotic Effect of Ulva Rigida Meal on the Intestinal Integrity and Serum Cholesterol and Triglyceride Content in Broilers. J. Appl. Phycol. 2019, 31, 3265–3273. [Google Scholar] [CrossRef]
- Nissen, S.H.; Juul, L.; Bruhn, A.; Søndergaard, J.; Dalsgaard, T.K. The Biochemical Composition and Its Relation to Color of Ulva Spp. upon Harvest Time. J. Appl. Phycol. 2024, 36, 2095–2107. [Google Scholar] [CrossRef]
- Jansen, H.M.; Bernard, M.S.; Nederlof, M.A.J.; van der Meer, I.M.; van der Werf, A. Seasonal Variation in Productivity, Chemical Composition and Nutrient Uptake of Ulva spp. (Chlorophyta) Strains. J. Appl. Phycol. 2022, 34, 1649–1660. [Google Scholar] [CrossRef]
- Pappou, S.; Dardavila, M.M.; Savvidou, M.G.; Louli, V.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from Ulva lactuca. Appl. Sci. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Suchinina, T.V.; Shestakova, T.S.; Petrichenko, V.M.; Novikova, V.V. Solvent Polarity Effect on the Composition of Biologically Active Substances, UV Spectral Characteristics, and Antibacterial Activity of Euphrasia Brevipila Herb Extracts. Pharm. Chem. J. 2011, 44, 683–686. [Google Scholar] [CrossRef]
- Holzinger, A.; Lütz, C.; Karsten, U. Desiccation Stress Causes Structural and Ultrastructural Alterations in the Aeroterrestrial Green Alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) Isolated from an Alpine Soil Crust. J. Phycol. 2011, 47, 591–602. [Google Scholar] [CrossRef]
- Narayanan, M. Biorefinery Products from Algal Biomass by Advanced Biotechnological and Hydrothermal Liquefaction Approaches. Discov. Appl. Sci. 2024, 6, 146. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Markham, J.; Templeton, D.W.; Christensen, E.D.; Wychen, S.V.; Vadelius, E.W.; Chen-Glasser, M.; Dong, T.; Davis, R.; Pienkos, P.T. Development of Algae Biorefinery Concepts for Biofuels and Bioproducts; a Perspective on Process-Compatible Products and Their Impact on Cost-Reduction. Energy Environ. Sci. 2017, 10, 1716–1738. [Google Scholar] [CrossRef]
- Pinheiro, V.F.; Marçal, C.; Abreu, H.; Lopes da Silva, J.A.; Silva, A.M.S.; Cardoso, S.M. Physicochemical Changes of Air-Dried and Salt-Processed Ulva Rigida over Storage Time. Molecules 2019, 24, 2955. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Nassar, H.N.; Ismail, A.R.; Ali, H.R.; Ali, B.A.; Abdelsalam, K.M.; Mubarak, M. A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products. Sustainability 2023, 15, 7319. [Google Scholar] [CrossRef]
