Waste Nutshell Particulate Biocomposites with Geopolymer Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Biocomposite Characterization
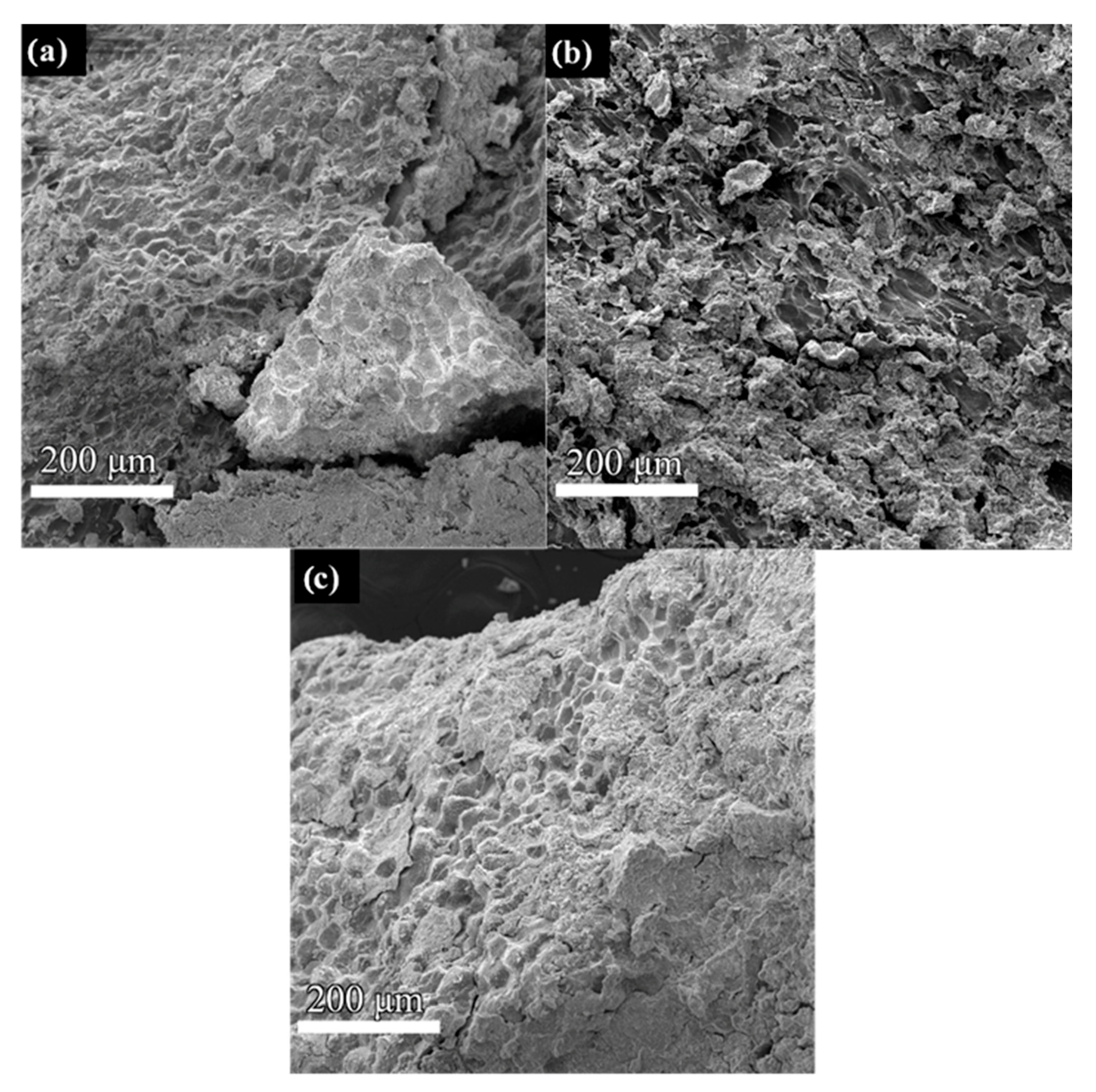
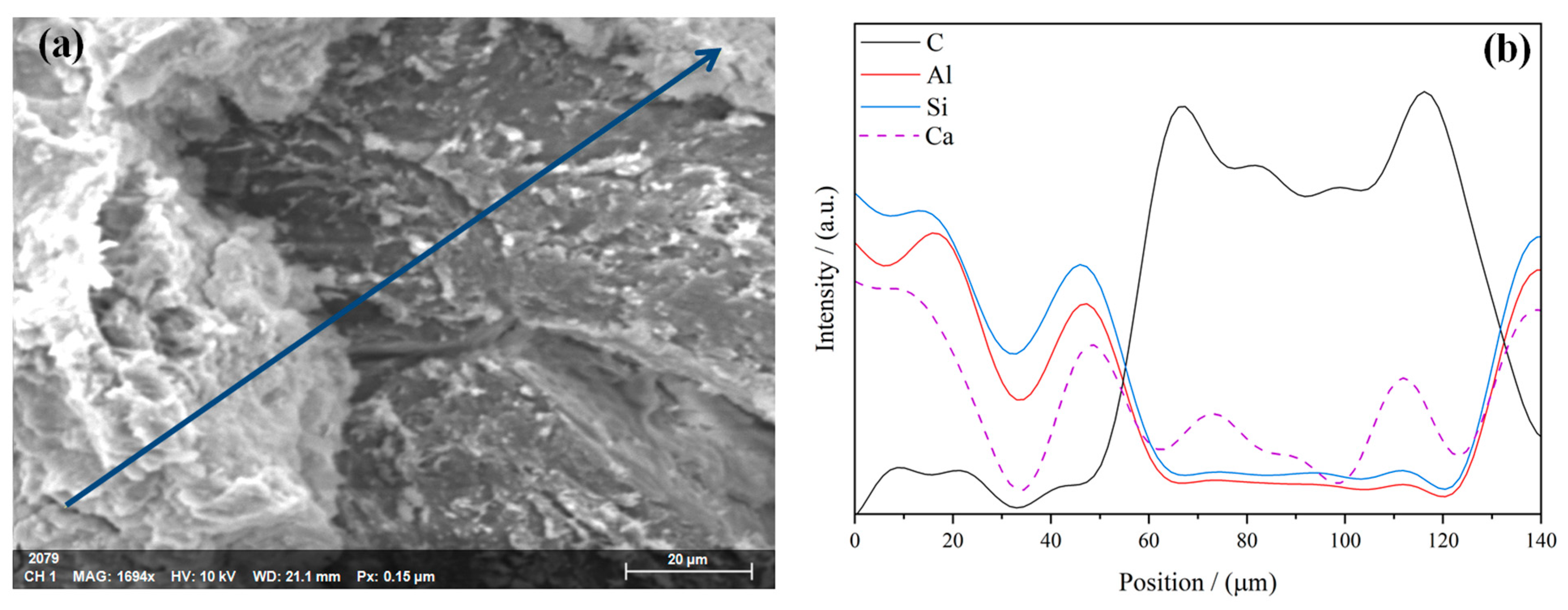
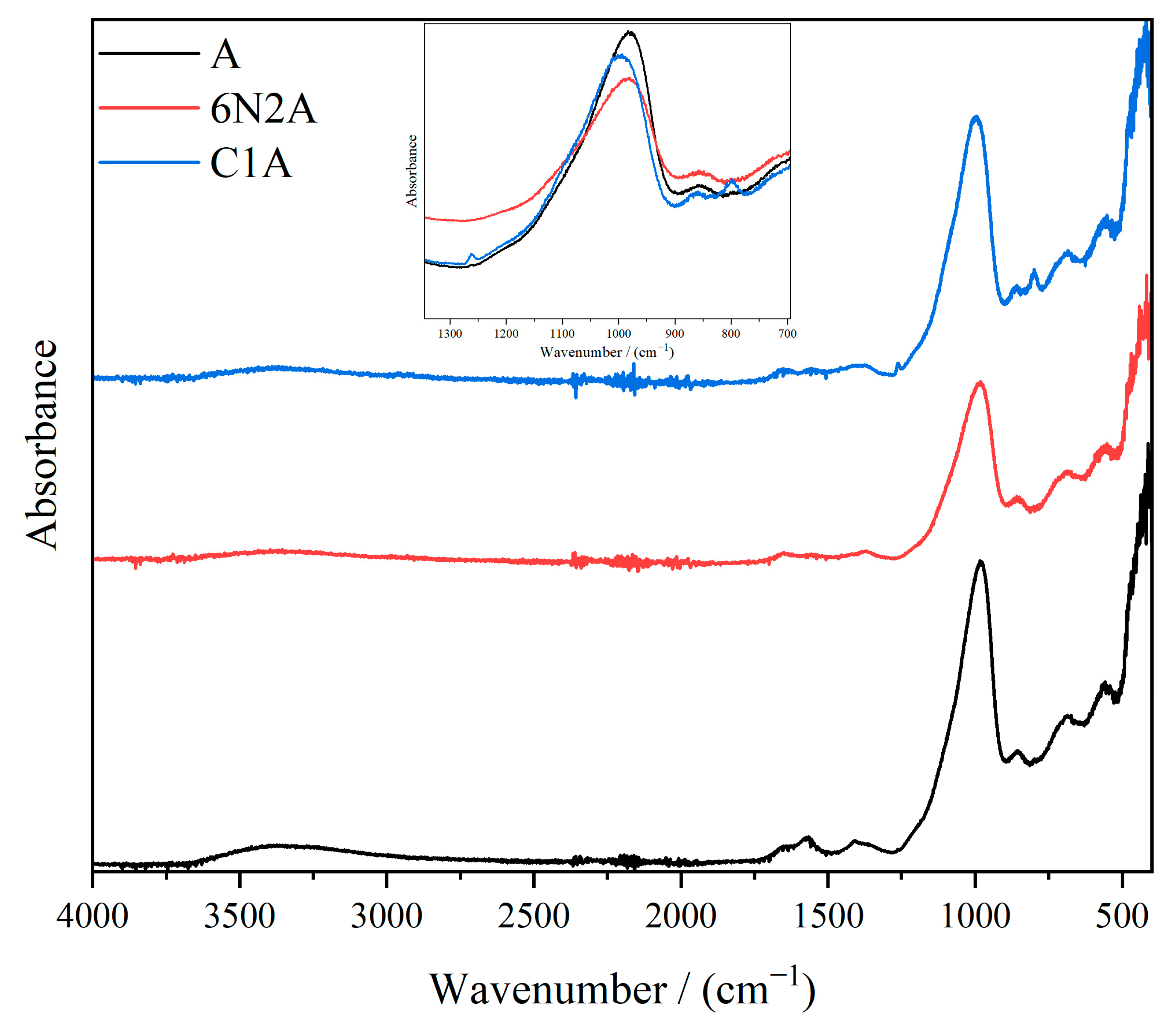
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, K.; Zhang, J.; Zeng, Y. Knowledge domain and emerging trends of agricultural waste management in the field of social science: A scientometric review. Sci. Total Environ. 2019, 670, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.; Korjakins, A.; Bajare, D.; Zimele, Z.; Sahmenko, G. Bio-based construction panels for low carbon development. Energy Procedia 2018, 147, 220–226. [Google Scholar] [CrossRef]
- Demirbas, A. Calculation of higher heating values of biomass fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- McNeill, D.C.; Pal, A.K.; Nath, D.; Rodriguez-Uribe, A.; Mohatny, A.K.; Pilla, S.; Gregori, S.; Dick, P.; Misra, M. Upcycling of ligno-cellulosic nutshells waste biomass in biodegradable plastic-based biocomposites uses—A comprehensive review. Compos. Part C Open Access 2024, 14, 100478. [Google Scholar] [CrossRef]
- Cruz-Lopez, L.P.; Martins, J.; Esteves, B.; Teixeira De Lemos, L. New products from hazelnut shell. In Proceedings of the ECOWOOD 2012, 5th International Conference on Environmentally-Compatible Forest Products, Porto, Portugal, 5–7 September 2012. [Google Scholar]
- Demirbas, A. Fuel characteristics of olive husk and walnut, hazelnut, sunflower, and almond shells. Energy Sources 2002, 24, 215–221. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Messaoudi, Y.; Smichi, N.; Bouachir, F.; Gargouri, M. Fractionation and biotransformation of lignocelluloses-based wastes for bioethanol, xylose and vanillin production. Waste Biomass Valoriz. 2017, 10, 357–367. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A. Short natural-fibre reinforced polyethylene and natural rubber composites: Effect of silane coupling agents and fibres loading. Compos. Sci. Technol. 2007, 67, 1627–1639. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Podgorbunskikh, E.M.; Ryabchikova, E.I.; Lomovsky, O.I. The role of mechanical action in the process of the thermomechanical isolation of lignin. Cellulose 2018, 25, 1–5. [Google Scholar] [CrossRef]
