Metal-Ion Batteries
Definition
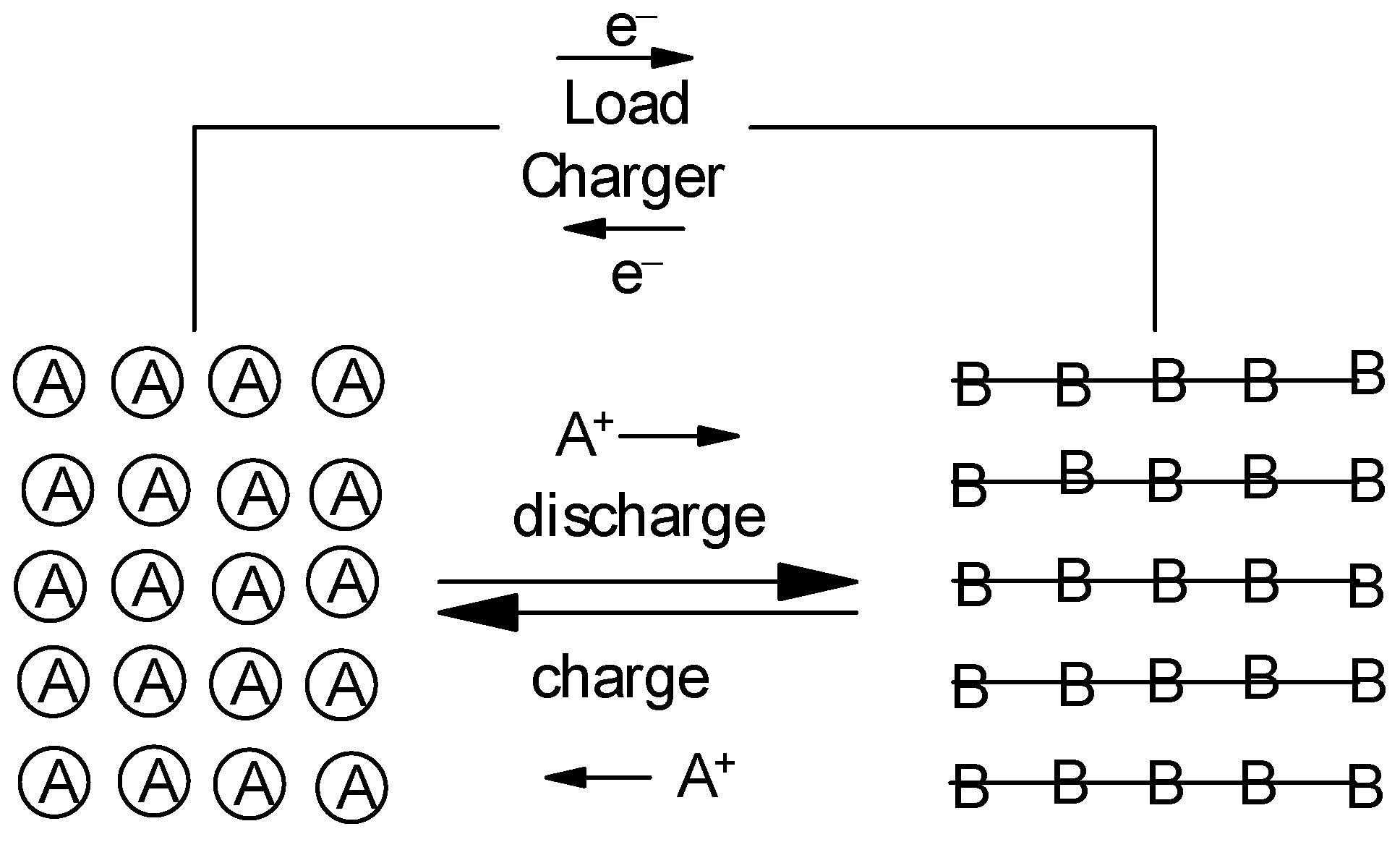
:1. Introduction
2. State of the Art
2.1. Monovalent Cations
2.2. Divalent Cations
2.3. Trivalent Cations
3. Trends and Developments
3.1. Cathode Materials
3.2. Metallic Anodes
3.3. Electrolyte Challenges
4. Conclusions and Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Wu, Y.; Holze, R. Electrochemical Energy Conversion and Storage; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Wu, Y.; Holze, R. Self-discharge in supercapacitors: Causes, effects and therapies: An overview. Electrochem. Energy Technol. 2021, 7, 1–37. [Google Scholar]
- Holze, R. Self-discharge of batteries: Causes, Mechanisms and Remedies. Adv. Mater. Sci. Technol. 2022. submitted. [Google Scholar]
- Biemolt, J.; Jungbacker, P.; van Teijlingen, T.; Yan, N.; Rothenberg, G. Beyond lithium-based batteries. Materials 2020, 13, 425. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Sun, M.; Liu, J. Recent progress in electrode materials for aqueous sodium and potassium ion batteries. Mater. Chem. Front. 2021, 5, 7384–7402. [Google Scholar] [CrossRef]
- Zhao, L.F.; Hu, Z.; Lai, W.H.; Tao, Y.; Peng, J.; Miao, Z.C.; Wang, Y.X.; Chou, S.L. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Wang, F.; Noori, A.; Mousavi, M.F.; Xia, X.; Zhang, Y. Recent Advances in Carbon Anodes for Sodium-Ion Batteries. Chem. Rec. 2022, 22, e202200083. [Google Scholar] [CrossRef]
- Lach, J.; Wróbel, K.; Wróbel, J.; Czerwński, A. Applications of carbon in rechargeable electrochemical power sources: A review. Energies 2021, 14, 2649. [Google Scholar] [CrossRef]
- Li, M.; Du, Z.; Khaleel, M.A.; Belharouak, I. Materials and engineering endeavors towards practical sodium-ion batteries. Energy Storage Mater. 2020, 25, 520–536. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. State-of-the-art electrode materials for sodium-ion batteries. Materials 2020, 13, 3453. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Mass‚, R.; Uchaker, E.; Cao, G. Beyond Li ion: Electrode materials for sodium and magnesium-ion batteries. In Nanomaterials for Energy Conversion and Storage; World Scientific: Singapore, 2017; pp. 639–755. [Google Scholar]
- Dong, S.; Lv, N.; Wu, Y.; Zhang, Y.; Zhu, G.; Dong, X. Titanates for sodium-ion storage. Nano Today 2022, 42, 101349. [Google Scholar] [CrossRef]
- Meng, Y.; Nie, C.; Guo, W.; Liu, D.; Chen, Y.; Ju, Z.; Zhuang, Q. Inorganic cathode materials for potassium ion batteries. Mater. Today Energy 2022, 25, 100982. [Google Scholar] [CrossRef]
- Liu, Z.G.; Du, R.; He, X.X.; Wang, J.C.; Qiao, Y.; Li, L.; Chou, S.L. Recent Progress on Intercalation-Based Anode Materials for Low-Cost Sodium-Ion Batteries. Chem. Sus. Chem. 2021, 14, 3724–3743. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, S.; Hou, Y.; Sun, S.; Zhang, J.; Zhao, Y. Recent Progress in Amorphous Carbon-Based Materials for Anodes of Sodium-Ion Batteries: Synthesis Strategies, Mechanisms, and Performance. Chem. Sus. Chem. 2021, 14, 3693–3723. [Google Scholar] [CrossRef]