- Trivedi, N.; Baghel, R.S.; Bothwell, J.; Gupta, V.; Reddy, C.R.K.; Lali, A.M.; Jha, B. An Integrated Process for the Extraction of Fuel and Chemicals from Marine Macroalgal Biomass. Sci. Rep. 2016, 6, 30728. [Google Scholar] [CrossRef] [PubMed]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of Protein Extraction with a Stream of Byproducts from Marine Macroalgae: A Model Forms the Basis for Marine Bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Fertahi, S.; Elalami, D.; Tayibi, S.; Bargaz, A.; Barakat, A. Multifunctional Agricultural Inputs Based on Biochar Impregnated with Algae Residues Extracts: Promoting Effect on Tomato Growth. Algal Res. 2024, 81, 103577. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Postma, P.R.; Cerezo-Chinarro, O.; Akkerman, R.J.; Olivieri, G.; Wijffels, R.H.; Brandenburg, W.A.; Eppink, M.H.M. Biorefinery of the Macroalgae Ulva lactuca: Extraction of Proteins and Carbohydrates by Mild Disintegration. J. Appl. Phycol. 2018, 30, 1281–1293. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Levkov, K.; Livney, Y.D.; Israel, A.; Golberg, A. High-Voltage Pulsed Electric Field Preprocessing Enhances Extraction of Starch, Proteins, and Ash from Marine Macroalgae Ulva Ohnoi. ACS Sustain. Chem. Eng. 2019, 7, 1753–17463. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Borges, L.A.; Ferreira, N.L.B.; Clerici, M.T.P.S. Seaweed as a Potential New Source for Starch, Produced in the Sea: A Short Review. Starch-Stärke 2023, 75, 2200130. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid Composition, Fatty Acids and Sterols in the Seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An Analysis from Nutritional, Chemotaxonomic, and Antiproliferative Activity Perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Peña-Rodríguez, A.; Mawhinney, T.P.; Ricque-Marie, D.; Cruz-Suárez, L.E. Chemical Composition of Cultivated Seaweed Ulva Clathrata (Roth) C. Agardh. Food Chem. 2011, 129, 491–498. [Google Scholar] [CrossRef]
- Nelson, M.M.; Phleger, C.F.; Nichols, P.D. Seasonal Lipid Composition in Macroalgae of the Northeastern Pacific Ocean. Bot. Mar. 2002, 45, 58–65. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.-E.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of Their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.M.P.; Gouveia, A.; Rema, P.; Matos, J.; Gomes, E.F.; Pinto, I.S. Evaluation of Three Seaweeds Gracilaria Bursa-Pastoris, Ulva rigida and Gracilaria cornea as Dietary Ingredients in European Sea Bass (Dicentrarchus labrax) Juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Ragonese, C.; Tedone, L.; Beccaria, M.; Torre, G.; Cichello, F.; Cacciola, F.; Dugo, P.; Mondello, L. Characterisation of Lipid Fraction of Marine Macroalgae by Means of Chromatography Techniques Coupled to Mass Spectrometry. Food Chem. 2014, 145, 932–940. [Google Scholar] [CrossRef]