- Peŕez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Faster methane production after sequential extrusion and enzymatic hydrolysis of vine trimming shoots. Environ. Chem. Lett. 2017, 16, 295–299. [Google Scholar] [CrossRef]
- Contreras-Hernández, M.G.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, J.G.; Rocha-Guzmán, N.E.; Lara-Ceniceros, T.E.; Contreras-Esquivel, J.C. Effect of ultrasound pre-treatment on the physicochemical composition of Agave durangensis leaves and potential enzyme production. Bioresour. Technol. 2018, 249, 439–446. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Sun, R.C.; Geng, Z.C.; Fowler, P.; Baird, M.S. Characteristics of degraded hemicellulosic polymers obtained from steam exploded wheat straw. Carbohydr. Polym. 2005, 60, 15–26. [Google Scholar] [CrossRef]
- Li, X.; Tabil, G.L.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Jędrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and chemical pretreatment of lignocellulosic biomass. In Second and Third Generation of Feedstocks, 1st ed.; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 143–196. [Google Scholar]
- Ali, A.; Khubab, S.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2016, 47, 2153–2183. [Google Scholar] [CrossRef]
- Symington, M.C.; Banks, W.M.; West, O.D.; Pethrick, R.A. Tensile testing of cellulose based natural fibers for structural composite applications. J. Compos. Mater. 2009, 43, 1083–1108. [Google Scholar] [CrossRef]
- Verma, D.; Goh, K.L. Effect of Mercerization/Alkali Surface Treatment of Natural Fibres and Their Utilization in Polymer Composites: Mechanical and Morphological Studies. J. Compos. Sci. 2021, 5, 175. [Google Scholar] [CrossRef]
