Advancements in Sustainable Biochar Production from Waste: Pathways for Renewable Energy Generation and Environmental Remediation
Abstract
1. Introduction
2. Feedstock for Biochar Production
3. Biochar Production Methods
3.1. Pyrolysis
3.2. Torrefaction
3.3. Hydrothermal Carbonization
3.4. Gasification
4. Biochar Characteristics
5. Techniques for the Modification of Biochar
5.1. Physical Activation
5.2. Chemical Modification
5.3. Biological Modification
5.4. Modification Through Doping or Co-Doping
6. Applications of Biochar
6.1. Water and Wastewater Treatment
6.1.1. Removal of Nutrients
6.1.2. Heavy Metal Removal
6.1.3. Organic Compounds
6.2. Soil Amendment
6.2.1. Soil Properties
6.2.2. Dynamics of Microbial Communities
6.2.3. Fertility of the Soil and the Growth of Plants
6.2.4. Soil Decontamination
Adsorption of Heavy Metals
Removal of Organic Pollutants
6.3. Renewable Energy Production
6.3.1. Utilization of Biomaterials in Microbial Fuel Cells (MFCs) to Produce Bioelectricity
Creation of an Anodic Electrode
Creation of the Cathodic Electrode
6.3.2. Incorporating Biochar into the Production of Biodiesel
6.3.3. Generation of Biohydrogen
The Use of Biochar as a Catalyst in the Water-Splitting Process to Generate Hydrogen
The Use of Biochar as a Catalyst in the Process of Methane Steam Reforming for Hydrogen Production
The Incorporation of Biochar in Anaerobic Digestion for the Production of Hydrogen
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.M.-C.; Bodirsky, B.L.; Krueger, T.; Mishra, A.; Popp, A. The world’s growing municipal solid waste: Trends and impacts. Environ. Res. Lett. 2020, 15, 074021. [Google Scholar] [CrossRef]
- Ustohalova, V. Management and Export of Wastes: Human Health Implications. In Encyclopedia of Environmental Health; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 603–611. [Google Scholar] [CrossRef]
- Spokas, K.; Koskinen, W.; Baker, J.; Reicosky, D. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.; Nasrullah, M. A comprehensive assessment of the method for producing biochar, its characterization, stability, and potential applications in regenerative economic sustainability—A review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Olugbenga, O.S.; Adeleye, P.G.; Oladipupo, S.B.; Adeleye, A.T.; John, K.I. Biomass-derived biochar in wastewater treatment- a circular economy approach. Waste Manag. Bull. 2023, 1, 1–14. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Seo, J.Y.; Tokmurzin, D.; Lee, D.; Lee, S.H.; Seo, M.W.; Park, Y.-K. Production of biochar from crop residues and its application for biofuel production processes—An overview. Bioresour. Technol. 2022, 361, 127740. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Palai, A.K.; Kumar, A.; Bhatia, R.K.; Patel, A.K.; Thakur, V.K.; Yang, Y.-H. Trends in renewable energy production employing biomass-based biochar. Bioresour. Technol. 2021, 340, 125644. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, L.; Rui, X.; Hu, J.; Zhu, N. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Kaushal, P.; Vijay, V.K. Process optimization of rice straw-derived activated biochar and biosorption of heavy metals from drinking water in rural areas. Appl. Surf. Sci. Adv. 2023, 18, 100481. [Google Scholar] [CrossRef]
- Zuo, W.; Wang, S.; Zhou, Y.; Ma, S.; Yin, W.; Shan, Y.; Wang, X. Conditional remediation performance of wheat straw biochar on three typical Cd-contaminated soils. Sci. Total. Environ. 2023, 863, 160998. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Meng, F.; Zhang, M.; Liu, Q. Effects of different low temperature pretreatments on properties of corn stover biochar for precursors of sulfonated solid acid catalysts. Bioresour. Technol. 2022, 357, 127342. [Google Scholar] [CrossRef] [PubMed]
- Azman, N.A.; Fuzi, S.F.Z.M.; Manas, N.H.A.; Azelee, N.I.W.; Masngut, N. Improved heterocyclic aromatic hydrocarbon compound adsorption using functionalised rice husk biochar. Bioresour. Technol. Rep. 2023, 25, 101704. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Tan, L.; Li, Q.; Zhang, C.; Wei, X.; Wang, Q.; Zheng, X.; Xu, Y. Coconut shell and its biochar as fertilizer amendment applied with organic fertilizer: Efficacy and course of actions on eliminating antibiotic resistance genes in agricultural soil. J. Hazard. Mater. 2022, 437, 129322. [Google Scholar] [CrossRef]
- Ponnuchamy, M.; Kapoor, A.; Jacob, M.M.; Awasthi, A.; Mukhopadhyay, M.; Nandagobu, S.; Raghav, A.; Arvind, D.; Chakraborty, P.; Prabhakar, S. Adsorptive removal of endocrine disruptor bisphenol A from aqueous environment using sugarcane bagasse derived biochar. J. Taiwan Inst. Chem. Eng. 2023, 166, 105216. [Google Scholar] [CrossRef]
- Adesemuyi, M.F.; Adebayo, M.A.; Akinola, A.O.; Olasehinde, E.F.; Adewole, K.A.; Lajide, L. Preparation and characterisation of biochars from elephant grass and their utilisation for aqueous nitrate removal: Effect of pyrolysis temperature. J. Environ. Chem. Eng. 2020, 8, 104507. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Nabil, G.M.; El-Mallah, N.M.; Bassiouny, H.I.; Kumar, S.; Abdel-Fattah, T.M. Kinetics, isotherm, and thermodynamic studies of the adsorption of reactive red 195 A dye from water by modified Switchgrass Biochar adsorbent. J. Ind. Eng. Chem. 2016, 37, 156–167. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Newton, R.A.; Mamirova, A. Miscanthus biochar value chain—A review. J. Environ. Manag. 2021, 290, 112611. [Google Scholar] [CrossRef]
- Crespo-Barreiro, A.; Gómez, N.; González-Arias, J.; Ortiz-Liébana, N.; González-Andrés, F.; Cara-Jiménez, J. Scaling-Up of the Production of Biochar from Olive Tree Pruning for Agricultural Use: Evaluation of Biochar Characteristics and Phytotoxicity. Agriculture 2023, 13, 1064. [Google Scholar] [CrossRef]
- Wahi, R.; Zuhaidi, N.; Yahaya, S.; Lam, S.; Imran-Shaukat, M.; Aziz, S.; Ngaini, Z.; Pauzan, A.M. Engineered microwave biochar from sago bark waste for heavy metals adsorption. Mater. Today Proc. 2022, 57, 1403–1414. [Google Scholar] [CrossRef]
- Hu, W.; Wang, J.; Hu, J.; Schuler, J.; Grushecky, S.; Nan, N.; Smith, W.; Jiang, C. Thermodegradation of naturally decomposed forest logging residues: Characteristics, kinetics, and thermodynamics. Bioresour. Technol. 2023, 376, 128821. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Y.; Ou, Y.; Zhong, Q.; Tang, P.; Zhang, Y.; Yi, L.; Li, Q.; Huang, Z.; Jiang, T. Synergistic recycling of biochar from sawdust pyrolysis and waste coke breeze to produce metallurgical quality biocoke with syngas as a by-product. Fuel 2023, 354, 129365. [Google Scholar] [CrossRef]
- Ba, Y.; Cen, K.; Wang, L.; Jia, D.; Kan, T.; Chen, D. Study on synergistic effect of co-pyrolysis of wood chips and its gasification tar on the biochar and subsequent activated carbon upgrading using response surface method. Ind. Crop. Prod. 2023, 205, 117558. [Google Scholar] [CrossRef]
- Struhs, E.; Mirkouei, A.; You, Y.; Mohajeri, A. Techno-economic and environmental assessments for nutrient-rich biochar production from cattle manure: A case study in Idaho, USA. Appl. Energy 2020, 279, 115782. [Google Scholar] [CrossRef]
- Gómez, E.M.P.; Domínguez, R.E.; López, D.A.; Téllez, J.F.; Marino, M.D.; Almada, N.; Gange, J.M.; Moyano, E.L. Chicken litter: A waste or a source of chemicals? Fast pyrolysis and hydrothermal conversion as alternatives in the valorisation of poultry waste. J. Anal. Appl. Pyrolysis 2023, 169, 105796. [Google Scholar] [CrossRef]
- Azeem, M.; Jeyasundar, P.G.S.A.; Ali, A.; Riaz, L.; Khan, K.S.; Hussain, Q.; Kareem, H.A.; Abbas, F.; Latif, A.; Majrashi, A.; et al. Cow bone-derived biochar enhances microbial biomass and alters bacterial community composition and diversity in a smelter contaminated soil. Environ. Res. 2023, 216, 114278. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Ghojavand, S.; Farjadfard, S.; Ramavandi, B. Cadmium elimination from wastewater using potato peel biochar modified by ZIF-8 and magnetic nanoparticle. Colloid Interface Sci. Commun. 2023, 55, 100723. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, D.; Xia, D.; Li, Q. Orange peels biochar doping with Fe-Cu bimetal for PMS activation on the degradation of bisphenol A: A synergy of SO4−, OH, 1O2 and electron transfer. Chem. Eng. J. 2023, 471, 144832. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, K.; Shen, S.; Hu, H.; Chang, M.; Chen, D.; Wu, Y.; Yuan, H.; Wang, Y. Engineering banana-peel-derived biochar for the rapid adsorption of tetracycline based on double chemical activation. Resour. Conserv. Recycl. 2023, 190, 106821. [Google Scholar] [CrossRef]
