Subcritical Water Processing of Grape Pomace (Vitis vinifera L.): Kinetic Evaluation of Sugar Production and By-Product Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
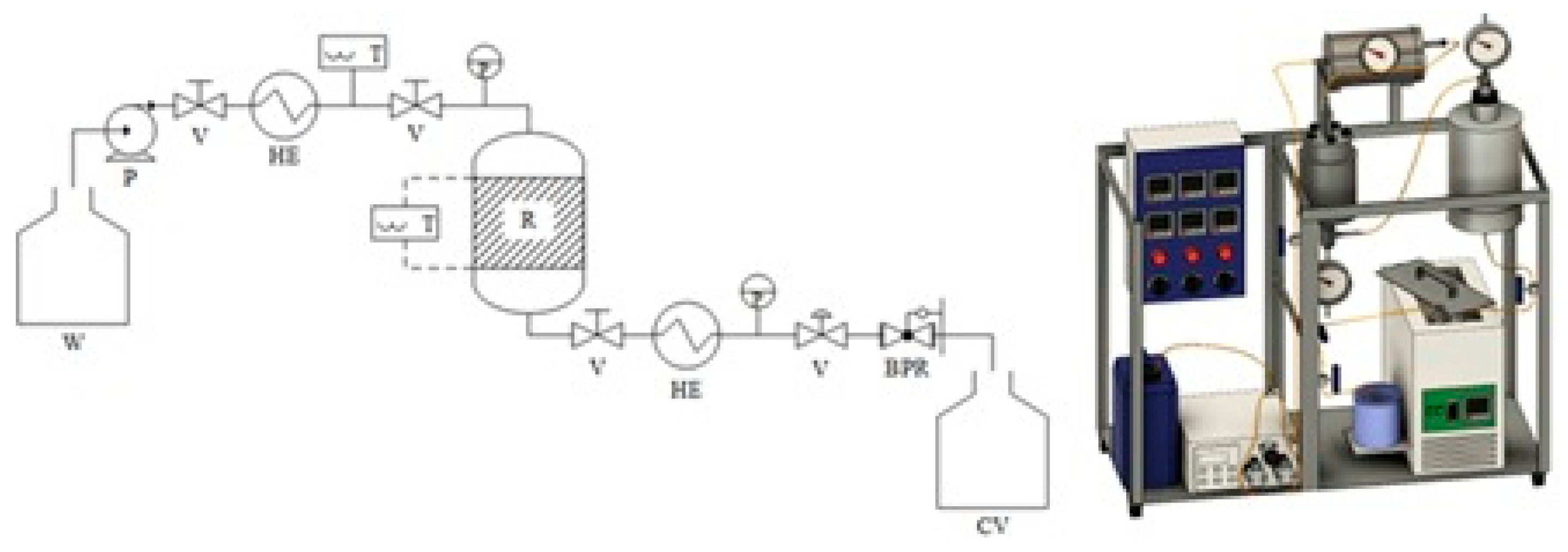
2.2. Semi-Continuous Flow-Through Subcritical Water Processing
2.3. Analytical Methods
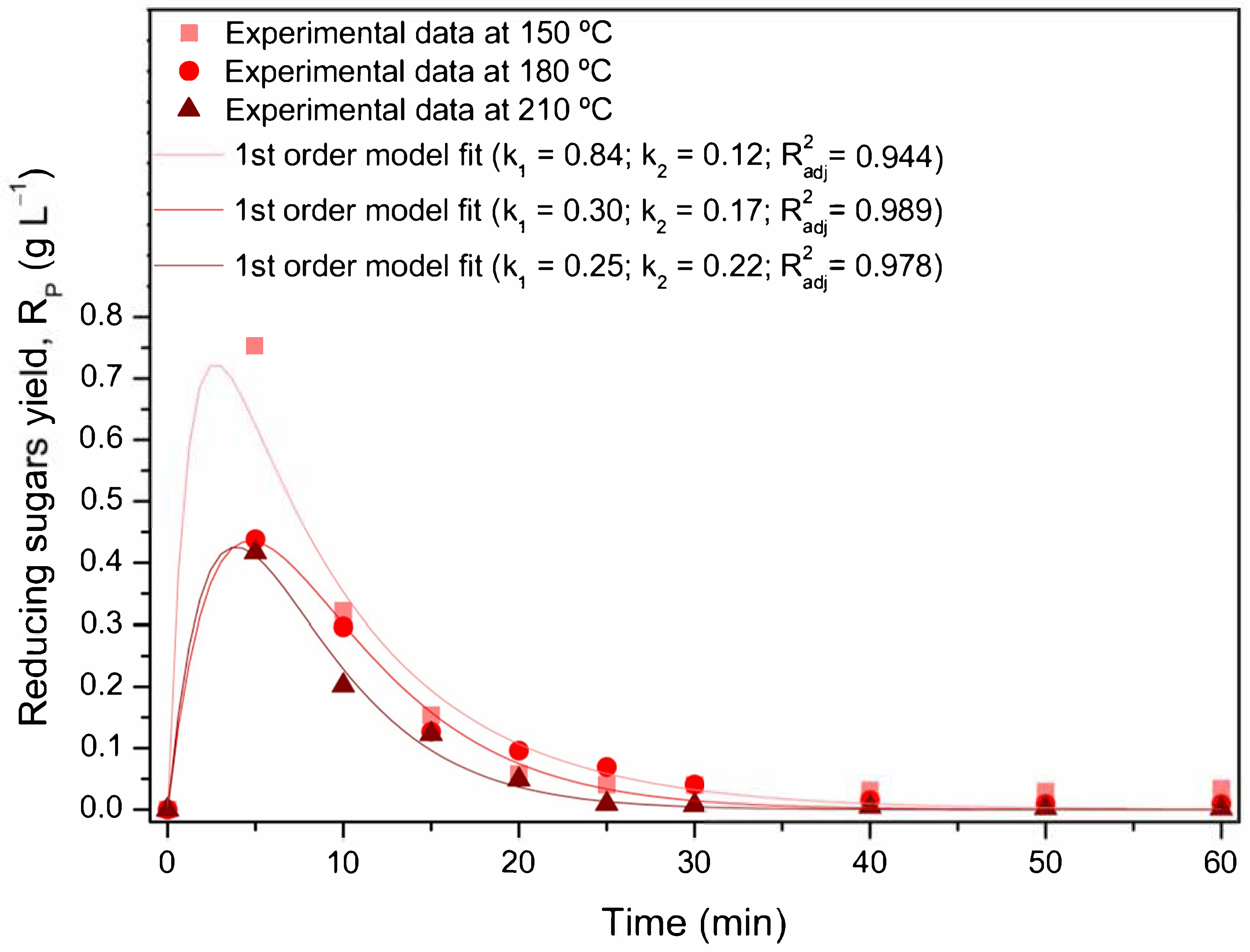
2.4. Kinetic Modeling of Subcritical Water Processing
2.5. Characterization of Hydrolysate Residue After Subcritical Water Processing
3. Results and Discussion
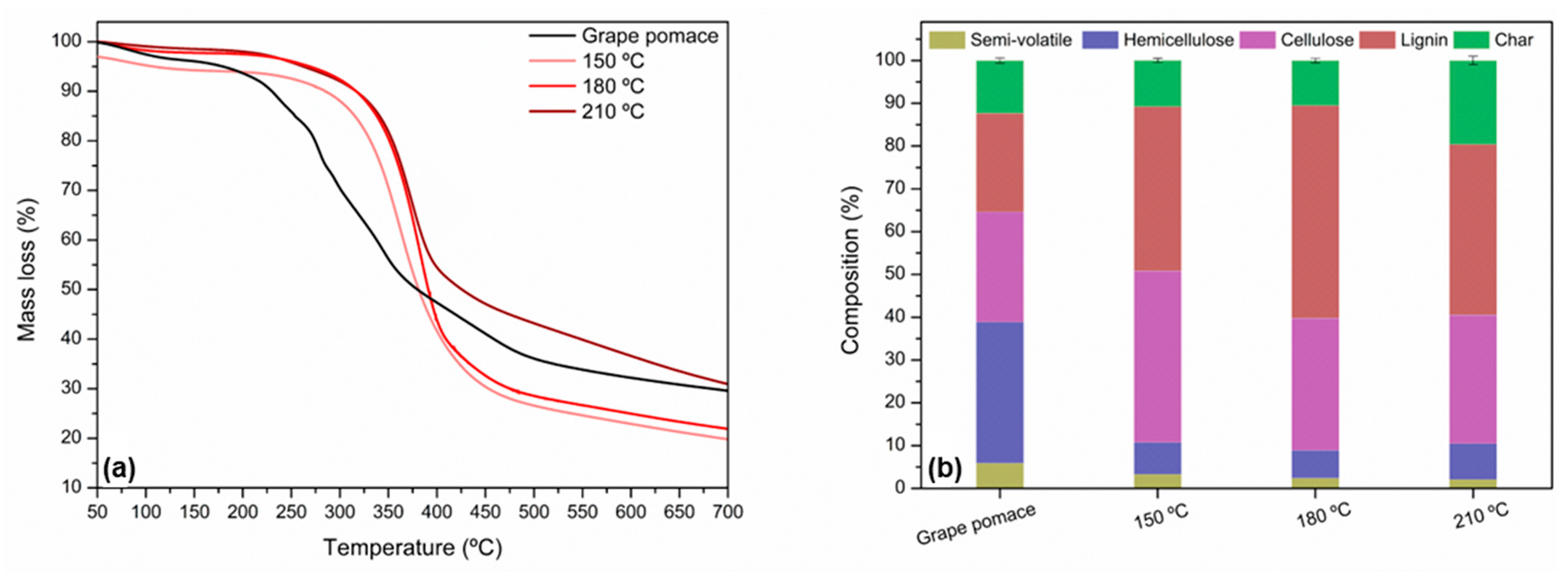
3.1. Lyophilized Grape Pomace Characterization
3.2. Characterization of Grape Pomace Hydrolysates
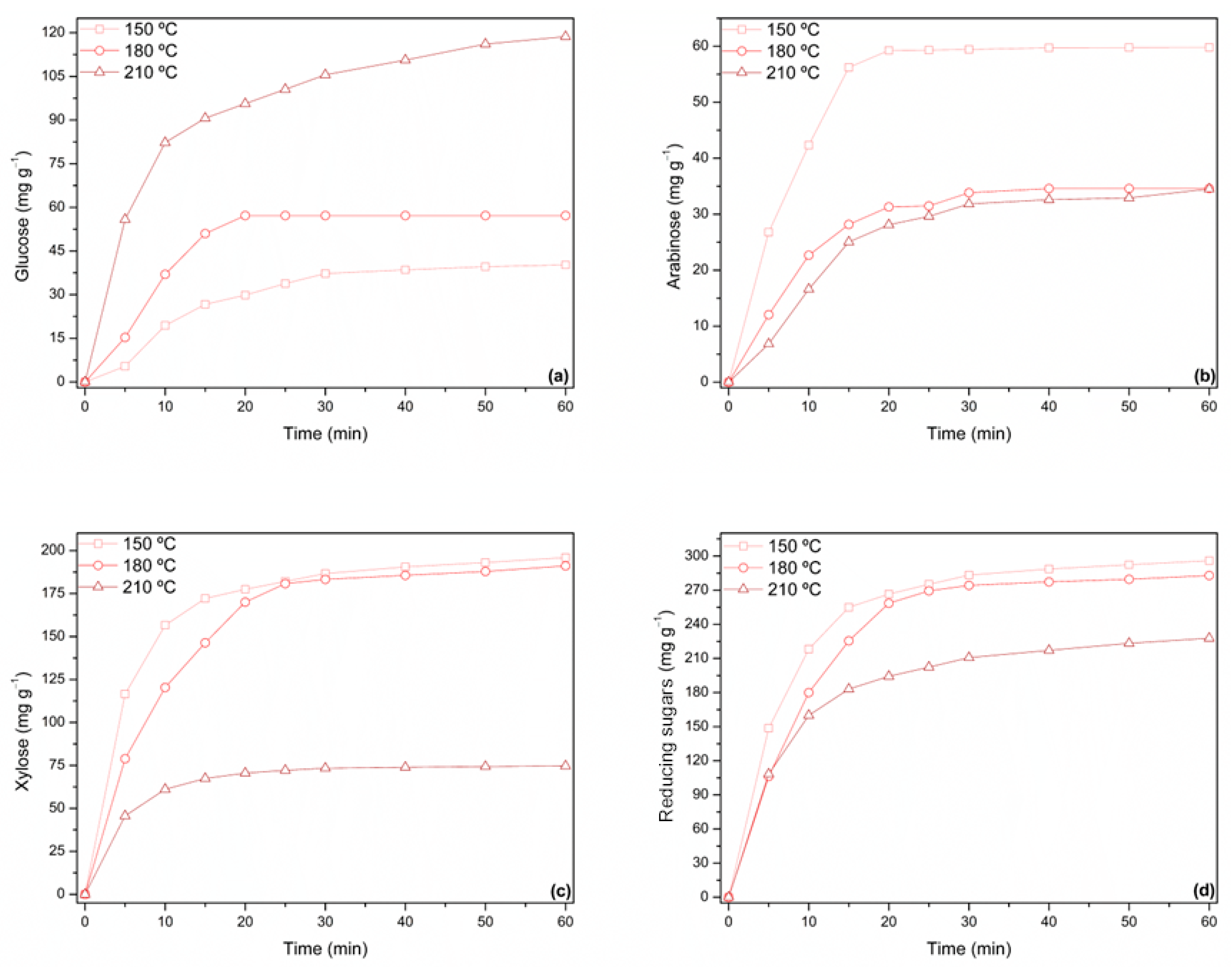
3.2.1. Sugars
3.2.2. Inhibitors and Degradation Products
3.3. Kinetic Study of Grape Pomace Hydrolysis in Subcritical Water Processing