- Hasan, A.; Rabbi, M.S.; Billah, M.M. Making the lignocellulosic fibers chemically compatible for composite: A comprehensive review. Clean. Mater. 2022, 4, 100078. [Google Scholar] [CrossRef]
- Woźniak, A.; Kuligowski, K.; Świerczek, L.; Ceinan, A. Review of Lignocellulosic Biomass Pretreatment Using Physical, Thermal and Chemical Methods for Higher Yields in Bioethanol Production. Sustainability 2025, 17, 287. [Google Scholar] [CrossRef]
- Anggono, J.; Sugondo, S.; Henrico, S.; Purwaningsih, H. Effect of Alkali Treatment Using Calcium Hydroxide and the Fiber Length on the Strength Of Sugarcane Bagasse Fibers-Polypropylene Composites. Appl. Mech. Mater. 2015, 815, 106–110. [Google Scholar] [CrossRef]
- Abson, N.I.; Allen, G.C.; Ball, R.J.; El-Turki, A. Linseed Fibre Lime Composites—Effect of Fibre Pretreatment. In Proceedings of the 11th International Conference on Non-Conventional Materials and Technologies (NOCMAT 2009), Bath, UK, 6–9 September 2009. [Google Scholar]
- Pawłowska, A.; Stepczyńska, M.; Walczak, M. Flax fibres modified with a natural plant agent used as a reinforcement for the polylactide-based biocomposites. Ind. Crops. Prod. 2022, 184, 115061. [Google Scholar] [CrossRef]
- Stepczyńska, M.; Rytlewski, P.; Moraczewski, K.; Pawłowska, A.; Karasiewicz, T. Novel Biocomposite of Starch and Flax Fiber Modified with Tannic Acid with Biocidal Properties. Polymers 2024, 16, 1108. [Google Scholar] [CrossRef] [PubMed]
- Dyer, T. Interaction of phenolic brownfield contaminants with hydrating Portland cement. Mag. Concr. Res. 2013, 65, 987–1002. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Bai, T.; Wang, H.; Du, F.; Zhang, Y.; Cai, L.; Jiang, C.; Wang, W. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers Structure, Processing, Properties and Industrial Applications, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix design and mechanical properties of geopolymer and alkali activated concrete: Review of the state-of-the-art and the development of a new unified approach. Constr. Build. Mater. 