- Rathod, N.; Jain, S.; Patel, M.R. Thermodynamic analysis of biochar produced from groundnut shell through slow pyrolysis. Energy Nexus 2023, 9, 100177. [Google Scholar] [CrossRef]
- Odeyemi, S.O.; Iwuozor, K.O.; Emenike, E.C.; Odeyemi, O.T.; Adeniyi, A.G. Valorization of waste cassava peel into biochar: An alternative to electrically-powered process. Total. Environ. Res. Themes 2023, 6, 100029. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Zheng, C.-H.; Huang, Y.-Y. Removal of chlortetracycline from water using spent tea leaves-based biochar as adsorption-enhanced persulfate activator. Chemosphere 2022, 286, 131770. [Google Scholar] [CrossRef]
- AAlabdrabalnabi, A.; Gautam, R.; Sarathy, S.M. Machine learning to predict biochar and bio-oil yields from co-pyrolysis of biomass and plastics. Fuel 2022, 328, 125303. [Google Scholar] [CrossRef]
- Sotoudehnia, F.; Rabiu, A.B.; Alayat, A.; McDonald, A.G. Characterization of bio-oil and biochar from pyrolysis of waste corrugated cardboard. J. Anal. Appl. Pyrolysis 2020, 145, 104722. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Kong, D.; Lian, L.; Liu, Y. Review on application of algae-based biochars in environmental remediation: Progress, challenge and perspectives. J. Environ. Chem. Eng. 2023, 11, 111263. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Li, X.; Zhu, K.; Jiang, Z.; Yuan, H.; Yan, K. One-step solvothermal construction of coral reef-like FeS2/biochar to activate peroxymonosulfate for efficient organic pollutant removal. Sep. Purif. Technol. 2023, 308, 122976. [Google Scholar] [CrossRef]
- Luo, M.; Wang, L.; Li, H.; Bu, Y.; Zhao, Y.; Cai, J. Hierarchical porous biochar from kelp: Insight into self-template effect and highly efficient removal of methylene blue from water. Bioresour. Technol. 2023, 372, 128676. [Google Scholar] [CrossRef]
- Khan, R.; Shukla, S.; Kumar, M.; Zuorro, A.; Pandey, A. Sewage sludge derived biochar and its potential for sustainable environment in circular economy: Advantages and challenges. Chem. Eng. J. 2023, 471, 144495. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.; Nasrullah, M. Advanced techniques in the production of biochar from lignocellulosic biomass and environmental applications. Clean. Mater. 2022, 6, 100137. [Google Scholar] [CrossRef]
- A Bapat, S.; Jaspal, D.K. Surface-modified Water Hyacinth (Eichhornia crassipes) over Activated Carbon for Wastewater Treatment: A Comparative Account. S. Afr. J. Chem. 2020, 73, 70–80. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, G.; Li, J.; Yang, Q.; Huang, S.; Cai, J. Hydrothermal conversion of bamboo shoot shell to biochar: Preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J. Anal. Appl. Pyrolysis 2019, 143, 104694. [Google Scholar] [CrossRef]
- Ukanwa, K.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Contescu, C.I.; Adhikari, S.P.; Gallego, N.C.; Evans, N.D.; Biss, B.E. Activated Carbons Derived from High-Temperature Pyrolysis of Lignocellulosic Biomass. C 2018, 4, 51. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes 2021, 9, 1154. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, Limitations, Co-Benefits, and Trade-Offs of Biochar Applications to Soils for Climate Change Mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Homagain, K.; Shahi, C.; Luckai, N.; Sharma, M. Life cycle cost and economic assessment of biochar-based bioenergy production and biochar land application in Northwestern Ontario, Canada. For. Ecosyst. 2016, 3, 21. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, D.J.P.; Narula, A.; Chistie, S.M.; Naik, S.U. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Uday, V.; Harikrishnan, P.; Deoli, K.; Zitouni, F.; Mahlknecht, J.; Kumar, M. Current trends in production, morphology, and real-world environmental applications of biochar for the promotion of sustainability. Bioresour. Technol. 2022, 359, 127467. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P.J.I.C. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crops Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Mahboubi, A.; Taherzadeh, M.J. Use of organic wastes and industrial by-products to produce filamentous fungi with potential as aqua-feed ingredients. Sustainability 2018, 10, 3296. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Ok, Y.S.; Peng, W.; Chong, C.T.; Ma, N.L.; Chase, H.A.; Liew, Z.; Yusup, S.; Kwon, E.E.; et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sustain. Energy Rev. 2019, 115, 109359. [Google Scholar] [CrossRef]
- Yu, X.; Han, Z.; Fang, S.; Chang, C.; Han, X. Optimized Preparation of High Value-Added Activated Carbon and Its Adsorption Properties for Methylene Blue. Int. J. Chem. React. Eng. 2019, 17, 20180267. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Manyà, J.J.; García-Morcate, D.; González, B. Adsorption performance of physically activated biochars for postcombustion CO2 capture from dry and humid flue gas. Appl. Sci. 2020, 10, 376. [Google Scholar] [CrossRef]
- Yadav, M.; Joshi, C.; Paritosh, K.; Thakur, J.; Pareek, N.; Masakapalli, S.K.; Vivekanand, V. Organic waste conversion through anaerobic digestion: A critical insight into the metabolic pathways and microbial interactions. Metab. Eng. 2022, 69, 323–337. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment—Conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Ha, J.H.; Lee, I.-G. Study of a method to effectively remove char byproduct generated from fast pyrolysis of lignocellulosic biomass in a bubbling fluidized bed reactor. Processes 2020, 8, 1407. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, G.; Chen, H.; Li, X. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment. Chemosphere 2018, 191, 64–71. [Google Scholar] [CrossRef]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M.; Gadonneix, P.; Celzard, A.; Fierro, V. Hydrothermal pre-treatment, an efficient tool to improve activated carbon performances. Ind. Crop. Prod. 2019, 140, 111717. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. Environ. Pollut. 2021, 268, 115910. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.-H.; Nguyen, D.D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.; Gopinath, A.; Ranjith, N.; Akre, A.P.; Sreedharan, V.; Kumar, M.S. Potential role of biochar in advanced oxidation processes: A sustainable approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Umenweke, G.C.; Afolabi, I.C.; Epelle, E.I.; Okolie, J.A. Machine learning methods for modeling conventional and hydrothermal gasification of waste biomass: A review. Bioresour. Technol. Rep. 2022, 17, 100976. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.-H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef]
- Singh, J.K.; Chaurasia, B.; Dubey, A.; Noguera, A.M.F.; Gupta, A.; Kothari, R.; Upadhyaya, C.P.; Kumar, A.; Hashem, A.; Alqarawi, A.A.; et al. Biological characterization and instrumental analytical comparison of two biorefining pretreatments for water hyacinth (Eichhornia crassipes) biomass hydrolysis. Sustainability 2021, 13, 245. [Google Scholar] [CrossRef]
- Putro, J.N.; Ju, Y.H.; Soetaredjo, F.E.; Santoso, S.P.; Ismadji, S. Biosorption of dyes. In Green Chemistry and Water Remediation: Research and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–133. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Iwata, Y. The preliminary study of water-retention related properties of biochar produced from various feedstock at different pyrolysis temperatures. Materials 2019, 12, 1732. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Liu, H.; Evrendilek, F.; Buyukada, M. Pyrolysis of water hyacinth biomass parts: Bioenergy, gas emissions, and by-products using TG-FTIR and Py-GC/MS analyses. Energy Convers. Manag. 2020, 207, 112552. [Google Scholar] [CrossRef]
- Kandanelli, R.; Meesala, L.; Kumar, J.; Raju, C.S.K.; Peddy, V.R.; Gandham, S.; Kumar, P. Cost effective and practically viable oil spillage mitigation: Comprehensive study with biochar. Mar. Pollut. Bull. 2018, 128, 32–40. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Rene, E.R.; Dupont, C.; Wongrod, S.; van Hullebusch, E.D. Anaerobic Digestion of Fruit Waste Mixed With Sewage Sludge Digestate Biochar: Influence on Biomethane Production. Front. Energy Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H.; et al. Biochar industry to circular economy. Sci. Total. Environ. 2021, 757, 143820. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent advances in photocatalytic removal of organic and inorganic pollutants in air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.; Nidheesh, P.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, S.; LaminKa-Ot, A.; Joshi, S.; Mandal, T.; Halder, G. Biosorptive uptake of Fe2+, Cu2+ and As5+ by activated biochar derived from Colocasia esculenta: Isotherm, kinetics, thermodynamics, and cost estimation. J. Adv. Res. 2016, 7, 597–610. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.-C.; Cho, J.-S.; Lee, S.-E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.-I.; Vithanage, M.; Park, Y.-K.; Lee, J.; Kwon, E.E. Modification of biochar properties using CO2. Chem. Eng. J. 2019, 372, 383–389. [Google Scholar] [CrossRef]