- Martins, M.; Oliveira, R.; Coutinho, J.A.P.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Pinto, D.C.G.A.; Ventura, S.P.M. Recovery of Pigments from Ulva rigida. Sep. Purif. Technol. 2021, 255, 117723. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, C.R.K.; Jha, B. Comparative Evaluation and Selection of a Method for Lipid and Fatty Acid Extraction from Macroalgae. Anal. Biochem. 2011, 415, 134–144. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed Proteins: Biochemical, Nutritional Aspects and Potential Uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Malvis Romero, A.; Picado Morales, J.J.; Klose, L.; Liese, A. Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva Fenestrata. Molecules 2023, 28, 6781. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J.; Gonçalves, A.M. Seaweed Proteins: A Step towards Sustainability? Nutrients 2024, 16, 1123. [Google Scholar] [CrossRef]
- Juel, N.; Juul, L.; Tanambell, H.; Dalsgaard, T.K. Extraction and Purification of Seaweed Protein from Ulva sp.—Challenges to Overcome. LWT 2024, 198, 115944. [Google Scholar] [CrossRef]
- Juul, L.; Danielsen, M.; Nebel, C.; Steinhagen, S.; Bruhn, A.; Jensen, S.K.; Undeland, I.; Dalsgaard, T.K. Ulva fenestrata Protein–Comparison of Three Extraction Methods with Respect to Protein Yield and Protein Quality. Algal Res. 2021, 60, 102496. [Google Scholar] [CrossRef]
- Nissen, S.H.; Juul, L.; Stødkilde, L.; Bruhn, A.; Ambye-Jensen, M.; Dalsgaard, T.K. Pilot-Scale Protein Extraction of Green Seaweed (Ulva spp.) Whole Biomass and Pulp–Investigating Biochemical Composition and Protein Digestibility in a Rat Trial. Food Bioprod. Process. 2024, 148, 353–364. [Google Scholar] [CrossRef]
- Polikovsky, M.; Gillis, A.; Steinbruch, E.; Robin, A.; Epstein, M.; Kribus, A.; Golberg, A. Biorefinery for the Co-Production of Protein, Hydrochar and Additional Co-Products from a Green Seaweed Ulva Sp. with Subcritical Water Hydrolysis. Energy Convers. Manag. 2020, 225, 113380. [Google Scholar] [CrossRef]
- Bonitati, J.; Elliott, W.B.; Miles, P.G. Interference by Carbohydrate and Other Substances in the Estimation of Protein with the Folin-Ciocalteu Reagent. Anal. Biochem. 1969, 31, 399–404. [Google Scholar] [CrossRef]