2020, 256, 119380. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Z.; Liu, X. Comprehensive Understanding of Aluminosilicate Phosphate Geopolymers: A Critical Review. Materials 2022, 15, 5961. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Yi, Z.; Du, F.; Zhang, Y. The properties and latest application of geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 472, 012029. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Farhan, K.Z.; Johari, M.A.M.; Demirboğa, R. Impact of fiber reinforcements on properties of geopolymer composites: A review. J. Build. Eng. 2021, 44, 106628. [Google Scholar] [CrossRef]
- Silva, F.J.; Thaumaturgo, C. Fibre reinforcement and fracture response in geopolymeric mortars. Fatigue Fract. Eng. Mater. Struct. 2003, 26, 167–172. [Google Scholar] [CrossRef]
- Silva, G.; Kim, S.; Bertolotti, B.; Nakamatsu, J. Optimization of a reinforced geopolymer composite using natural fibers and construction wastes. Contr. Build. Mater. 2020, 258, 119697. [Google Scholar] [CrossRef]
- Korniejenko, K.; Łach, M.; Dogan-Saglamtimur, N.; Furtos, G.; Mikuła, J. The overview of mechanical properties of short natural fiber reinforced geopolymer composites. Environ. Res. Technol. 2020, 3, 28–39. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium bio-composites in industrial design and architecture: Comparative review and experimental analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Roper, D.S.; Kutyla, G.P.; Kriven, W.M. Properties of Cork Particle Reinforced Sodium Geopolymer Composites. In Proceedings of the 40th International Conference on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 24–29 January 2016. [Google Scholar]
- Brleković, F.; Mužina, K.; Kurajica, S. The Influence of Alkaline Pretreatment of Waste Nutshell for Use in Particulate Biocomposites. J. Compos. Sci. 2024, 8, 26. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.A.; Rafiq, S.; Mohammed, A.S.; Mosavi, A.; Sor, N.M.; Qaidi, S.M.A. Compressive Strength of Sustainable Geopolymer Concrete Composites: A State-of-the-Art Review. Sustainability 2021, 13, 13502. [Google Scholar] [CrossRef]