- Soares, O.; Rocha, R.; Gonçalves, A.; Figueiredo, J.; Órfão, J.; Pereira, M. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Peiris, C.; Wathudura, P.D.; Gunatilake, S.R.; Gajanayake, B.; Wewalwela, J.J.; Abeysundara, S.; Vithanage, M. Effect of acid modified tea-waste biochar on crop productivity of red onion (Allium cepa L.). Chemosphere 2022, 288, 132551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Zhang, D.-L.; Yi, W.-M.; Liu, Y. Effects of acid modification on the structure and adsorption NH4+-N properties of biochar. Renew. Energy 2021, 169, 1343–1350. [Google Scholar] [CrossRef]
- He, X.; Hong, Z.-N.; Jiang, J.; Dong, G.; Liu, H.; Xu, R.-K. Enhancement of Cd(II) adsorption by rice straw biochar through oxidant and acid modifications. Environ. Sci. Pollut. Res. 2021, 28, 42787–42797. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Wang, Y.; Li, R.-X.; Cao, H.-L.; Li, Y.-F.; Lü, J. Impacts of temperatures and phosphoric-acid modification to the physicochemical properties of biochar for excellent sulfadiazine adsorption. Biochar 2022, 4, 14. [Google Scholar] [CrossRef]
- An, Q.; Zhu, S.; Li, Z.; Deng, S.; Zhao, B.; Meng, F.; Jin, N.; Ren, X. Sorption and transport of Mn2+ in soil amended with alkali-modified pomelo biochar. Environ. Sci. Pollut. Res. 2021, 28, 56552–56564. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Z.; Zhu, Y.; Zhu, Y.; Jiang, Y.; Wang, Y.; Gao, H. Adsorption characteristics of modified rice straw biochar for Zn and in-situ remediation of Zn contaminated soil. Environ. Technol. Innov. 2021, 22, 101388. [Google Scholar] [CrossRef]
- Li, J.-H.; Lv, G.-H.; Bai, W.-B.; Liu, Q.; Zhang, Y.-C.; Song, J.-Q. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. Desalination Water Treat. 2016, 57, 4681–4693. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, L. Sorption of phenanthrene to biochar modified by base. Front. Environ. Sci. Eng. 2017, 12, 1. [Google Scholar] [CrossRef]
- Wang, J.; Kang, Y.; Duan, H.; Zhou, Y.; Li, H.; Chen, S.; Tian, F.; Li, L.; Drosos, M.; Dong, C.; et al. Remediation of Cd2+ in aqueous systems by alkali-modified (Ca) biochar and quantitative analysis of its mechanism. Arab. J. Chem. 2022, 15, 103750. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Guo, J.; Li, Y.; Zhang, H.; Zhu, J.; Xie, X. Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves. Water Sci. Technol. 2017, 77, 1127–1136. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Yuan, L.; Zhou, D. Comparison of adsorption behavior studies of Cd2+ by vermicompost biochar and KMnO4-modified vermicompost biochar. J. Environ. Manag. 2020, 256, 109959. [Google Scholar] [CrossRef]
- Ao, H.; Cao, W.; Hong, Y.; Wu, J.; Wei, L. Adsorption of sulfate ion from water by zirconium oxide-modified biochar derived from pomelo peel. Sci. Total. Environ. 2020, 708, 135092. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, M.; Qu, C.; Yu, D.; Chen, C.; Wang, M.; Tan, W. Quantitative analysis of Pb adsorption on sulfhydryl-modified biochar. Biochar 2021, 3, 37–49. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Wu, F.; Zhang, C.; Yang, L. Engineered biochar from biofuel residue: Characterization and its silver removal potential. ACS Appl. Mater. Interfaces 2015, 7, 10634–10640. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ngambia, A.; Ifthikar, J.; Shahib, I.I.; Jawad, A.; Shahzad, A.; Zhao, M.; Wang, J.; Chen, Z.; Chen, Z. Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(II) composite. Sci. Total. Environ. 2019, 691, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; González, A.; Pis, J.; Rubiera, F.; Pevida, C. Production of microporous biochars by single-step oxidation: Effect of activation conditions on CO2 capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef]
- Ahmed, A.; Kurian, J.; Raghavan, V. Biochar influences on agricultural soils, crop production, and the environment: A review. Environ. Rev. 2016, 24, 495–502. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, S.; Liu, J.; Wang, B.; Liu, F.; Feng, Y. Surface properties of activated sludge-derived biochar determine the facilitating effects on Geobacter co-cultures. Water Res. 2018, 142, 441–451. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Wahla, A.Q.; Anwar, S.; Mueller, J.A.; Arslan, M.; Iqbal, S. Immobilization of metribuzin degrading bacterial consortium MB3R on biochar enhances bioremediation of potato vegetated soil and restores bacterial community structure. J. Hazard. Mater. 2020, 390, 121493. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Qi, X.; Gou, J.; Chen, X.; Xiao, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Zhao, Y.; Gu, Y.; Wang, Y.; Yu, J.; Xu, H. The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci. Total. Environ. 2019, 686, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B Environ. 2019, 254, 312–320. [Google Scholar] [CrossRef]
- Gao, J.; Han, D.; Xu, Y.; Liu, Y.; Shang, J. Persulfate activation by sulfide-modified nanoscale iron supported by biochar (S-nZVI/BC) for degradation of ciprofloxacin. Sep. Purif. Technol. 2020, 235, 116202. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, J.; Huang, D.; Feng, Y.; Zhang, X.; Zhang, S.; Chen, H. Influence of different precursors on the characteristic of nitrogen-enriched biochar and SO2 adsorption properties. Chem. Eng. J. 2020, 385, 123932. [Google Scholar] [CrossRef]
- Luo, H.; Lin, Q.; Zhang, X.; Huang, Z.; Fu, H.; Xiao, R.; Liu, S.-S. Determining the key factors of nonradical pathway in activation of persulfate by metal-biochar nanocomposites for bisphenol A degradation. Chem. Eng. J. 2020, 391, 123555. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Li, M.; Sang, W.; Qiu, Y.; Xie, C. Activation of persulfate by manganese oxide-modified sludge-derived biochar to degrade Orange G in aqueous solution. Environ. Pollut. Bioavailab. 2019, 31, 70–79. [Google Scholar] [CrossRef]
- Arslanoğlu, H.; Kaya, S.; Tümen, F. Cr(VI) adsorption on low-cost activated carbon developed from grape marc-vinasse mixture. Part. Sci. Technol. 2019, 38, 768–781. [Google Scholar] [CrossRef]
- Kamali, M.; Costa, M.E.; Capela, I. Nitrate Removal And Nitrogen Sequestration From Polluted Waters Using Zero-Valent Iron Nanoparticles Synthesized under Ultrasonic Irradiation. In Advanced Materials for Wastewater Treatment; Wiely: Hoboken, NJ, USA, 2017. [Google Scholar]
- Kamali, M. An opinion on multi-criteria decision-making analysis for sustainability-based spatial planning practices.time to improve? J. Settl. Spat. Plan. 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- Tong, Y.; McNamara, P.J.; Mayer, B.K. Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium. Environ. Sci. Water Res. Technol. 2019, 5, 821–838. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; He, H.; Fang, Y.; Dong, H.; Lü, J.; Li, J.; Li, Y. Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: Influence of biochar porosity and molecular size of antibiotics. J. Mol. Liq. 2019, 274, 353–361. [Google Scholar] [CrossRef]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of Pyrolysis Temperature and Feedstock on Surface Charge and Functional Group Chemistry of Biochars. J. Environ. Qual. 2018, 47, 452–461. [Google Scholar] [CrossRef]
- Moradi-Choghamarani, F.; Moosavi, A.A.; Baghernejad, M. Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J. Therm. Anal. Calorim. 2019, 138, 331–342. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. MnO2-decorated biochar composites of coconut shell and rice husk: An efficient lithium ions adsorption-desorption performance in aqueous media. Chemosphere 2020, 260, 127500. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef]
- Bolton, L.; Joseph, S.; Greenway, M.; Donne, S.; Munroe, P.; Marjo, C.E. Phosphorus adsorption onto an enriched biochar substrate in constructed wetlands treating wastewater. Ecol. Eng. 2019, 142, 100005. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Li, A.-Y.; Deng, H.; Ye, C.-H.; Wu, Y.-Q.; Linmu, Y.-D.; Hang, H.-L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Yang, Q.; Yan, M.; Li, X.; Chen, F.; Zhong, Y.; Yin, H.; Chen, S.; Fu, J.; Wang, D.; et al. Synergistic adsorption and electrocatalytic reduction of bromate by Pd/N-doped loofah sponge-derived biochar electrode. J. Hazard. Mater. 2020, 386, 121651. [Google Scholar] [CrossRef]