- Juul, L.; Steinhagen, S.; Bruhn, A.; Jensen, S.K.; Undeland, I.; Dalsgaard, T.K. Combining Pressing and Alkaline Extraction to Increase Protein Yield from Ulva fenestrata Biomass. Food Bioprod. Process. 2022, 134, 80–85. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the Green Macroalgae Ulva lactuca: Isolation, Characterization, Optotracing, and Production of Cellulose Nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Yousuf, W.E.; Kenawy, E.-R.; Mohamed, T.M. Biosynthesis of Cellulose from Ulva lactuca, Manufacture of Nanocellulose and Its Application as Antimicrobial Polymer. Sci. Rep. 2023, 13, 10188. [Google Scholar] [CrossRef]
- Jmel, M.A.; Anders, N.; Ben Messaoud, G.; Marzouki, M.N.; Spiess, A.; Smaali, I. The Stranded Macroalga Ulva lactuca as a New Alternative Source of Cellulose: Extraction, Physicochemical and Rheological Characterization. J. Clean. Prod. 2019, 234, 1421–1427. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and Characterization of Seaweed Cellulose Derived Carboxymethyl Cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Pari, R.F.; Uju, U.; Hardiningtyas, S.D.; Ramadhan, W.; Wakabayashi, R.; Goto, M.; Kamiya, N. Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design. Mar. Drugs 2025, 23, 56. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Glasson, C.R.K.; Magnusson, M.; Sims, I.M.; Hinkley, S.F.R.; de Nys, R.; Carnachan, S.M. Ulvans Are Not Equal-Linkage and Substitution Patterns in Ulvan Polysaccharides Differ with Ulva Morphology. Carbohydr. Polym. 2024, 333, 121962. [Google Scholar] [CrossRef]
- Mhatre, A.; Gore, S.; Mhatre, A.; Trivedi, N.; Sharma, M.; Pandit, R.; Anil, A.; Lali, A. Effect of Multiple Product Extractions on Bio-Methane Potential of Marine Macrophytic Green Alga Ulva lactuca. Renew. Energy 2019, 132, 742–751. [Google Scholar] [CrossRef]
- Córdoba, V.; Bavio, M.; Acosta, G. Biomethane Production Modelling from Third-Generation Biomass. Renew. Energy 2024, 234, 121211. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Sims, I.M.; Carnachan, S.M.; de Nys, R.; Magnusson, M. A Cascading Biorefinery Process Targeting Sulfated Polysaccharides (Ulvan) from Ulva ohnoi. Algal Res. 2017, 27, 383–391. [Google Scholar] [CrossRef]
- Robic, A.; Gaillard, C.; Sassi, J.-F.; Lerat, Y.; Lahaye, M. Ultrastructure of Ulvan: A Polysaccharide from Green Seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.-F.; Lerat, Y.; Lahaye, M. Structure and Interactions of Ulvan in the Cell Wall of the Marine Green Algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Pizzoferrato, L.; Liquori, A.M. A Physico-Chemical Study on the Polysaccharide Ulvan from Hot Water Extraction of the Macroalga Ulva. Int. J. Biol. Macromol. 1999, 25, 309–315. [Google Scholar] [CrossRef]