3.4. Solid Residue Characterization After SWP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green Processing and Biotechnological Potential of Grape Pomace: Current Trends and Opportunities for Sustainable Biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Abreu, T.; Sousa, P.; Gonçalves, J.; Hontman, N.; Teixeira, J.; Câmara, J.S.; Perestrelo, R. Grape Pomace as a Renewable Natural Biosource of Value-Added Compounds with Potential Food Industrial Applications. Beverages 2024, 10, 45. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Barroso, T.L.C.T.; Sganzerla, W.G.; Costa, J.M.; Saia, F.T.; Colpini, L.M.S.; Forster-Carneiro, T. Subcritical Water Hydrolysis of Grape Pomace as a Sustainable Pretreatment for Anaerobic Digestion in a Biorefinery Concept. Fuel 2024, 363, 130899. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Sganzerla, W.G.; Barroso, T.L.C.T.; Maciel-Silva, F.W.; Colpini, L.M.S.; Bittencourt, P.R.S.; Rostagno, M.A.; Forster-Carneiro, T. Improving the Semi-Continuous Flow-through Subcritical Water Hydrolysis of Grape Pomace (Vitis vinifera L.) by PH and Temperature Control. J. Supercrit. Fluids 2023, 196, 105894. [Google Scholar] [CrossRef]
- Vedovatto, F.; Ugalde, G.; Bonatto, C.; Bazoti, S.F.; Treichel, H.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Subcritical Water Hydrolysis of Soybean Residues for Obtaining Fermentable Sugars. J. Supercrit. Fluids 2021, 167, 105043. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Qi, F.; Zhou, J.; Cen, K. Subcritical Water Hydrolysis of Rice Straw for Reducing Sugar Production with Focus on Degradation By-Products and Kinetic Analysis. Bioresour. Technol. 2015, 186, 8–14. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, E.J.; Ryu, W.B.; Hong, G.P. Characterization of Temperature-Dependent Subcritical Water Hydrolysis Pattern of Strong and Floury Rice Cultivars and Potential Utilizations of Their Hydrolysates. Food Chem. 2024, 445, 138737. [Google Scholar] [CrossRef]
- Gallon, R.; Draszewski, C.P.; Santos, J.A.A.; Wagner, R.; Brondani, M.; Zabot, G.L.; Tres, M.V.; Hoffmann, R.; Castilhos, F.; Abaide, E.R.; et al. Obtaining Oil, Fermentable Sugars, and Platform Chemicals from Butia odorata Seed Using Supercritical Fluid Extraction and Subcritical Water Hydrolysis. J. Supercrit. Fluids 2023, 203, 106062. [Google Scholar] [CrossRef]
- ASTM-E870; Standard Test Methods for Analysis of Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2006.