- Mathurasa, L.; Damrongsiri, S. Low cost and easy rice husk modification to efficiently enhance ammonium and nitrate adsorption. Int. J. Recycl. Org. Waste Agric. 2018, 7, 143–151. [Google Scholar] [CrossRef]
- Takaya, C.A.; Parmar, K.R.; Fletcher, L.A.; Ross, A.B. Biomass-Derived Carbonaceous Adsorbents for Trapping Ammonia. Agriculture 2019, 9, 16. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Ismadji, S.; Tong, D.S.; Soetaredjo, F.E.; Ayucitra, A.; Yu, W.H.; Zhou, C.H. Bentonite hydrochar composite for removal of ammonium from Koi fish tank. Appl. Clay Sci. 2016, 119, 146–154. [Google Scholar] [CrossRef]
- An, Q.; Li, Z.; Zhou, Y.; Meng, F.; Zhao, B.; Miao, Y.; Deng, S. Ammonium removal from groundwater using peanut shell based modified biochar: Mechanism analysis and column experiments. J. Water Process. Eng. 2021, 43, 102219. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars. Environ. Sci. Pollut. Res. 2017, 25, 25638–25647. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Mahmood, I.B. Recovery of NH4+ by corn cob produced biochars and its potential application as soil conditioner. Front. Environ. Sci. Eng. 2014, 8, 825–834. [Google Scholar] [CrossRef]
- Jung, K.-W.; Hwang, M.-J.; Ahn, K.-H.; Ok, Y.-S. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int. J. Environ. Sci. Technol. 2015, 12, 3363–3372. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef]
- Sarkhot, D.V.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of Biochar for Sorption of Ammonium and Phosphate from Dairy Effluent. J. Environ. Qual. 2013, 42, 1545–1554. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef]
- Kim, D.W.; Suhaimi, M.A.; Kim, B.M.; Cho, M.H.; Chen, F.F. Rough cut machining for impellers with 3-axis and 5-axis NC machines. In Lecture Notes in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2013; Volume 7, pp. 609–616. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Wu, N.; Gao, M.; Tan, Y. Combined toxicity of pyrethroid insecticides and heavy metals: A review. Environ. Chem. Lett. 2019, 17, 1693–1706. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremediat. 2020, 22, 111–126. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Fang, J.; Zhang, M.; Chen, H.; Zhou, Y.; Creamer, A.E.; Sun, Y.; Yang, L. Characterization and environmental applications of clay–biochar composites. Chem. Eng. J. 2014, 242, 136–143. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total. Environ. 2020, 711, 134847. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wang, Y.; Kasiulienė, A.; Huang, C.; Ai, P. Cadmium removal potential by rice straw-derived magnetic biochar. Clean Technol. Environ. Policy 2017, 19, 761–774. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, D.; Ge, X. CO2-looping in biomass pyrolysis or gasification. Sustain. Energy Fuels 2017, 1, 1700–1729. [Google Scholar] [CrossRef]
- Shen, Z.; Tian, D.; Zhang, X.; Tang, L.; Su, M.; Zhang, L.; Li, Z.; Hu, S.; Hou, D. Mechanisms of biochar assisted immobilization of Pb2+ by bioapatite in aqueous solution. Chemosphere 2018, 190, 260–266. [Google Scholar] [CrossRef]
- Amin, M.; Chetpattananondh, P. Biochar from extracted marine Chlorella sp. residue for high efficiency adsorption with ultrasonication to remove Cr(VI), Zn(II) and Ni(II). Bioresour. Technol. 2019, 289, 121578. [Google Scholar] [CrossRef]
- Zhang, W.; Du, W.; Wang, F.; Xu, H.; Zhao, T.; Zhang, H.; Ding, Y.; Zhu, W. Comparative study on Pb2+ removal from aqueous solutions using biochars derived from cow manure and its vermicompost. Sci. Total. Environ. 2020, 716, 137108. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Lian, W.; Yang, L.; Joseph, S.; Shi, W.; Bian, R.; Zheng, J.; Li, L.; Shan, S.; Pan, G. Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II). Bioresour. Technol. 2020, 317, 124011. [Google Scholar] [CrossRef]
- Lee, M.-E.; Park, J.H.; Chung, J.W. Comparison of the lead and copper adsorption capacities of plant source materials and their biochars. J. Environ. Manag. 2019, 236, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Ok, Y.S. Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Liu, L.; Ju, M.; Zheng, K. Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials 2018, 11, 299. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Van Hien, N.; Valsami-Jones, E.; Vinh, N.C.; Phu, T.T.; Tam, N.T.T.; Lynch, I. Effectiveness of different biochar in aqueous zinc removal: Correlation with physicochemical characteristics. Bioresour. Technol. Rep. 2020, 11, 100466. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.-W.; Tsang, D.C.; Bolan, N.; Rinklebe, J.; Song, H. Fabrication of engineered biochar from paper mill sludge and its application into removal of arsenic and cadmium in acidic water. Bioresour. Technol. 2017, 246, 69–75. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, Y.; Vithanage, M.; Shaheen, S.M.; Rinklebe, J.; Alessi, D.S.; Hou, C.-H.; Hashimoto, Y.; Withana, P.A.; Ok, Y.S. Arsenic removal from water and soils using pristine and modified biochars. Biochar 2022, 4, 55. [Google Scholar] [CrossRef]
- Liu, J.; Luo, K.; Li, X.; Yang, Q.; Wang, D.; Wu, Y.; Chen, Z.; Huang, X.; Pi, Z.; Du, W.; et al. The biochar-supported iron-copper bimetallic composite activating oxygen system for simultaneous adsorption and degradation of tetracycline. Chem. Eng. J. 2020, 402, 126039. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total. Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.U.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Wathukarage, A.; Herath, I.; Iqbal, M.C.M.; Vithanage, M. Mechanistic understanding of crystal violet dye sorption by woody biochar: Implications for wastewater treatment. Environ. Geochem. Health 2019, 41, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Faheem; Du, J.; Bao, J.; Hassan, M.A.; Irshad, S.; Talib, M.A. Multi-functional Biochar Novel Surface Chemistry for Efficient Capture of Anionic Congo Red Dye: Behavior and Mechanism. Arab. J. Sci. Eng. 2019, 44, 10127–10139. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Woo, S.H. Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour. Technol. 2017, 224, 206–213. [Google Scholar] [CrossRef]
- Acemioğlu, B. Removal of a reactive dye using NaOH-activated biochar prepared from peanut shell by pyrolysis process. Int. J. Coal Prep. Util. 2022, 42, 671–693. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Emran, M.; El-Sadek, M.H.; El-Shanshory, A.A.; Soliman, H.M.A.; Akl, M.A.; Rashad, M. Enhanced removal of cationic dye by eco-friendly activated biochar derived from rice straw. Appl. Water Sci. 2020, 10, 45. [Google Scholar] [CrossRef]
- Blachnio, M.; Derylo-Marczewska, A.; Charmas, B.; Zienkiewicz-Strzalka, M.; Bogatyrov, V.; Galaburda, M. Activated Carbon from Agricultural Wastes for Adsorption of Organic Pollutants. Molecules 2020, 25, 5105. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, D.; Chen, J.; Chen, Y.; Chen, W. Enhanced adsorption of aromatic chemicals on boron and nitrogen co-doped single-walled carbon nanotubes. Environ. Sci. Nano 2017, 4, 558–564. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Wang, X.; Wang, K.; Zhu, L.; Wang, S. Nitrogen-Doped Hierarchical Porous Biochar Derived from Corn Stalks for Phenol-Enhanced Adsorption. Energy Fuels 2019, 33, 12459–12468. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Chen, S.S.; Cao, Y.; Tsang, D.C.; Tessonnier, J.-P.; Shang, J.; Hou, D.; Shen, Z.; Zhang, S.; Ok, Y.S.; Wu, K.C.-W. Effective Dispersion of MgO Nanostructure on Biochar Support as a Basic Catalyst for Glucose Isomerization. ACS Sustain. Chem. Eng. 2020, 8, 6990–7001. [Google Scholar] [CrossRef]
- Zhai, Y.; Dai, Y.; Guo, J.; Zhou, L.; Chen, M.; Yang, H.; Peng, L. Novel biochar@CoFe2O4/Ag3PO4 photocatalysts for highly efficient degradation of bisphenol a under visible-light irradiation. J. Colloid Interface Sci. 2020, 560, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S.; Gonçalves, M.G.; Veiga, P.A.d.S.; Matos, T.T.d.S.; Peralta-Zamora, P.; Mangrich, A.S. TiO2 supported on Salvinia molesta biochar for heterogeneous photocatalytic degradation of Acid Orange 7 dye. J. Environ. Chem. Eng. 2019, 7, 102879. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Huang, Z.; He, K.; Zeng, G.; Chen, A.; Yuan, L.; Peng, M.; Huang, T.; Chen, G. Preparation of silver-nanoparticle-loaded magnetic biochar/poly(dopamine) composite as catalyst for reduction of organic dyes. J. Colloid Interface Sci. 2019, 555, 460–469. [Google Scholar] [CrossRef]