- Morelli, A.; Puppi, D.; Chiellini, F. Chapter 16—Perspectives on Biomedical Applications of Ulvan. In Seaweed Polysaccharides; Venkatesan, J., Anil, S., Kim, S.-K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 305–330. ISBN 978-0-12-809816-5. [Google Scholar]
- Chi, Y.; Li, H.; Fan, L.; Du, C.; Zhang, J.; Guan, H.; Wang, P.; Li, R. Metal-Ion-Binding Properties of Ulvan Extracted from Ulva clathrata and Structural Characterization of Its Complexes. Carbohydr. Polym. 2021, 272, 118508. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.-F.; Lahaye, M. Impact of Stabilization Treatments of the Green Seaweed Ulva rotundata (Chlorophyta) on the Extraction Yield, the Physico-Chemical and Rheological Properties of Ulvan. Carbohydr. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Barthélemy, J.-P.; Paquot, M.; Blecker, C.; Attia, H. Impact of Extraction Procedures on the Chemical, Rheological and Textural Properties of Ulvan from Ulva lactuca of Tunisia Coast. Food Hydrocoll. 2014, 40, 53–63. [Google Scholar] [CrossRef]
- Kraithong, S.; Bunyameen, N.; Theppawong, A.; Ke, X.; Lee, S.; Zhang, X.; Huang, R. Potentials of Ulva spp.-Derived Sulfated Polysaccharides as Gelling Agents with Promising Therapeutic Effects. Int. J. Biol. Macromol. 2024, 273, 132882. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a Bioactive Marine Sulphated Polysaccharide as a Key Constituent of Hybrid Biomaterials: A Review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Cappelli, A.; Gigli, E.; Romagnoli, F.; Simoni, S.; Blumberga, D.; Palerno, M.; Guerriero, E. Co-Digestion of Macroalgae for Biogas Production: An LCA-Based Environmental Evaluation. Energy Procedia 2015, 72, 3–10. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization Potential of Microalgae for Biofuels Production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Helmes, R.J.K.; López-Contreras, A.M.; Benoit, M.; Abreu, H.; Maguire, J.; Moejes, F.; Burg, S.W.K. van den Environmental Impacts of Experimental Production of Lactic Acid for Bioplastics from Ulva spp. Sustainability 2018, 10, 2462. Sustainability 2018, 10, 2462. [Google Scholar] [CrossRef]