- Welch, B.L. The generalization of ‘student’s’ problem when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef]
- Ahad, N.A.; Yahaya, S.S.S. Sensitivity Analysis of Welch’s t-Test. In Proceedings of the 21st National Symposium on Mathematical Sciences (SKSM), Penang, Malaysia, 6–8 November 2013. [Google Scholar]
- Datatab.net. Available online: https://datatab.net/tutorial/t-distribution (accessed on 10 November 2024).
- Davidovits, J. Geopolymer Chemistry and Application, 3rd ed.; Institut Geopolymere: Saint-Quentin, France, 2011. [Google Scholar]
- Lin, H.; Liu, H.; Li, Y.; Kong, X. Properties and reaction mechanism of phosphoric acid activated metakaolin geopolymer at varied curing temperatures. Cem. Concr. Res. 2021, 144, 106425. [Google Scholar] [CrossRef]
- Alsina, O.L.S.; de Carvalho, L.H.; Ramos Filho, F.G.; d’Almeida, J.R.M. Immersion Temperature Effects on the Water Absorption Behavior of Hybrid Lignocellulosic Fiber Reinforced-Polyester Matrix Composites. Polym. Plast. Technol. Eng. 2007, 46, 515–520. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Pang, E. Strength and Microstructure of Geopolymer Based on Fly Ash and Metakaolin. Materials 2022, 15, 3732. [Google Scholar] [CrossRef]
- Yuan, J.; Li, L.; He, P.; Chen, Z.; Lao, C.; Jia, D.; Zhou, Y. Effects of kinds of alkali-activated ions on geopolymerization process of geopolymer cement pastes. Constr. Build. Mater. 2021, 293, 123536. [Google Scholar] [CrossRef]
- Boca Santa, R.A.A.; Gracher Riella, H.; Cabral Kuhnen, N. Comparative study between geopolymers enabled with sodium and potassium hydroxides. In Proceedings of the 6th International Conference on Mechanics and Materials in Design, Delgada, Portugal, 26–30 July 2015. [Google Scholar]
- Sore, S.O.; Messan, A.; Prud’Homme, E.; Escadeillas, G.; Tsobnang, F. Comparative Study on Geopolymer Binders Based on Two Alkaline Solutions (NaOH and KOH). J. Miner. Mater. Charact. Eng. 2020, 8, 407–420. [Google Scholar] [CrossRef]