- Chen, M.; Bao, C.; Hu, D.; Jin, X.; Huang, Q. Facile and low-cost fabrication of ZnO/biochar nanocomposites from jute fibers for efficient and stable photodegradation of methylene blue dye. J. Anal. Appl. Pyrolysis 2019, 139, 319–332. [Google Scholar] [CrossRef]
- Trinh, B.-S.; Le, P.T.K.; Werner, D.; Phuong, N.H.; Luu, T.L. Rice Husk Biochars Modified with Magnetized Iron Oxides and Nano Zero Valent Iron for Decolorization of Dyeing Wastewater. Processes 2019, 7, 660. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Hung, C.-M.; Nguyen, T.-B.; Chang, J.-H.; Wang, T.-H.; Wu, C.-H.; Lin, Y.-L.; Chen, C.-W.; Dong, C.-D. Efficient Heterogeneous Activation of Persulfate by Iron-Modified Biochar for Removal of Antibiotic from Aqueous Solution: A Case Study of Tetracycline Removal. Catalysts 2019, 9, 49. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Li, J.; Hao, Q.; Li, X.; Liu, F. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols. Environ. Pollut. 2020, 257, 113610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, L.; Guo, X.; Han, Z.; Ji, L.; He, Q.; Han, L.; Sun, K. Mechanism of biochar as a biostimulation strategy to remove polycyclic aromatic hydrocarbons from heavily contaminated soil in a coking plant. Geoderma 2020, 375, 114497. [Google Scholar] [CrossRef]
- You, X.; Suo, F.; Yin, S.; Wang, X.; Zheng, H.; Fang, S.; Zhang, C.; Li, F.; Li, Y. Biochar decreased enantioselective uptake of chiral pesticide metalaxyl by lettuce and shifted bacterial community in agricultural soil. J. Hazard. Mater. 2021, 417, 126047. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.O.; Bernardino, C.A.R.; Mahler, C.F.; Santelli, R.E.; Braz, B.F.; Borges, R.C.; Veloso, M.C.d.C.; Romeiro, G.A.; Cincotto, F.H. Biochar Generated from Agro-Industry Sugarcane Residue by Low Temperature Pyrolysis Utilized as an Adsorption Agent for the Removal of Thiamethoxam Pesticide in Wastewater. Water Air Soil Pollut. 2021, 232, 67. [Google Scholar] [CrossRef]
- Ndoun, M.C.; Knopf, A.; Preisendanz, H.E.; Vozenilek, N.; Elliott, H.A.; Mashtare, M.L.; Velegol, S.; Veith, T.L.; Williams, C.F. Fixed bed column experiments using cotton gin waste and walnut shells-derived biochar as low-cost solutions to removing pharmaceuticals from aqueous solutions. Chemosphere 2023, 330, 138591. [Google Scholar] [CrossRef]
- Filipinas, J.Q.; Rivera, K.K.P.; Ong, D.C.; Pingul-Ong, S.M.B.; Abarca, R.R.M.; de Luna, M.D.G. Removal of sodium diclofenac from aqueous solutions by rice hull biochar. Biochar 2021, 3, 189–200. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef]
- Peng, Y.; Tong, W.; Xie, Y.; Hu, W.; Li, Y.; Zhang, Y.; Wang, Y. Yeast biomass-induced Co2P/biochar composite for sulfonamide antibiotics degradation through peroxymonosulfate activation. Environ. Pollut. 2021, 268, 115930. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, W.; Yin, Y.; Chen, Z.; Qiu, R.; Simonnot, M.-O.; Wang, X. Adsorption-reduction removal of Cr(VI) by tobacco petiole pyrolytic biochar: Batch experiment, kinetic and mechanism studies. Bioresour. Technol. 2018, 268, 149–157. [Google Scholar] [CrossRef]
- Sun, P.; Li, Y.; Meng, T.; Zhang, R.; Song, M.; Ren, J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018, 147, 91–100. [Google Scholar] [CrossRef]
- Fu, D.; Chen, Z.; Xia, D.; Shen, L.; Wang, Y.; Li, Q. A novel solid digestate-derived biochar-Cu NP composite activating H2O2 system for simultaneous adsorption and degradation of tetracycline. Environ. Pollut. 2017, 221, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.M.; Ponnuchamy, M.; Roshin, A.; Kapoor, A. Adsorptive removal of oxytetracycline hydrochloride using bagasse-based biochar powder and beads. Chemosphere 2024, 363, 143016. [Google Scholar] [CrossRef] [PubMed]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Marshall, J.; Muhlack, R.; Morton, B.J.; Dunnigan, L.; Chittleborough, D.; Kwong, C.W. Pyrolysis Temperature Effects on Biochar–Water Interactions and Application for Improved Water Holding Capacity in Vineyard Soils. Soil Syst. 2019, 3, 27. [Google Scholar] [CrossRef]
- Albanese, L.; Baronti, S.; Liguori, F.; Meneguzzo, F.; Barbaro, P.; Vaccari, F.P. Hydrodynamic cavitation as an energy efficient process to increase biochar surface area and porosity: A case study. J. Clean. Prod. 2019, 210, 159–169. [Google Scholar] [CrossRef]
- Verheijen, F.G.; Zhuravel, A.; Silva, F.C.; Amaro, A.; Ben-Hur, M.; Keizer, J.J. The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma 2019, 347, 194–202. [Google Scholar] [CrossRef]
- Vilas-Boas, A.C.M.; Tarelho, L.A.C.; Kamali, M.; Hauschild, T.; Pio, D.T.; Jahanianfard, D.; Gomes, A.P.D.; Matos, M.A.A. Biochar from slow pyrolysis of biological sludge from wastewater treatment: Characteristics and effect as soil amendment. Biofuels Bioprod. Biorefining 2021, 15, 1054–1072. [Google Scholar] [CrossRef]
- Bordoloi, S.; Kumar, H.; Hussain, R.; Karangat, R.; Lin, P.; Sreedeep, S.; Zhu, H.-H. Assessment of hydro-mechanical properties of biochar-amended soil sourced from two contrasting feedstock. Biomass-Convers. Biorefin. 2020, 14, 5803–5818. [Google Scholar] [CrossRef]
- Hallin, I.; Douglas, P.; Doerr, S.; Matthews, I.; Bryant, R.; Charbonneau, C. The potential of biochar to remove hydrophobic compounds from model sandy soils. Geoderma 2017, 285, 132–140. [Google Scholar] [CrossRef]
- Fernelius, K.J.; Madsen, M.D.; Hopkins, B.G.; Bansal, S.; Anderson, V.J.; Eggett, D.L.; Roundy, B.A. Post-fire interactions between soil water repellency, soil fertility and plant growth in soil collected from a burned piñon-juniper woodland. J. Arid. Environ. 2017, 144, 98–109. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, H.; Lu, W.; Zhang, D.; Ren, X.; Yu, W.; Wu, J.; Zhou, L.; Han, X.; Yi, W.; et al. Properties evaluation of biochar/high-density polyethylene composites: Emphasizing the porous structure of biochar by activation. Sci. Total. Environ. 2020, 737, 139770. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, S.A.; Doyle, R.; Bowman, J.P. Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, K.; Chen, B. Linking hydrophobicity of biochar to the water repellency and water holding capacity of biochar-amended soil. Environ. Pollut. 2019, 253, 779–789. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Senbayram, M.; Saygan, E.P.; Chen, R.; Aydemir, S.; Kaya, C.; Wu, D.; Bladogatskaya, E. Effect of biochar origin and soil type on the greenhouse gas emission and the bacterial community structure in N fertilised acidic sandy and alkaline clay soil. Sci. Total. Environ. 2019, 660, 69–79. [Google Scholar] [CrossRef]
- Olmo, M.; Villar, R.; Salazar, P.; Alburquerque, J.A. Changes in soil nutrient availability explain biochar’s impact on wheat root development. Plant Soil 2016, 399, 333–343. [Google Scholar] [CrossRef]
- Joseph, S.; Pow, D.; Dawson, K.; Rust, J.; Munroe, P.; Taherymoosavi, S.; Mitchell, D.R.; Robb, S.; Solaiman, Z.M. Biochar increases soil organic carbon, avocado yields and economic return over 4 years of cultivation. Sci. Total. Environ. 2020, 724, 138153. [Google Scholar] [CrossRef]
- Feng, N.; Ghoveisi, H.; Bitton, G.; Bonzongo, J.-C.J. Removal of phyto-accessible copper from contaminated soils using zero valent iron amendment and magnetic separation methods: Assessment of residual toxicity using plant and MetPLATE™ studies. Environ. Pollut. 2016, 219, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hussain, Q.; Zhu, J.; Fu, Q.; Houben, D.; Hu, H. Efficiency of KOH-modified rice straw-derived biochar for reducing cadmium mobility, bioaccessibility and bioavailability risk index in red soil. Pedosphere 2020, 30, 874–882. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The Interfacial Behavior between Biochar and Soil Minerals and Its Effect on Biochar Stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Dilmaghani, M.R.; Malakouti, M.J.; Neilsen, G.H.; Fallahi, E. Interactive Effects of Potassium and Calcium on K/Ca Ratio and Its Consequences on Apple Fruit Quality in Calcareous Soils of Iran. J. Plant Nutr. 2004, 27, 1149–1162. [Google Scholar] [CrossRef]
- Syuhada, A.; Shamshuddin, J.; Fauziah, C.; Rosenani, A.; Arifin, A. Biochar as soil amendment: Impact on chemical properties and corn nutrient uptake in a Podzol. Can. J. Soil Sci. 2016, 96, 400–412. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Obia, A.; Mulder, J.; Martinsen, V.; Cornelissen, G.; Børresen, T. In situ effects of biochar on aggregation, water retention and porosity in light-textured tropical soils. Soil Tillage Res. 2016, 155, 35–44. [Google Scholar] [CrossRef]