- Ghosh, S.; Greiserman, S.; Chemodanov, A.; Slegers, P.M.; Belgorodsky, B.; Epstein, M.; Kribus, A.; Gozin, M.; Chen, G.-Q.; Golberg, A. Polyhydroxyalkanoates and Biochar from Green Macroalgal Ulva sp. Biomass Subcritical Hydrolysates: Process Optimization and a Priori Economic and Greenhouse Emissions Break-Even Analysis. Sci. Total Environ. 2021, 770, 145281. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel Macroalgae (Seaweed) Biorefinery Systems for Integrated Chemical, Protein, Salt, Nutrient and Mineral Extractions and Environmental Protection by Green Synthesis and Life Cycle Sustainability Assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Golberg, A.; Polikovsky, M.; Epstein, M.; Slegers, P.M.; Drabik, D.; Kribus, A. Hybrid Solar-Seaweed Biorefinery for Co-Production of Biochemicals, Biofuels, Electricity, and Water: Thermodynamics, Life Cycle Assessment, and Cost-Benefit Analysis. Energy Convers. Manag. 2021, 246, 114679. [Google Scholar] [CrossRef]
- Kulikova, Y.; Ilinykh, G.; Sliusar, N.; Babich, O.; Bassyouni, M. Life Cycle Assessments of Biofuel Production from Beach-Cast Seaweed by Pyrolysis and Hydrothermal Liquefaction. Energy Convers. Manag. X 2024, 23, 100647. [Google Scholar] [CrossRef]
- Bin Abu Sofian, A.D.A.; Lim, H.R.; Manickam, S.; Ang, W.L.; Show, P.L. Towards a Sustainable Circular Economy: Algae-Based Bioplastics and the Role of Internet-of-Things and Machine Learning. ChemBioEng Rev. 2024, 11, 39–59. [Google Scholar] [CrossRef]
- de Carvalho, M.M.; de Freitas, R.A.; Ducatti, D.R.B.; Ferreira, L.G.; Gonçalves, A.G.; Colodi, F.G.; Mazepa, E.; Aranha, E.M.; Noseda, M.D.; Duarte, M.E.R. Modification of Ulvans via Periodate-Chlorite Oxidation: Chemical Characterization and Anticoagulant Activity. Carbohydr. Polym. 2018, 197, 631–640. [Google Scholar] [CrossRef]
- Bang, T.H.; Van, T.T.T.; Hung, L.X.; LY, B.M.; Nhut, N.D.; Thuy, T.T.T.; Huy, B.T. Nanogels of Acetylated Ulvan Enhance the Solubility of Hydrophobic Drug Curcumin. Bull. Mater. Sci. 2019, 42, 1. [Google Scholar] [CrossRef]
- Chen, X.; Yue, Z.; Winberg, P.C.; Lou, Y.-R.; Beirne, S.; Wallace, G.G. 3D Bioprinting Dermal-like Structures Using Species-Specific Ulvan. Biomater. Sci. 2021, 9, 2424–2438. [Google Scholar] [CrossRef]
- Wang, W.; Huang, W.-C.; Zheng, J.; Xue, C.; Mao, X. Preparation and Comparison of Dialdehyde Derivatives of Polysaccharides as Cross-Linking Agents. Int. J. Biol. Macromol. 2023, 236, 123913. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, S.; Zhu, Y.; Ning, Y.; Chen, T.; Yang, Y.; Qi, B.; Huang, Y.; Li, Y. Crosslinking of Gelatin Schiff Base Hydrogels with Different Structural Dialdehyde Polysaccharides as Novel Crosslinkers: Characterization and Performance Comparison. Food Chem. 2024, 456, 140090. [Google Scholar] [CrossRef]