- Khan, T.H. Titrimetric Determination of Reducing Sugars with Copper(II) Sulphate. Analyst 1979, 104, 261. [Google Scholar] [CrossRef]
- Stallcup, O.T. Composition of Crude Fiber in Certain Roughages. J. Dairy Sci. 1958, 41, 963–968. [Google Scholar] [CrossRef]
- Soest, P.J. Van Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. AOAC Int. 1990, 73, 491–497. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Wine, R.H. Determination of Lignin and Cellulose in Acid-Detergent Fiber with Permanganate. J. AOAC Int. 1968, 51, 780–785. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric Analysis as a New Method to Determine the Lignocellulosic Composition of Biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Oliveira, M.; Teixeira, B.M.M.; Toste, R.; Borges, A.D.S. Transforming Wine By-Products into Energy: Evaluating Grape Pomace and Distillation Stillage for Biomass Pellet Production. Appl. Sci. 2024, 14, 7313. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Barroso, T.L.C.T.; Ferreira, V.C.; Forster Carneiro, T. Characterization of Benitaka Grape Pomace (Vitis vinifera L.): An Analysis of Its Properties for Future Biorefinery Applications. Waste 2025, 3, 4. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Stafussa, A.P.; Makara, C.N.; Branco, I.G.; Maciel, G.M.; Haminiuk, C.W.I. Exploratory Analysis of Bioactive Compounds and Antioxidant Potential of Grape (Vitis vinifera) Pomace. Acta Scientiarum. Technol. 2022, 44, e56934. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.d.; Lima, A.d.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical Composition and Bioactive Compounds of Grape Pomace (Vitis vinifera L.), Benitaka Variety, Grown in the Semiarid Region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.F.E.; Ghafoor, K.; Banjanin, T.; Osman, M.A.; Gassem, M.A.; Alqah, H.A.S. Chemical Composition, Bioactive Compounds, Mineral Contents, and Fatty Acid Composition of Pomace Powder of Different Grape Varieties. J. Food Process Preserv. 2020, 44, e14539. [Google Scholar] [CrossRef]
- Khandelwal, K.; Seraj, S.; Nanda, S.; Azargohar, R.; Dalai, A.K. Subcritical Water Conversion of Biomass to Biofuels, Chemicals and Materials: A Review. Environ. Chem. Lett. 2024, 22, 2191–2211. [Google Scholar] [CrossRef]
- Costa, B.S.Y.; da Cunha, H.N.; Draszewski, C.P.; Martins-Vieira, J.C.; Brondani, M.; Zabot, G.L.; Tres, M.V.; de Castilhos, F.; Abaide, E.R.; Mayer, F.D.; et al. Sequential Process of Subcritical Water Hydrolysis and Hydrothermal Liquefaction of Butia Capitata Endocarp to Obtain Fermentable Sugars, Platform Chemicals, Bio-Oil, and Biochar. Appl. Biochem. Biotechnol. 2024, 196, 4317–4336. [Google Scholar] [CrossRef]
- Branca, C.; Blasi, D.; Mango, C.; Hrablay, I. Products and Kinetics of Glucomannan Pyrolysis. Ind. Eng. Chem. Res. 2013, 52, 5030–5039. [Google Scholar] [CrossRef]
- de Azevedo, A.R.; dos Santos, M.S.N.; Wancura, J.H.C.; Oro, C.E.D.; Pfeifenberg, R.; Zabot, G.L.; Tres, M.V. Semi-Continuous Subcritical Hydrolysis of Orange Waste Biomasses for Integrated Production of Fermentable Sugars and Platform Chemicals. Chem. Eng. Process. Process Intensif. 2024, 197, 109719. [Google Scholar] [CrossRef]