- Jeffery, S.; Meinders, M.B.; Stoof, C.R.; Bezemer, T.M.; van de Voorde, T.F.; Mommer, L.; van Groenigen, J.W. Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 2015, 251–252, 47–54. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Straw and straw biochar differently affect phosphorus availability, enzyme activity and microbial functional genes in an Ultisol. Sci. Total. Environ. 2022, 805, 150325. [Google Scholar] [CrossRef]
- Kamran, M.A.; Xu, R.-K.; Li, J.-Y.; Jiang, J.; Shi, R.-Y. Impacts of chicken manure and peat-derived biochars and inorganic P alone or in combination on phosphorus fractionation and maize growth in an acidic ultisol. Biochar 2019, 1, 283–291. [Google Scholar] [CrossRef]
- Zhao, W.-R.; Li, J.-Y.; Deng, K.-Y.; Shi, R.-Y.; Jiang, J.; Hong, Z.-N.; Qian, W.; He, X.; Xu, R.-K. Effects of crop straw biochars on aluminum species in soil solution as related with the growth and yield of canola (Brassica napus L.) in an acidic Ultisol under field condition. Environ. Sci. Pollut. Res. 2020, 27, 30178–30189. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhao, F.; Lan, X.; Nawaz, M.; Shohag, J.I. Role of Coconut Shell Biochar on Soil Properties, Microbial Diversity and Nitrogen Mineralization in Tropical Latosol. Pol. J. Environ. Stud. 2023, 33, 1487–1496. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; Pena, A.N.L.; de Oliveira, W.R.; Fernandes, L.A.; Colen, F.; Ferreira, E.A.; Veloso, M.d.D.; Frazão, L.A. Filter Cake Biochar as a Soil Conditioner Cultivated with Native Cerrado Species: Effect on Soil Chemical and Microbiological Properties. Floresta Ambient 2023, 30, e20220075. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, S.; Biswas, S.; Naik, S.K.; Dey, S.; Sengupta, A.; Dutta, A.K. Rice-pigeon pea biochar influences nutrient cycling, microbial dynamics, and onion performance in organic production system: Insights from Alfisols of the Eastern Plateau and Hill Region of India. Discov. Sustain. 2024, 5, 403. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Pyrolysis temperature affects phosphorus availability of rice straw and canola stalk biochars and biochar-amended soils. J. Soils Sediments 2021, 21, 2817–2830. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Lu, S. Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Yu, J.; Deem, L.M.; Crow, S.E.; Deenik, J.L.; Penton, C.R. Biochar application influences microbial assemblage complexity and composition due to soil and bioenergy crop type interactions. Soil Biol. Biochem. 2018, 117, 97–107. [Google Scholar] [CrossRef]
- Wu, C.; Shi, L.; Xue, S.; Li, W.; Jiang, X.; Rajendran, M.; Qian, Z. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci. Total. Environ. 2019, 647, 1158–1168. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, D.; Liu, Y.; Xu, H.; Nan, H.; Li, D.; Kan, Y.; Cao, X. Biochar as simultaneous shelter, adsorbent, pH buffer, and substrate of Pseudomonas citronellolis to promote biodegradation of high concentrations of phenol in wastewater. Water Res. 2020, 172, 115494. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Zhou, F.; Ding, J.; Li, T.; Zhang, X. Plant communities are more sensitive than soil microbial communities to multiple environmental changes in the Eurasian steppe. Glob. Ecol. Conserv. 2020, 21, e00779. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Li, X.; Lu, W.; Li, J. Function prediction and network analysis to investigate the response of microbial communities to a single environmental factor. J. Freshw. Ecol. 2020, 35, 271–289. [Google Scholar] [CrossRef]
- Randolph, P.; Bansode, R.; Hassan, O.; Rehrah, D.; Ravella, R.; Reddy, M.; Watts, D.; Novak, J.; Ahmedna, M. Effect of biochars produced from solid organic municipal waste on soil quality parameters. J. Environ. Manag. 2017, 192, 271–280. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, C.; Chen, X.; Hopkins, I.; Zhang, X.; Han, Z.; Jiang, F.; Billy, G. The crucial factors of soil fertility and rapeseed yield—A five year field trial with biochar addition in upland red soil, China. Sci. Total. Environ. 2019, 649, 1467–1480. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.-E.; Lee, Y.H.; Tsang, D.C.; Rinklebe, J.; Kwon, E.E.; Ok, Y.S. Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-Ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Adrees, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Qayyum, M.F.; Nawaz, R. Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar] [CrossRef]
- Lal, S.; Kumar, R.; Ahmad, S.; Dixit, V.K.; Berta, G. Exploring the survival tactics and plant growth promising traits of root-associated bacterial strains under Cd and Pb stress: A modelling based approach. Ecotoxicol. Environ. Saf. 2019, 170, 267–277. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Li, Z.-G.; Liu, X.-D.; Wang, B.-C.; Zhou, G.-L.; Huang, X.-X.; Lin, C.-F.; Wang, A.-H.; Brooks, M. Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol. Eng. 2017, 98, 183–188. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Li, P.; Xian, Q.; Tang, X. Biochar’s impact on dissolved organic matter (DOM) export from a cropland soil during natural rainfalls. Sci. Total. Environ. 2019, 650, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Haris, A.A.; Bhat, R.; Kumar, V.K.; Muralidharan, K.; John, K.S.; Surendran, U. A comparative assessment of nutrient partitioning in healthy and root (wilt) disease affected coconut palms grown in an Entisol of humid tropical Kerala. Trees 2021, 35, 621–635. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.; Rodríguez-Ramos, I.; Leyva-Ramos, R.; Mendoza-Mendoza, E.; Villela-Martínez, D. Effect of surface area and physical–chemical properties of graphite and graphene-based materials on their adsorption capacity towards metronidazole and trimethoprim antibiotics in aqueous solution. Chem. Eng. J. 2020, 402, 126155. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Liu, X.; Qiu, W.; Song, Z. Effects of Fe-Mn modified biochar composite treatment on the properties of As-polluted paddy soil. Environ. Pollut. 2019, 244, 600–607. [Google Scholar] [CrossRef]
- Beiyuan, J.; Awad, Y.M.; Beckers, F.; Tsang, D.C.; Ok, Y.S.; Rinklebe, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 2017, 178, 110–118. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef]
- Shen, Z.; Hou, D.; Zhao, B.; Xu, W.; Ok, Y.S.; Bolan, N.S.; Alessi, D.S. Stability of heavy metals in soil washing residue with and without biochar addition under accelerated ageing. Sci. Total Environ. 2018, 619–620, 185–193. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Akmal, M.; Riaz, M.; Hu, H.; Ijaz, S.S.; Iqbal, M.; Abro, S.; Mehmood, S.; Ahmad, M. Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J. Soils Sediments 2018, 18, 874–886. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, H.; Wang, Y.; Huang, H.; Chen, Y. Characteristic of heavy metals in biochar derived from sewage sludge. J. Mater. Cycles Waste Manag. 2016, 18, 725–733. [Google Scholar] [CrossRef]
- Xing, J.; Li, L.; Li, G.; Xu, G. Feasibility of sludge-based biochar for soil remediation: Characteristics and safety performance of heavy metals influenced by pyrolysis temperatures. Ecotoxicol. Environ. Saf. 2019, 180, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Suitability of marginal biomass-derived biochars for soil amendment. Sci. Total. Environ. 2016, 547, 314–322. [Google Scholar] [CrossRef]
- Herath, H.M.S.K.; Camps-Arbestain, M.; Hedley, M.; Kirschbaum, M.; Wang, T.; van Hale, R. Experimental evidence for sequestering C with biochar by avoidance of CO2 emissions from original feedstock and protection of native soil organic matter. GCB Bioenergy 2015, 7, 512–526. [Google Scholar] [CrossRef]
- Xia, T.; Qi, Y.; Liu, J.; Qi, Z.; Chen, W.; Wiesner, M.R. cation-inhibited transport of graphene oxide nanomaterials in saturated porous media: The hofmeister effects. Environ. Sci. Technol. 2017, 51, 828–837. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Yin, Y.-F.; He, X.-H.; Gao, R.; Ma, H.-L.; Yang, Y.-S. Effects of rice straw and its biochar addition on soil labile carbon and soil organic carbon. J. Integr. Agric. 2014, 13, 491–498. [Google Scholar] [CrossRef]
- Awad, Y.M.; Lee, S.S.; Kim, K.-H.; Ok, Y.S.; Kuzyakov, Y. Carbon and nitrogen mineralization and enzyme activities in soil aggregate-size classes: Effects of biochar, oyster shells, and polymers. Chemosphere 2018, 198, 40–48. [Google Scholar] [CrossRef]
- IBI, version 2.1. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. International Biochar Initiative: Norfolk, VA, USA, 2015. Available online: https://biochar-international.org/biochar-standards/ (accessed on 20 September 2024).