- Ren, Y.; Aierken, A.; Zhao, L.; Lin, Z.; Jiang, J.; Li, B.; Wang, J.; Hua, J.; Tu, Q. hUC-MSCs Lyophilized Powder Loaded Polysaccharide Ulvan Driven Functional Hydrogel for Chronic Diabetic Wound Healing. Carbohydr. Polym. 2022, 288, 119404. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Sapalidis, A.; Kikionis, S.; Aggelidou, E.; Demiri, E.; Kritis, A.; Ioannou, E.; Roussis, V. Hybrid Sponge-like Scaffolds Based on Ulvan and Gelatin: Design, Characterization and Evaluation of Their Potential Use in Bone Tissue Engineering. Materials 2020, 13, 1763. [Google Scholar] [CrossRef]
- Morelli, A.; Betti, M.; Puppi, D.; Bartoli, C.; Gazzarri, M.; Chiellini, F. Enzymatically Crosslinked Ulvan Hydrogels as Injectable Systems for Cell Delivery. Macromol. Chem. Phys. 2016, 217, 581–590. [Google Scholar] [CrossRef]
- Colodi, F.G.; Ducatti, D.R.B.; Noseda, M.D.; de Carvalho, M.M.; Winnischofer, S.M.B.; Duarte, M.E.R. Semi-Synthesis of Hybrid Ulvan-Kappa-Carrabiose Polysaccharides and Evaluation of Their Cytotoxic and Anticoagulant Effects. Carbohydr. Polym. 2021, 267, 118161. [Google Scholar] [CrossRef] [PubMed]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and Characterization of Ulvan Polysaccharides-Based Hydrogel Films for Potential Wound Dressing Applications. DDDT 2021, 15, 4213–4226. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Pippa, N.; Ioannou, E.; Demetzos, C.; Roussis, V. Development of Ulvan-Containing Liposomes as Antibacterial Drug Delivery Platforms. J. Funct. Biomater. 2022, 13, 186. [Google Scholar] [CrossRef]
- Don, T.-M.; Liu, L.-M.; Chen, M.; Huang, Y.-C. Crosslinked Complex Films Based on Chitosan and Ulvan with Antioxidant and Whitening Activities. Algal Res. 2021, 58, 102423. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, H.; Feng, T.; Bu, X.; Cui, C.; Yang, F.; Yu, C. Advancing Soy Protein Isolate-Ulvan Film Physicochemical Properties and Antioxidant Activities through Strategic High-Pressure Homogenization Technique. Ind. Crops Prod. 2024, 215, 118704. [Google Scholar] [CrossRef]
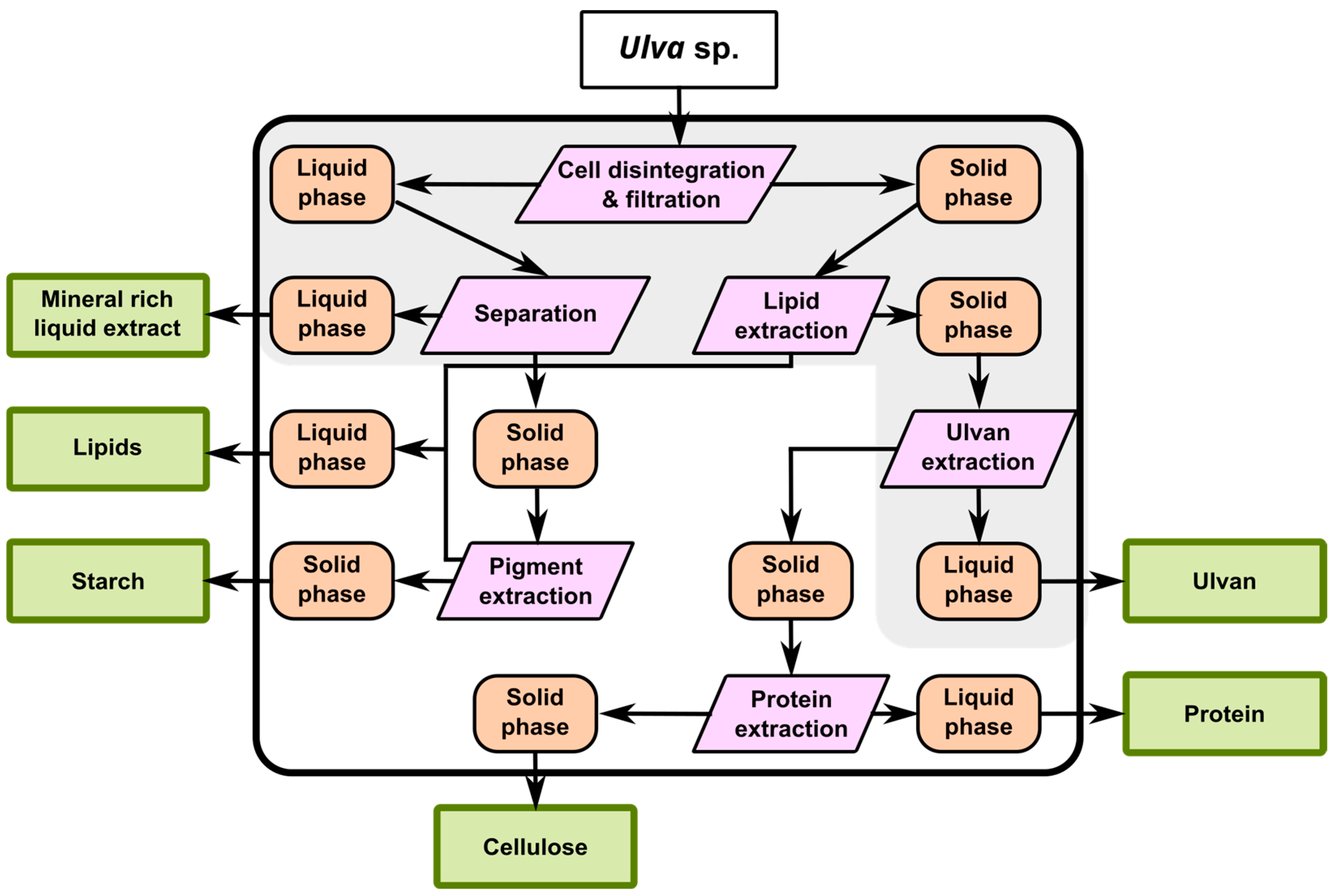
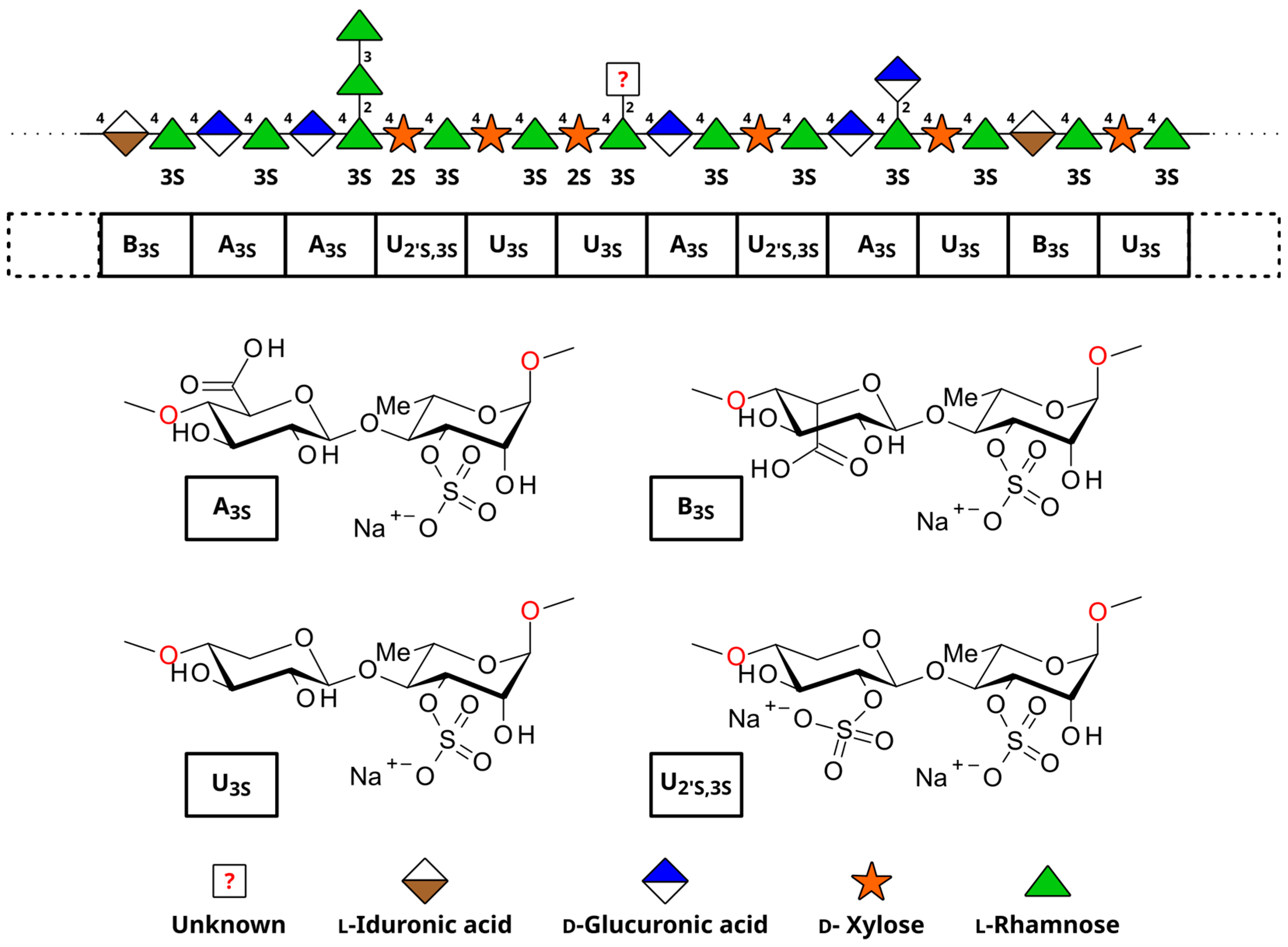
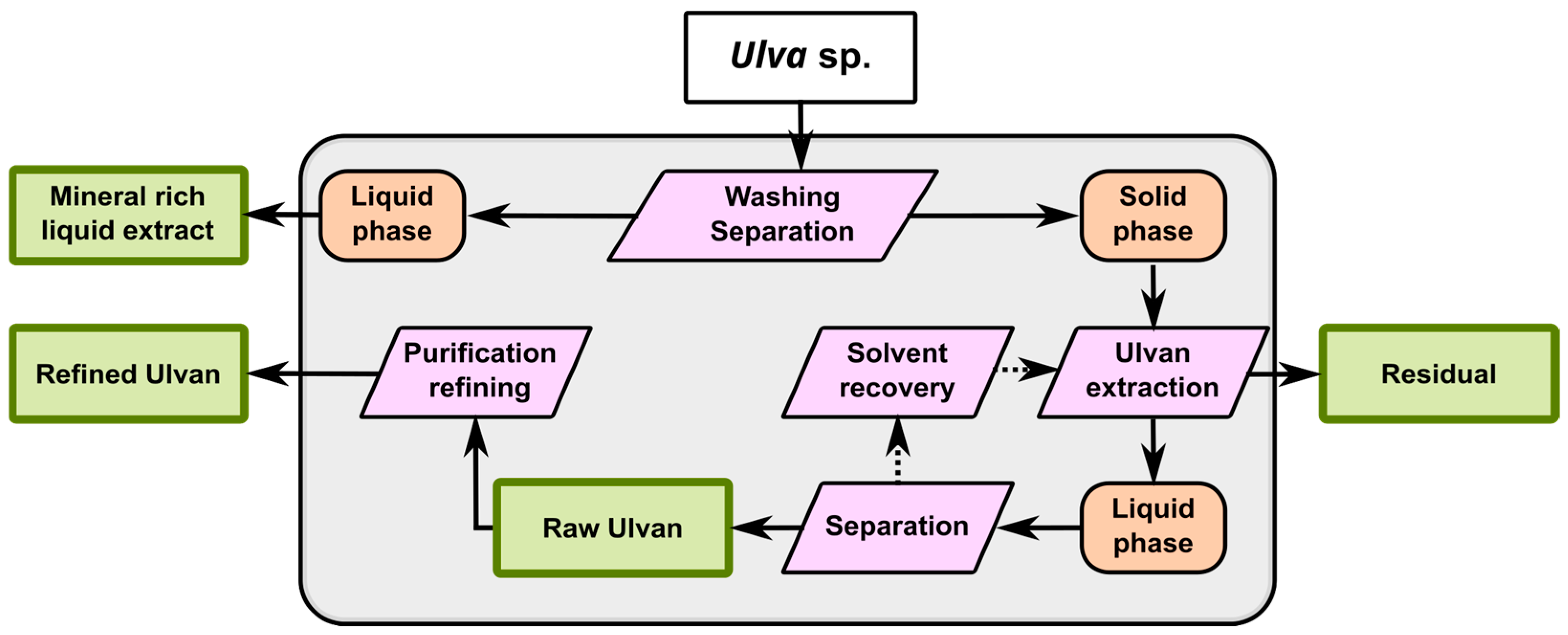
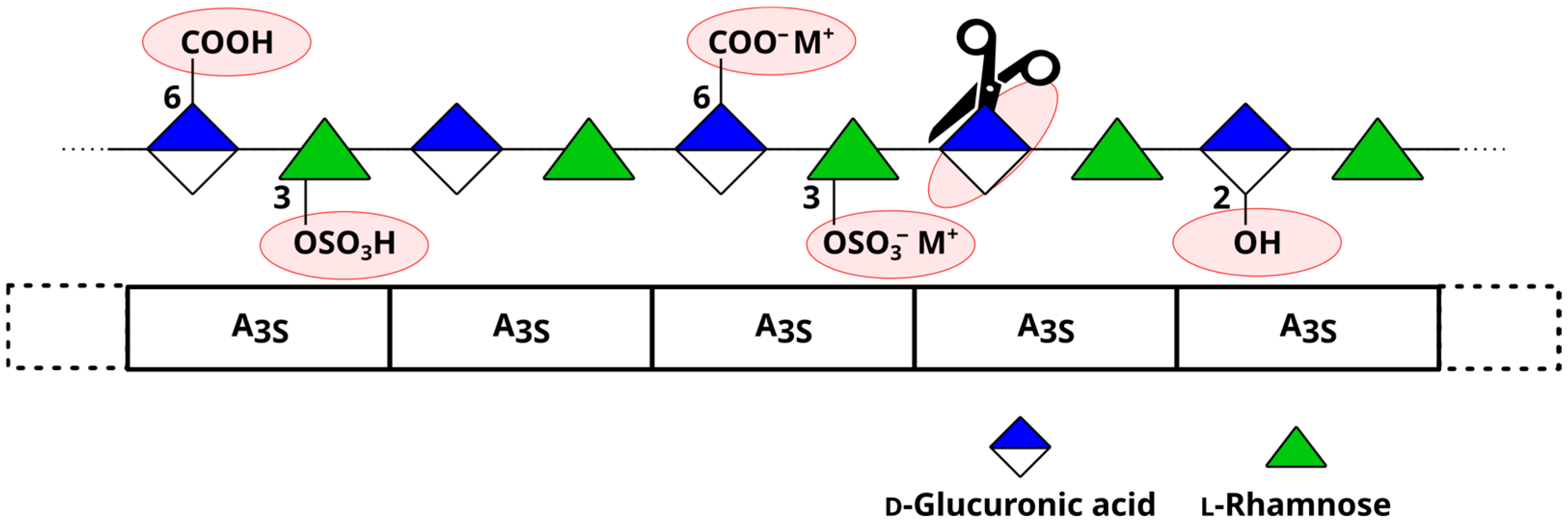
| Species | Location | Month | Lipid | Protein | Carbohydrates | Ash | Ref. |
|---|---|---|---|---|---|---|---|
| U. lactuca | SE of India | / | 1.6% | 3.2% | 25.0% | / | [22] |
| India | / | 3.3% | 8.4% | 35.3% | / | ||
| Hong Kong | Dec | 1.6% | 7.0% | 14.6% 1 | 21.3% | ||
| Philippines | May | 0.7% | 4.0% | 55.0% | 17.3% 2 | ||
| The Netherlands | Dec | 0.3–5.0% | 24.6% | 20.0% | 15.9% | [23] | |
| Tunisia | Jul | 7.9% | 8.5% | 54.0% | 19.5% | [24] | |
| Ulva rigida | Mexico | / | / | 8.7% | 55.0% | 30.1% | [25] |