- Chen, Y.; de Lima, L.M.; Li, Z.; Ma, B.; Lotenbach, B.; Yin, S.; Yu, Q.; Ye, G. Synthesis, solubility and thermodynamic properties of N-A-S-H gels with various target Si/Al ratios. Cem. Concr. Res. 2024, 180, 107484. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Blanco-Varela, M.T.; Palomo, A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol-Gel Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Rao, F.; Tian, X.; Lin, S. Synergistic gel formation in geopolymers of superior mechanical strength synthesized with volcanic ash and slag. Environ. Sci. Pollut. Res. 2024, 30, 26244–26255. [Google Scholar] [CrossRef] [PubMed]
- Slaný, M.; Kuzielová, E.; Žemlička, M.; Matejdes, M.; Struhárová, A.; Palou, M.T. Metabentonite and metakaolin-based geopolymers/zeolites: Relation between kind of clay, calcination temperature and concentration of alkaline activator. J. Therm. Anal. Calorim. 2023, 148, 10531–10547. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W. Identification of silicooxygen rings in SiO2 based on IR spectra. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2000, 56, 1819. [Google Scholar] [CrossRef]
- Giacon, V.M.; Rebelo, V.S.M.; dos Santos, G.M.; Sanches, E.A.; Fiorelli, J.; dos Santos Costella, A.M.; de Melo, G.M.M.; Brito, L.M.A.F. Influence of Mercerization on the Physical and Mechanical Properties of Polymeric Composites Reinforced with Amazonian Fiber. Fiber. Polym. 2021, 22, 1950–1956. [Google Scholar] [CrossRef]
- Kochova, K.; Gauvin, F.; Schollbach, K.; Brouwers, H.J.H. Using alternative waste coir fibres as a reinforcement in cement-fibre composites. Constr. Build. Mater. 2020, 231, 117121. [Google Scholar] [CrossRef]
- Fragmat.eu. Available online: https://en.fragmat.eu/thermal-insulation/composite-panels-for-general-use/drvolit-d-2 (accessed on 15 May 2025).


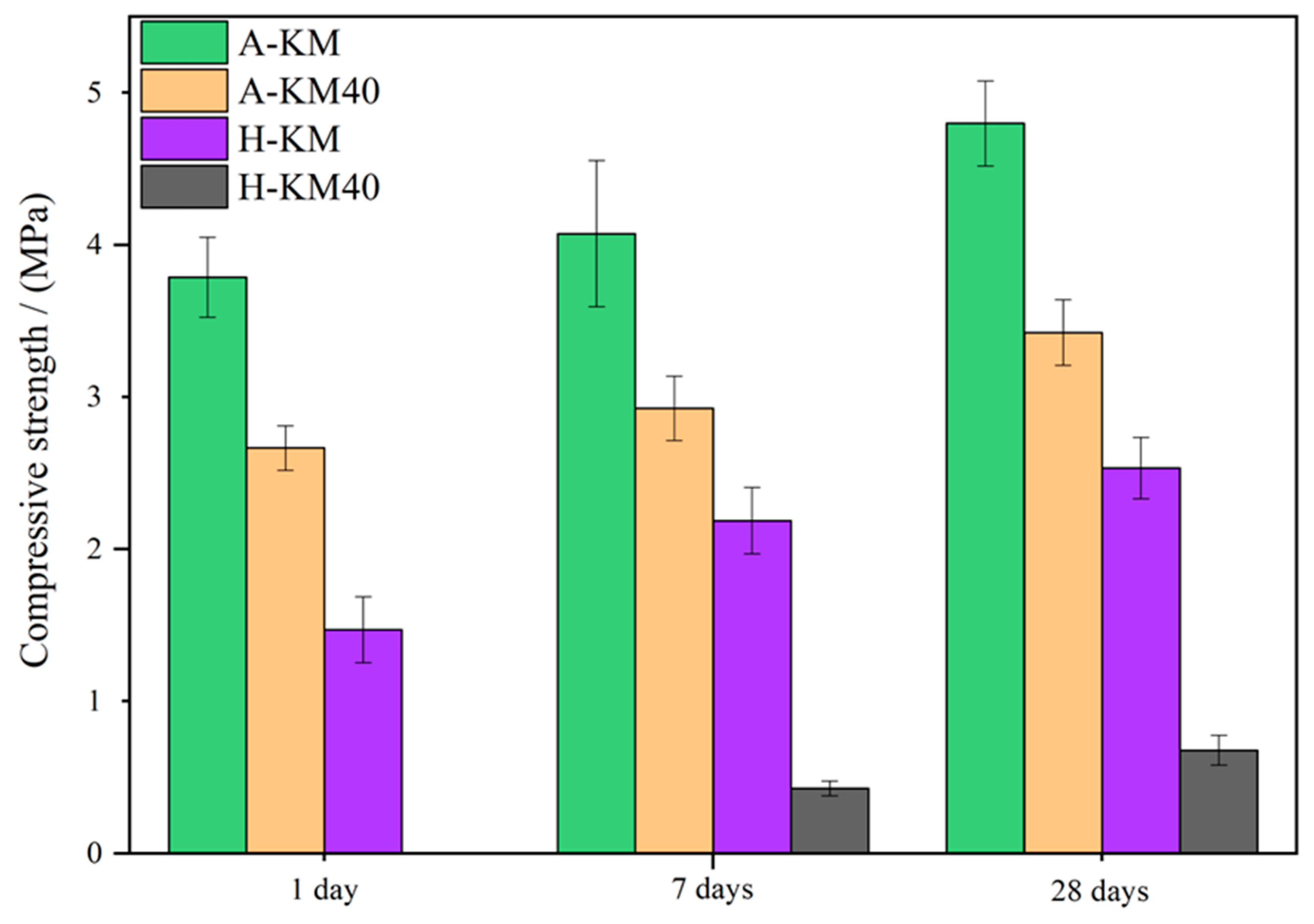
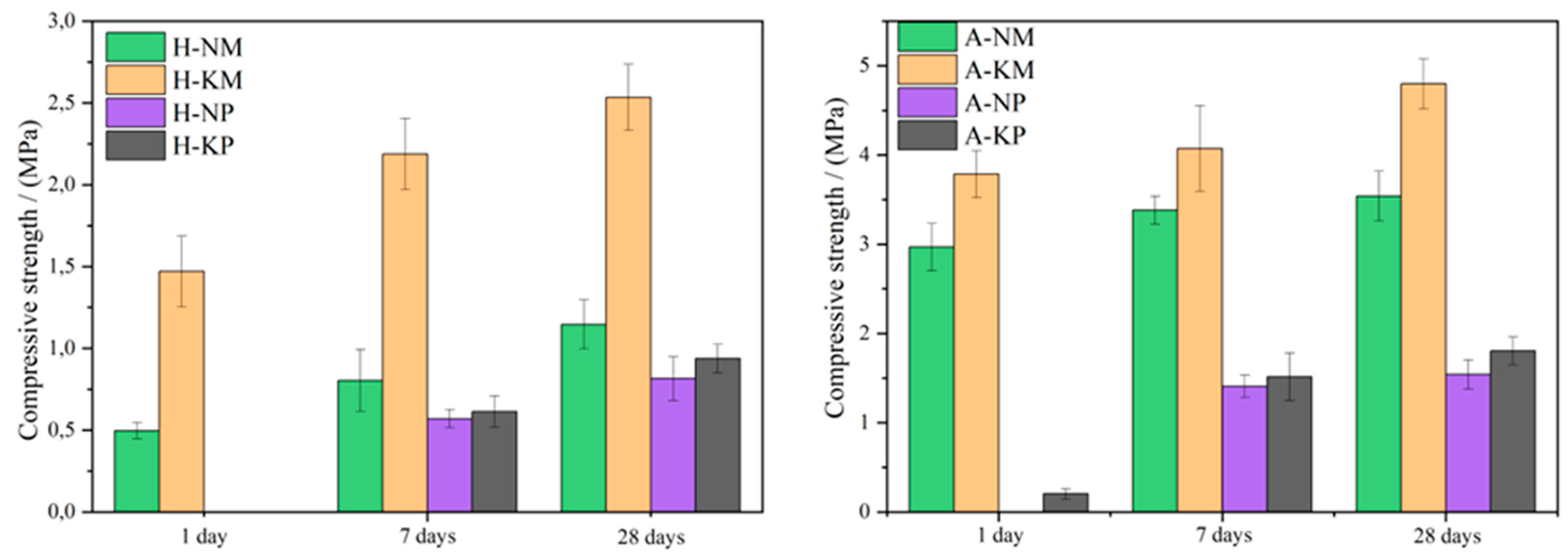