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Sharma, P.; Pandey, A.; Bui, X.T.; Zhang, X. Performance of a dual-chamber microbial fuel cell as biosensor for on-line measuring ammonium nitrogen in synthetic municipal wastewater. Sci. Total. Environ. 2021, 795, 148755. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, T.; Fu, P.; Tang, J.; Zhou, S. Conversion of sewage sludge into high-performance bifunctional electrode materials for microbial energy harvesting. J. Mater. Chem. A 2015, 3, 8475–8482. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sathe, S.; Dubey, B.; Ghangrekar, M. Waste-derived biochar: Applications and future perspective in microbial fuel cells. Bioresour. Technol. 2020, 312, 123587. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Mohan, S.V. Concomitant use of Azolla derived bioelectrode as anode and hydrolysate as substrate for microbial fuel cell and electro-fermentation applications. Sci. Total. Environ. 2020, 707, 135851. [Google Scholar] [CrossRef]
- Cao, C.; Wei, L.; Su, M.; Wang, G.; Shen, J. Low-cost adsorbent derived and in situ nitrogen/iron co-doped carbon as efficient oxygen reduction catalyst in microbial fuel cells. Bioresour. Technol. 2016, 214, 348–354. [Google Scholar] [CrossRef]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Appl. Sci. 2020, 2, 1307. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Yang, J.; Sun, J.; Li, H.; Han, B.; Zhao, S.; Shi, Y.; Feng, Y.; Tang, Z.; et al. Bread-derived 3D macroporous carbon foams as high performance free-standing anode in microbial fuel cells. Biosens. Bioelectron. 2018, 122, 217–223. [Google Scholar] [CrossRef]
- Huggins, T.M.; Latorre, A.; Biffinger, J.C.; Ren, Z.J. Biochar based microbial fuel cell for enhanced wastewater treatment and nutrient recovery. Sustainability 2016, 8, 169. [Google Scholar] [CrossRef]
- Santoro, C.; Babanova, S.; Artyushkova, K.; Cornejo, J.A.; Ista, L.; Bretschger, O.; Marsili, E.; Atanassov, P.; Schuler, A.J. Influence of anode surface chemistry on microbial fuel cell operation. Bioelectrochemistry 2015, 106, 141–149. [Google Scholar] [CrossRef]
- Silva, A.V.; Edel, M.; Gescher, J.; Paquete, C.M. Exploring the Effects of bolA in Biofilm Formation and Current Generation by Shewanella oneidensis MR-1. Front. Microbiol. 2020, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Gajda, I.; Ieropoulos, I. Microbial fuel cells (MFC) and microalgae; Photo microbial fuel cell (PMFC) as complete recycling machines. Sustain. Energy Fuels 2019, 3, 2546–2560. [Google Scholar] [CrossRef]
- Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Investigating the specific role of external load on the performance versus stability trade-off in microbial fuel cells. Bioresour. Technol. 2020, 309, 123313. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Li, J.; Liao, Q.; Ye, D. Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J. Power Sources 2011, 196, 6029–6035. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Wang, B.; Xuan, J.; Wong, J.W.; Lee, P.K.; Leung, M.K. Interfacial electron transfer and bioelectrocatalysis of carbonized plant material as effective anode of microbial fuel cell. Electrochim. Acta 2015, 157, 314–323. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S. Biomass-Derived Carbon for Electrode Fabrication in Microbial Fuel Cells: A Review. Ind. Eng. Chem. Res. 2020, 59, 6391–6404. [Google Scholar] [CrossRef]
- Bose, D.; Sridharan, S.; Dhawan, H.; Vijay, P.; Gopinath, M. Biomass derived activated carbon cathode performance for sustainable power generation from Microbial Fuel Cells. Fuel 2019, 236, 325–337. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Jiang, Y.; Tan, G.; Xu, N.; Xu, Y. Enhanced power generation and wastewater treatment in sustainable biochar electrodes based bioelectrochemical system. Bioresour. Technol. 2017, 241, 841–848. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Qi, Y.; Kobayashi, N.; Tang, J. Nonactivated and Activated Biochar Derived from Bananas as Alternative Cathode Catalyst in Microbial Fuel Cells. Sci. World J. 2014, 2014, 832850. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Tang, J.; Wang, Y.; Cai, X.; Liu, H.; Lin, J.; Van der Bruggen, B.; Zhou, S. Hierarchically structured carbon materials derived from lotus leaves as efficient electrocatalyst for microbial energy harvesting. Sci. Total. Environ. 2019, 666, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Zhang, Z.; Xiang, P.; Zhu, H.; Zhou, B.; Sun, Z.; Zhou, S. One-step preparation of eggplant-derived hierarchical po-rous graphitic biochar as efficient oxygen reduction catalyst in microbial fuel cells. RSC Adv. 2020, 11, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; You, S.; Wang, W.; Liu, G.; Qi, D.; Chen, X.; Qu, J.; Ren, N. Biomass-derived porous fe3c/tungsten carbide/graphitic carbon nanocomposite for efficient electrocatalysis of oxygen reduction. ACS Appl. Mater. Interfaces 2016, 8, 32307–32316. [Google Scholar] [CrossRef]
- Sciarria, T.P.; de Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Goldfarb, J.L.; Adani, F. Metal-free activated biochar as an oxygen reduction reaction catalyst in single chamber microbial fuel cells. J. Power Sources 2020, 462, 228183. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Yang, Y.-H. An overview of microdiesel—A sustainable future source of renewable energy. Renew. Sustain. Energy Rev. 2017, 79, 1078–1090. [Google Scholar] [CrossRef]
- Watson, V.J.; Delgado, C.N.; Logan, B.E. Influence of chemical and physical properties of activated carbon powders on oxy-gen reduction and microbial fuel cell performance. Environ. Sci. Technol. 2013, 47, 6704–6710. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bhowmick, G.D.; Ghosh, D.; Dubey, B.; Pradhan, D.; Ghangrekar, M. Novel low-cost activated algal biochar as a cathode catalyst for improving performance of microbial fuel cell. Sustain. Energy Technol. Assess. 2020, 42, 100808. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ramavandi, B. Biodiesel production from edible oils using algal biochar/CaO/K2CO3 as a heterogeneous and recyclable catalyst. Renew. Energy 2021, 168, 1207–1216. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Ma, L. Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters. Processes 2020, 8, 1130. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.-M.; Pugazhendhi, A.; Awasthi, M.K.; Kumar, D.; Kumar, G.; Yoon, J.-J.; Yang, Y.-H. An overview on advancements in biobased transesterification methods for biodiesel production: Oil resources, extraction, biocatalysts, and process intensification technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Jung, J.-M.; Lee, J.; Choi, D.; Oh, J.-I.; Lee, S.-R.; Kim, J.-K.; Kwon, E.E. Biochar as porous media for thermally-induced non-catalytic transesterification to synthesize fatty acid ethyl esters from coconut oil. Energy Convers. Manag. 2017, 145, 308–313. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.-M.; Oh, J.-I.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour. Technol. 2017, 231, 59–64. [Google Scholar] [CrossRef]
- Mustapha, S.I.; Bux, F.; Isa, Y.M. Techno-economic analysis of biodiesel production over lipid extracted algae derived catalyst. Biofuels 2021, 13, 663–674. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, R.; Dong, G.; Xiang, M.; Hui, J.; Ou, J.; Qin, H. Biochar Nanocomposite Derived from Watermelon Peels for Electrocatalytic Hydrogen Production. ACS Omega 2021, 6, 2066–2073. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Zheng, X.; Wang, X.; Xu, Y.; Tang, H.; Kang, F.; Yang, Q.-H.; Luo, J. Biomass Organs Control the Porosity of Their Pyrolyzed Carbon. Adv. Funct. Mater. 2016, 27, 1604687. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, X.; Liu, T.; Wu, Z.; Wang, D. N, K Co-activated biochar-derived molybdenum carbide as efficient electrocatalysts for hydrogen evolution. Appl. Surf. Sci. 2020, 509, 144879. [Google Scholar] [CrossRef]
- Humagain, G.; MacDougal, K.; MacInnis, J.; Lowe, J.M.; Coridan, R.H.; MacQuarrie, S.; Dasog, M. Highly Efficient, Biochar-Derived Molybdenum Carbide Hydrogen Evolution Electrocatalyst. Adv. Energy Mater. 2018, 8, 1801461. [Google Scholar] [CrossRef]
- Harun, K.; Adhikari, S.; Jahromi, H. Hydrogen production via thermocatalytic decomposition of methane using carbon-based catalysts. RSC Adv. 2020, 10, 40882–40893. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.S.; Dhand, V.; Himabindu, V.; Anjaneyulu, Y. Production of hydrogen and carbon nanofibers through the decomposition of methane over activated carbon supported Ni catalysts. Int. J. Hydrogen Energy 2011, 36, 11702–11711. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Marzbali, M.H.; Chiang, K.; Surapaneni, A.; Shah, K. Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int. J. Hydrogen Energy 2020, 45, 29978–29992. [Google Scholar] [CrossRef]