- Ma, X.; Ma, Y.; Zhang, L.; Zhao, Y.; Lei, J.; Wang, Y.; Liu, M.; Lü, X.; Wang, X. Subcritical Water Pretreatment of De-Pectin Apple Pomace for Ethanol Conversion and Whole Components Utilization. Ind. Crops Prod. 2024, 216, 118720. [Google Scholar] [CrossRef]
- Yang, W.; Sen, A. One-Step Catalytic Transformation of Carbohydrates and Cellulosic Biomass to 2,5-Dimethyltetrahydrofuran for Liquid Fuels. ChemSusChem 2010, 3, 597–603. [Google Scholar] [CrossRef]
- Oikawa, A.; Otsuka, T.; Jikumaru, Y.; Yamaguchi, S.; Matsuda, F.; Nakabayashi, R.; Takashina, T.; Isuzugawa, K.; Saito, K.; Shiratake, K. Effects of Freeze-Drying of Samples on Metabolite Levels in Metabolome Analyses. J. Sep. Sci. 2011, 34, 3561–3567. [Google Scholar] [CrossRef]

| Parameter | Content | Unit |
|---|---|---|
| Moisture | 97.10 ± 3.30 | mg g−1 |
| Ash | 68.00 ± 2.70 | |
| Reducing sugar | 33.70 ± 2.40 | |
| Non-reducing sugar | 195.1 ± 10.50 | |
| Crude fiber | 773.3 ± 12.20 | |
| Acid detergent fiber | 510.3 ± 30.10 | |
| Neutral detergent fiber | 773.3 ± 21.80 | |
| Hemicellulose | 263.0 ± 16.80 | |
| Lignin | 241.2 ± 5.40 | |
| Cellulose | 269.1 ± 1.60 | |
| Elemental analysis | ||
| C | 50.25 | % |
| N | 3.33 | |
| H | 4.33 | |
| S | 0.77 | |
| O | 41.32 | |
| Compounds | Temperature (°C) | Unit | ||
|---|---|---|---|---|
| 150 | 180 | 210 | ||
| Glucose | 1.34 ± 0.08 | 1.91 ± 0.03 | 3.96 ± 0.11 | g L−1 |
| Arabinose | 1.99 ± 0.10 | 1.15 ± 0.99 | 1.15 ± 0.05 | |
| Xylose | 6.53 ± 0.14 | 6.37 ± 3.30 | 2.49 ± 0.04 | |
| Total | 9.87 ± 0.21 | 9.43 ± 7.40 | 7.59 ± 0.33 | |
| Compounds | Temperature (°C) | Unit | ||
|---|---|---|---|---|
| 150 | 180 | 210 | ||
| 5-HMF | n.d. | n.d. | 0.132 ± 0.01 | g L−1 |
| Furfural | n.d. | 0.400 ± 0.04 | 0.533 ± 0.09 | |
| Total | n.d. | 0.400 ± 0.04 | 0.665 ± 0.10 | |
| Parameter | Reducing Sugar Production (k1) | Reducing Sugar Decomposition (k1) | Unit |
|---|---|---|---|
| Ea | −34.9 | 17.2 | kJ mol−1 |
| A | 3.74 × 10−5 | 16.2 | min−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, L.E.N.; Sganzerla, W.G.; Matheus, L.R.; Ferreira, V.C.; Rostagno, M.A.; Forster-Carneiro, T. Subcritical Water Processing of Grape Pomace (Vitis vinifera L.): Kinetic Evaluation of Sugar Production and By-Product Formation. Biomass 2025, 5, 34. https://doi.org/10.3390/biomass5020034
Castro LEN, Sganzerla WG, Matheus LR, Ferreira VC, Rostagno MA, Forster-Carneiro T. Subcritical Water Processing of Grape Pomace (Vitis vinifera L.): Kinetic Evaluation of Sugar Production and By-Product Formation. Biomass. 2025; 5(2):34. https://doi.org/10.3390/biomass5020034
Chicago/Turabian StyleCastro, Luiz Eduardo Nochi, William Gustavo Sganzerla, Larissa Resende Matheus, Vanessa Cosme Ferreira, Mauricio Ariel Rostagno, and Tania Forster-Carneiro. 2025. "Subcritical Water Processing of Grape Pomace (Vitis vinifera L.): Kinetic Evaluation of Sugar Production and By-Product Formation" Biomass 5, no. 2: 34. https://doi.org/10.3390/biomass5020034
APA StyleCastro, L. E. N., Sganzerla, W. G., Matheus, L. R., Ferreira, V. C., Rostagno, M. A., & Forster-Carneiro, T. (2025). Subcritical Water Processing of Grape Pomace (Vitis vinifera L.): Kinetic Evaluation of Sugar Production and By-Product Formation. Biomass, 5(2), 34. https://doi.org/10.3390/biomass5020034