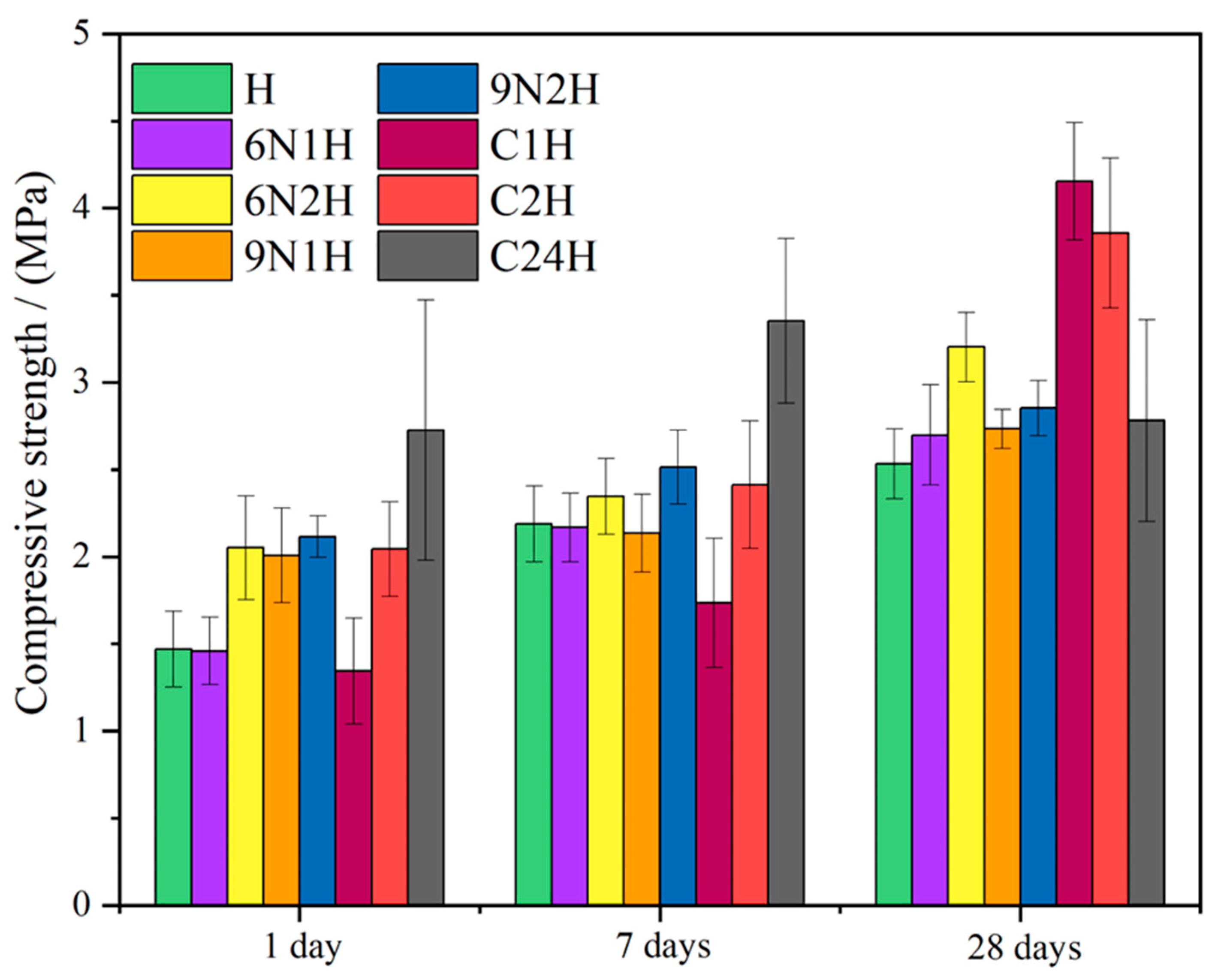
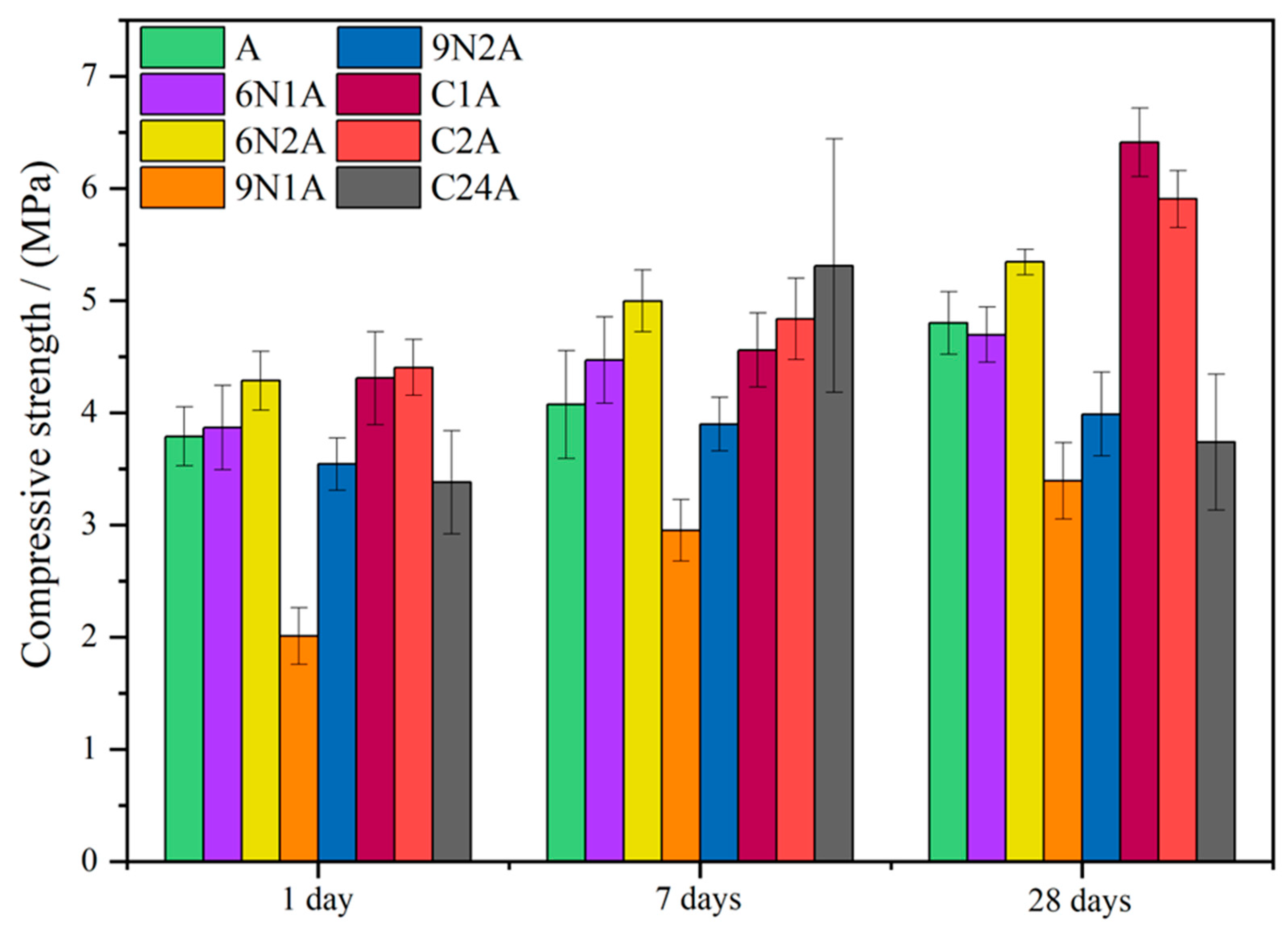
| Abbreviation | Description | Details |
|---|---|---|
| Waste nutshell | ||
| C | Concentration of sodium hydroxide used for pretreatment | 6 or 9 for 6 and 9 wt.% solutions |
| P | Pretreatment method | N for mercerization, C for pretreatment with milk of lime |
| H | Duration of pretreatment | 1 and 2 for 1 and 2.5 h at 80 °C, 24 for 24 h at room temperature |
| N | Type of nutshell in the biocomposite | H for hazelnut shell, A for almond shell, untreated designated as H or A |
| Geopolymers and curing | ||
| S | Type of activation solution used | N for sodium activation solution, K for potassium activation solution |
| P | Solid precursor used | P for fly ash, M for metakaolin |
| T | Curing temperature | No designation for room temperature, 40 for curing at 40 °C |
| df | tcrit | Null Hypothesis Rejected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 |
| A-1.5KM × A-2KM | 3.08 | 4.26 | 4.56 | 3.15 | 2.72 | 2.66 | 6.60 | 3.97 | 10.38 | + | + | + |
| H-1.5KM × H-2KM | 3.72 | 3.61 | 5.29 | 2.89 | 2.93 | 2.53 | 2.53 | 5.86 | 6.66 | − | + | + |
| df | tcrit | Null Hypothesis Rejected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 |
| A-KM × A-KM40 | 3.14 | 4.12 | 4.96 | 3.13 | 2.75 | 2.58 | 6.45 | 4.37 | 7.35 | + | + | + |
| H-KM × H-KM40 | / | 3.29 | 4.32 | / | 3.26 | 2.71 | / | 15.76 | 16.52 | / | + | + |
| df | tcrit | Null Hypothesis Rejected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 |
| H-NM × H-NP | / | 3.67 | 5.92 | / | 2.91 | 2.46 | / | 2.33 | 3.29 | / | − | + |
| H-KM × H-KP | / | 4.29 | 4.06 | / | 2.72 | 2.76 | / | 12.89 | 14.50 | / | + | + |
| A-NM × A-NP | / | 5.77 | 4.87 | / | 2.47 | 2.59 | / | 19.53 | 12.33 | / | + | + |
| A-KM × A-KP | 2.18 | 4.68 | 4.72 | 4.10 | 2.64 | 2.63 | 23.04 | 9.31 | 18.71 | + | + | + |
| df | tcrit | Null Hypothesis Rejected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 |
| H-NM × H-KM | 2.22 | 5.88 | 5.53 | 4.05 | 2.46 | 2.51 | 7.55 | 9.58 | 11.01 | + | + | + |