| Ulva spp. | Denmark | May | 1.0% | 20.6% | / | 41.2% | [26] |
| Jun | 1.7% | 6.5% | / | 36.9% | |||
| Aug | 10.7% | / | 35.5% | ||||
| The Netherlands | May | 0.4–1.3% | 8.0–22.0% | 35.0–45.0% | 20.0–15.0% | [27] |
| Species | Yield [w/w] | Reference |
|---|---|---|
| Ulva ohnoi | 45% | [9] |
| 29% | [22] | |
| Ulva tepida | 36% | [22] |
| Ulva fasciata | 35% | [34] |
| 26% | [35] |
| Macroelements | Microelements | Heavy Metals 1 |
|---|---|---|
| Na | B | Ni |
| K | Al | Cu |
| Ca | Cr | Zn |
| Mg | Mn | Mo |
| P | Fe | Ni |
| Co |
| Fat (% DW) | Fatty Acids (% DW) | 1 SFA (% DW) | 2 MUFA (% DW) | 3 PUFA (% DW) | Reference | |
|---|---|---|---|---|---|---|
| Ulva armoricana | 2.6 | 46.5 | 24.3 | 29.2 | [42] | |
| Ulva clathrata | 2–4 | 74–77 | 29–51 | 10–12 | 13–34 | [43] |
| Ulva lobata | 2–3 | 22–29 | 13–15 | 55–64 | [44] | |
| U. lactuca | 7–13 | 27–69 | 16–24 | 7–43 | [24,45] | |
| U. rigida | 1–6 | 29–37 | 21–22 | 33–34 | [46,47] |
| Reaction | Involved Moiety | Reactant | Reference |
|---|---|---|---|
| Oxidation | Saccharide unit | NaIO4; NaClO2 | [88] |
| Condensation | -OH | Acetic anhydride | [89] |
| -OH | Methacrylic anhydride | [90] | |
| Covalent cross-linking | Oxidized saccharides | Chitosan | [91] |
| Oxidized saccharides | Gelatine | [92] | |
| Oxidized saccharides | Chitosan | [93] | |
| -COOH; -OH | 1,4-Butanediol diglycidyl ether | [94] | |
| Functionalized -COOH | Tyramine | [95] | |
| Functionalized -COOH | kappa-carrabiose disaccharide | [96] | |
| Ionic cross-linking | -COO−; -SO3− | Boric acid | [97] |
| -SO3− | Phospholipids | [98] | |
| -COO−; -SO3− | Chitosan | [99] | |
| Soy protein | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottolina, G.; Zaccheria, F.; Paini, J. Resource Recovery from Green Tide Biomass: Sustainable Cascading Biorefinery Strategies for Ulva spp. Biomass 2025, 5, 41. https://doi.org/10.3390/biomass5030041
Ottolina G, Zaccheria F, Paini J. Resource Recovery from Green Tide Biomass: Sustainable Cascading Biorefinery Strategies for Ulva spp. Biomass. 2025; 5(3):41. https://doi.org/10.3390/biomass5030041
Chicago/Turabian StyleOttolina, Gianluca, Federica Zaccheria, and Jacopo Paini. 2025. "Resource Recovery from Green Tide Biomass: Sustainable Cascading Biorefinery Strategies for Ulva spp." Biomass 5, no. 3: 41. https://doi.org/10.3390/biomass5030041
APA StyleOttolina, G., Zaccheria, F., & Paini, J. (2025). Resource Recovery from Green Tide Biomass: Sustainable Cascading Biorefinery Strategies for Ulva spp. Biomass, 5(3), 41. https://doi.org/10.3390/biomass5030041