- Sugiarto, Y.; Sunyoto, N.M.; Zhu, M.; Jones, I.; Zhang, D. Effect of biochar in enhancing hydrogen production by mesophilic anaerobic digestion of food wastes: The role of minerals. Int. J. Hydrogen Energy 2021, 46, 3695–3703. [Google Scholar] [CrossRef]
- Sunyoto, N.M.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Zhao, W.; Zang, L. Improved Fermentative Hydrogen Production with the Addition of Calcium-Lignosulfonate-Derived Biochar. Energy Fuels 2019, 33, 7406–7414. [Google Scholar] [CrossRef]
- Sharma, P.; Melkania, U. Biochar-enhanced hydrogen production from organic fraction of municipal solid waste using co-culture of Enterobacter aerogenes and E. coli. Int. J. Hydrogen Energy 2017, 42, 18865–18874. [Google Scholar] [CrossRef]
- Li, W.; He, L.; Cheng, C.; Cao, G.; Ren, N. Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions. Bioresour. Technol. 2020, 306, 123088. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Deng, C.; Cheng, J.; Murphy, J.D. Low concentrations of furfural facilitate biohydrogen production in dark fermentation using Enterobacter aerogenes. Renew. Energy 2020, 150, 23–30. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Jain, R.; Praveenkumar, R.; Rintala, J.; Romar, H.; Konttinen, J. Adsorption of furfural from torrefaction condensate using torrefied biomass. Chem. Eng. J. 2018, 334, 558–568. [Google Scholar] [CrossRef]
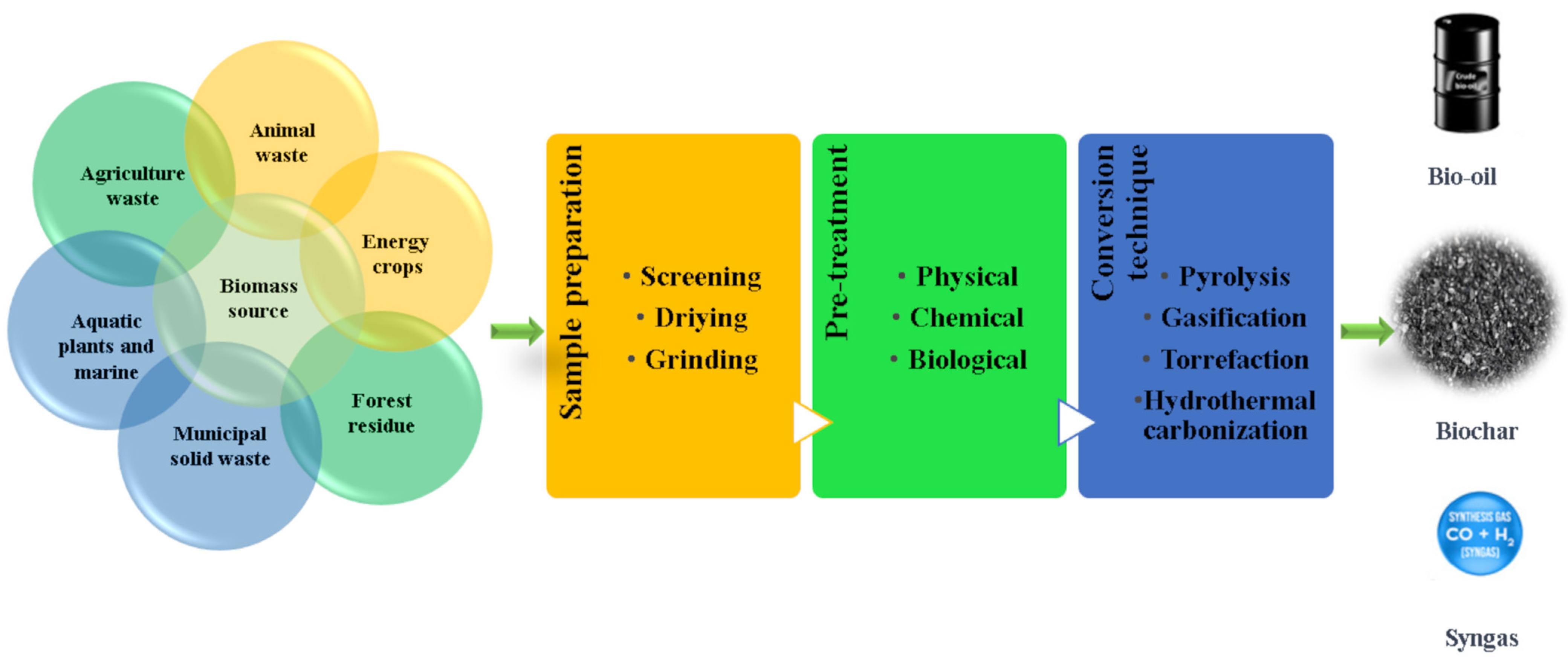

| Feedstock Type | Pyrolysis Temperature, °C | Nutrient Type | Initial Concentration | Adsorption Capacity | References |
|---|---|---|---|---|---|
| Rice Husk | 500 | NH4+ | 3000 mg N/L | 44.04 mg N/L | [147] |
| Oak Sawdust | 650 | NH4+ | 450 mg N/L | 32.7 mg N/L | [148] |
| Municipal Waste | 650 | NH4+ | 1000 mg N/L | 128.3 mg N/L | [149] |
| Cassava Peel | 500 | NH4+ | 200 mg N/L | 23.67 mg N/L | [150] |
| Peanut Shell | 500 | NH4+ | 60 mg N/L | 3.83 mg N/L | [151] |
| Wheat Straw | 550 | NH4+ | 40 mg N/L | 2.08 mg N/L | [152] |
| Corn Cob | 400 | NH4+ | 100 mg N/L | 1.09 mg N/L | [153] |
| Oak Wood | 450 | PO43− | 400 mg P/L | 5.5 mg P/L | [149] |
| Municipal Waste | 650 | PO43− | 400 mg P/L | 14.3 mg P/L | [149] |
| Peanut Shell | 700 | PO43− | 5 mg P/L | 0.613 | [154] |
| Orange Peel | 700 | PO43− | 2.4 mg P/L | 83.3% | [155] |
| CaCao Shell | 300–350 | PO43− | 0.1–50 mg P/L | 1 mg P/L | [156] |
| Brown Marine Macroalgae | 480 | PO43− | 200 mg P/L | 7 mg P/L | [157] |
| PressCake | 650 | PO43− | 400 mg P/L | 30 mg P/L | [149] |
| Mixed Hardwood | 300 | PO43− | 24 mg P/L | 0.48 mg P/L | [158] |
| Metals | Biochar Feedstock Type | Pyrolysis Temperature, °C | Biochar Dose, g/L | Adsorption Capacity, mg/g | References |
|---|---|---|---|---|---|
| Pb2+ | Cow Manure | 700 | - | 149.3 | [172] |
| Banana Peels | 600 | 2.5 | 247.1 | [173] | |
| Ragweed | 450 | - | 358.7 | [174] | |
| Gingko Leaf | 800 | 1 | 138.9 | [175] | |
| Cu2+ | Loofah | 700 | 0.5 | 54.68 | [176] |
| Banana Peels | 600 | 2.5 | 75.99 | [173] | |
| Ginkgo Leaf | 800 | 1 | 59.9 | [175] | |
| Cd2+ | Ragweed | 450 | - | 139 | [174] |
| Peanut Husk | - | 40 | 28.99 | [177] | |
| Green Waste | 600 | 2 | 6.72 | [178] | |
| Cr6+ | Rice Husk | 450–500 | 1 | 435.7 | [179] |
| Loofah | 700 | 0.5 | 30.14 | [176] | |
| Cr3+ | Sewage Sludge | 300 | 4 | - | [180] |
| Zn2+ | Rice Husk | 550 | 12.5 | 3.8 | [181] |
| As5+ | Papermill Sludge | 720 | 1 | 22.8 | [182] |
| AS3+ | Pine Wood | 400 | 10 | 1.78 | [183] |
| Organic Contaminants | Biochar Feedstock Type | Pyrolysis Temperature | Removal Efficiency % | References |
|---|---|---|---|---|
| Polyaromatic Hydrocarbons | Rice straw | 600 | 58.8 | [206] |
| Metalaxyl | Wood | 450 | 70.1 | [207] |
| Tiamethoxan | Sugarcane filter cake | 380 | 70 | [208] |
| Ibuprofen | Cotton gin waste | 700 | 50 | [209] |
| Diclofenac | Rice hull | 350 | 97 | [210] |
| Sulfamethoxasole | Bagasse | 600 | 83.3 | [211] |
| Sulfamethoxasole | Yeast | 900 | 98.97 | [212] |
| Norfloxacin | Wheat straw | 400 | 88.1 | [213] |
| Sulfadiazine | Corn straw | 350 | 74 | [214] |
| Tetracycline | Rice straw | 500 | 97 | [215] |
| Oxytetracycline | Sugarcane bagasse | 450 | 86 | [216] |
| Soil Type | Biochar Feedstock Type | Pyrolysis Temperature, °C | Biochar Properties | Impact of Incorporating Biochar into the Soil | References |
|---|---|---|---|---|---|
| Ultisol | Rice straw | 350 | pH = 9.94Total C = 48.71%O/TOC = 0.29 | Biochar had a more significant impact on phosphorus availability, soil chemical properties, microbial activity, and crop yields. It improved soil fertility by increasing available P, altering pH, and promoting beneficial microbial functional genes. | [244] |
| Chicken manure | 400 | pH = 9.97, EC = 5.03 (mS cm−1), total C = 28.8%, CEC = 95.7 (cmolc kg−1) | The addition of biochar increased soil pH and enhanced phosphorus (P) availability by raising water-extractable and labile P while reducing Fe- and Al-bound P fractions. Furthermore, it significantly improved maize growth, dry matter yield, and chlorophyll content. | [245] | |
| Peanut straw | 400–500 | pH = 10.54, alkalinity = 505 (cmolkg−1), total C = 44%, C/N = 15 | The application of biochar increased soil pH and reduced the concentrations of total Al, monomeric Al, and monomeric inorganic Al, resulting in higher canola seed and straw yields. | [246] | |
| Latosol | Coconut shell | 600 | A significant rise in the phosphorus (P), potassium (K), and magnesium (Mg) levels in the modified soil. | [247] | |
| Latosol | Filter cake | 550 | pH = 8.50, CE = 0.323 (mS cm−1), total C = 287.9 (g kg −1) | Biochar application improved soil chemical and microbiological properties, with the 1% dose enhancing pH, CEC, organic carbon, microbial biomass, and Ca content. Higher doses (4%) increased N and Mg levels, but 1% biochar and mineral fertilization showed the most significant overall benefits for soil quality. | [248] |
| Alfisol | Pigeon pea | 350–500 | pH = 8.52, EC = 2.16 g (dS m−1), total C = 69% | Biochar (BC) improved soil health by enhancing pH, carbon content, and nutrient cycling, which contributed to a 7–25% increase in onion growth, yield, and quality. | [249] |
| Canola stalk | 650 | pH = 11.0, TOC = 327 (g kg−1) | Biochar application in soil increased total P, Olsen P, and H2O-extractable P while enhancing Al-P, Fe-P, and Ca-P availability. Moreover, the positive correlation between biochar pH and soil P availability highlights its role in improving nutrient dynamics. | [250] | |
| Rice straw | 450 | pH = 10.63, TOC = 518.93 (g kg−1) | Biochar application enhances soil structure by increasing porosity, microporosity, and water-holding capacity, thereby improving plant-available water and soil resilience. | [251] |
| Electrode | Biochar Feedstock Type | Pyrolysis Conditions for Biochar Preparation | Power Density | References | |
|---|---|---|---|---|---|
| Temperature, °C | Time (h) | ||||
| Anode | Sewage Sludge | 900 | 2 h | 1069 mW/m2 | [286] |
| Microalgal | 900 | 1 h | 12.86 W/m3 | [305] | |
| Corn Straw | 900 | 1 h | 8.89 W/m3 | [306] | |
| Waste Food | 1000 | 1 h | - | [297] | |
| Deoiled Azolla Biomass | 600 | 3 h | - | [293] | |
| Cathode | Banana Peels | 900 | 2 h | 528.2 mW/m2 | [307] |
| Lotus Leaves | 900 | 2 h | 511.5 mW/m2 | [308] | |
| Eggplant | 800 | 1 h | 667 mW/m2 | [309] | |
| Pamelo Peel | 1000 | 1.5 h | 799 mW/m2 | [310] | |
| Olive Mill Waste | 800 | 45 min | 271 mW/m2 | [311] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derdag, S.M.; Ouazzani, N. Advancements in Sustainable Biochar Production from Waste: Pathways for Renewable Energy Generation and Environmental Remediation. Biomass 2025, 5, 32. https://doi.org/10.3390/biomass5020032
Derdag SM, Ouazzani N. Advancements in Sustainable Biochar Production from Waste: Pathways for Renewable Energy Generation and Environmental Remediation. Biomass. 2025; 5(2):32. https://doi.org/10.3390/biomass5020032
Chicago/Turabian StyleDerdag, Sara Mrhari, and Naaila Ouazzani. 2025. "Advancements in Sustainable Biochar Production from Waste: Pathways for Renewable Energy Generation and Environmental Remediation" Biomass 5, no. 2: 32. https://doi.org/10.3390/biomass5020032
APA StyleDerdag, S. M., & Ouazzani, N. (2025). Advancements in Sustainable Biochar Production from Waste: Pathways for Renewable Energy Generation and Environmental Remediation. Biomass, 5(2), 32. https://doi.org/10.3390/biomass5020032