- Gebert, F.; Knott, J.; Gorkin III, R.; Chou, S.L.; Dou, S.X. Polymer electrolytes for sodium-ion batteries. Energy Storage Mater. 2021, 36, 10–30. [Google Scholar] [CrossRef]
- Liu, M.; Ao, H.; Jin, Y.; Hou, Z.; Zhang, X.; Zhu, Y.; Qian, Y. Aqueous rechargeable sodium ion batteries: Developments and prospects. Mater. Today Energy 2020, 17, 100432. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, H.; Xue, M. Research Progress and Practical Challenges of Aqueous Sodium-Ion Batteries. Acta Chim. Sin. 2021, 79, 388–405. [Google Scholar] [CrossRef]
- Hijazi, H.; Desai, P.; Mariyappan, S. Non-Aqueous Electrolytes for Sodium-Ion Batteries: Challenges and Prospects Towards Commercialization. Batter. Supercaps 2021, 4, 881–896. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Zhu, K.; Zhang, Z.; Si, Y.; Wang, Y.; Jiao, L. Solid-State Electrolytes for Sodium Metal Batteries. Energy Fuels 2021, 35, 9063–9079. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, D.; Rui, X.; Yu, Y.; Huang, S. Advanced cathodes for potassium-ion battery. Curr. Opin. Electrochem. 2019, 18, 24–30. [Google Scholar] [CrossRef]
- Chu, S.; Guo, S.; Zhou, H. Advanced cobalt-free cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2021, 50, 13189–13235. [Google Scholar] [CrossRef] [PubMed]
- Holze, R. Conjugated Molecules and Polymers in Secondary Batteries: A Perspective. Molecules 2022, 27, 546. [Google Scholar] [CrossRef]
- Holguin, K.; Mohammadiroudbari, M.; Qin, K.; Luo, C. Organic electrode materials for non-aqueous, aqueous, and all-solid-state Na-ion batteries. J. Mater. Chem. A 2021, 9, 19083–19115. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Fan, L.; Hu, Y.; Rao, A.M.; Zhou, J.; Hou, Z.; Wang, C.; Lu, B. Prospects of Electrode Materials and Electrolytes for Practical Potassium-Based Batteries. Small Methods 2021, 5, 2101131. [Google Scholar] [CrossRef]
- Xu, Y.S.; Duan, S.Y.; Sun, Y.G.; Bin, D.S.; Tao, X.S.; Zhang, D.; Liu, Y.; Cao, A.M. Recent developments in electrode materials for potassium-ion batteries. J. Mater. Chem. A 2019, 7, 4334–4352. [Google Scholar] [CrossRef]
- An, Y.; Liu, Y.; Tian, Y.; Xu, X.; Ma, Y.; Wei, H.; Ma, C.; Feng, J. Recent development and prospect of potassium-ion batteries with high energy and high safety for post-lithium batteries. Funct. Mater. Lett. 2019, 12, 1930002. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Suo, G.; Wang, W.A.; Xi, K.; Iqbal, S.B. Improvement in potassium ion batteries electrodes: Recent developments and efficient approaches. J. Energy Chem. 2021, 62, 307–337. [Google Scholar] [CrossRef]
- John, B.; Anoopkumar, V.; Mercy, T.D. Potassium-ion batteries: Key to future large-scale energy storage? ACS Appl. Energy Mater. 2020, 3, 9478–9492. [Google Scholar]