| 6N1H-NM × 6N1H-KM | 5.96 | 5.98 | 3.95 | 2.44 | 2.45 | 2.77 | 2.84 | 1.42 | 2.85 | + | − | + |
| 6N2H-NM × 6N2H-KM | 3.48 | 4.50 | 4.20 | 2.98 | 2.67 | 2.74 | 3.31 | 0.97 | 3.23 | + | − | + |
| df | tcrit | Null Hypothesis Rejected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 | 1 | 7 | 28 |
| A-NM × A-KM | 3.99 | 3.63 | 6 | 2.78 | 2.936 | 2.45 | 3.82 | 2.74 | 6.39 | + | − | + |
| 6N1A-NM × 6N1A-KM | 4.63 | 4.51 | 4.74 | 2.65 | 2.67 | 2.62 | 1.28 | 2.69 | 3.24 | − | + | + |
| 6N2A-NM × 6N2A-KM | 5.97 | 3.60 | 4.83 | 2.45 | 2.93 | 2.61 | 1.81 | 4.4 | 9.00 | − | + | + |
| Sample | Hx6N2H | Hx9N2H | HxC1H | 6N2Hx9N2H | C1HxC2H | 6N2HxC1H |
|---|---|---|---|---|---|---|
| df | 4.48 | 5.67 | 4.92 | 4.79 | 5.66 | 4.87 |
| tcrit | 2.67 | 2.48 | 2.59 | 2.61 | 2.49 | 2.60 |
| 4.36 | 2.49 | 8.26 | 2.62 | 1.08 | 4.66 | |
| Null hypothesis rejected | + | + | + | + | − | + |
| Sample | Ax6N2A | Ax9N2A | AxC1A | 6N2Ax9N2A | C1AxC2A | 6N2AxC1A |
|---|---|---|---|---|---|---|
| df | 4.15 | 5.54 | 5.95 | 3.68 | 5.81 | 3.99 |
| tcrit | 2.74 | 2.50 | 2.45 | 2.90 | 2.47 | 2.78 |
| 3.54 | 3.47 | 7.80 | 6.83 | 2.55 | 6.44 | |
| Null hypothesis rejected | + | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brleković, F.; Mužina, K.; Haramina, T.; Kurajica, S. Waste Nutshell Particulate Biocomposites with Geopolymer Matrix. Biomass 2025, 5, 31. https://doi.org/10.3390/biomass5020031
Brleković F, Mužina K, Haramina T, Kurajica S. Waste Nutshell Particulate Biocomposites with Geopolymer Matrix. Biomass. 2025; 5(2):31. https://doi.org/10.3390/biomass5020031
Chicago/Turabian StyleBrleković, Filip, Katarina Mužina, Tatjana Haramina, and Stanislav Kurajica. 2025. "Waste Nutshell Particulate Biocomposites with Geopolymer Matrix" Biomass 5, no. 2: 31. https://doi.org/10.3390/biomass5020031
APA StyleBrleković, F., Mužina, K., Haramina, T., & Kurajica, S. (2025). Waste Nutshell Particulate Biocomposites with Geopolymer Matrix. Biomass, 5(2), 31. https://doi.org/10.3390/biomass5020031