- Wu, Z.; Zou, J.; Chen, S.; Niu, X.; Liu, J.; Wang, L. Potassium-ion battery cathodes: Past, present, and prospects. J. Power Sources 2021, 484, 229307. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An Initial Review of the Status of Electrode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.; Ji, X.; Jia, C.; Wang, H. Advancements and Challenges in Potassium Ion Batteries: A Comprehensive Review. Adv. Funct. Mater. 2020, 30, 1909486. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.; Shao, Y.; Shen, M.; Du, L.; Liao, S. Potassium-Ion Battery and Its Recent Research Progress. Prog. Chem. 2019, 31, 1329–1340. [Google Scholar]
- Eftekhari, A.; Jian, Z.; Ji, X. Potassium Secondary Batteries. ACS Appl. Mater. Interf. 2017, 9, 4404–4419. [Google Scholar] [CrossRef]
- Wang, B.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Liu, Q.; Li, C.C. Post-Lithium-Ion Battery Era: Recent Advances in Rechargeable Potassium-Ion Batteries. Chem. Eur. J. 2021, 27, 512–536. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.C.; Bianchini, M.; Seo, D.H.; Rodriguez-Garcia, J.; Ceder, G. Recent Progress and Perspective in Electrode Materials for K-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702384. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Wang, W.; Bayhan, Z.; Alshareef, H.N. Status of rechargeable potassium batteries. Nano Energy 2021, 83, 105792. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Recent Progress in Rechargeable Potassium Batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Zhang, J.; Lai, L.; Wang, H.; Chen, M.; Shen, Z.X. Energy storage mechanisms of anode materials for potassium ion batteries. Mater. Today Energy 2021, 21, 100747. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Kim, K.H.; Kim, J.; Jung, H.G.; Sun, Y.K. State-of-the-art anodes of potassium-ion batteries: Synthesis, chemistry, and applications. Chem. Sci. 2021, 12, 7623–7655. [Google Scholar] [CrossRef]
- Ma, L.; Lv, Y.; Wu, J.; Xia, C.; Kang, Q.; Zhang, Y.; Liang, H.; Jin, Z. Recent advances in anode materials for potassium-ion batteries: A review. Nano Res. 2021, 14, 4442–4470. [Google Scholar] [CrossRef]
- Guo, K.; Wang, W.; Jiao, S. Recent progress and prospective on layered anode materials for potassium-ion batteries. Int. J. Miner. Metall. Mater. 2022, 29, 1037–1052. [Google Scholar] [CrossRef]
- Li, W.; Bi, Z.; Zhang, W.; Wang, J.; Rajagopalan, R.; Wang, Q.; Zhang, D.; Li, Z. Advanced cathodes for potassium-ion batteries with layered transition metal oxides: A review. J. Mater. Chem. A 2021, 9, 8221–8247. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Henzie, J.; Zhang, J.; Ha, J.; Amin, M.A.; Hossain, M.S.S.A.; Jun, S.C.; Yamauchi, Y. Recent Advances and Perspectives of Battery-Type Anode Materials for Potassium Ion Storage. ACS Nano 2021, 15, 18931–18973. [Google Scholar] [CrossRef]
- Liao, J.; Han, Y.; Zhang, Z.; Xu, J.; Li, J.; Zhou, X. Recent Progress and Prospects of Layered Cathode Materials for Potassium-ion Batteries. Energy Environ. Mater. 2021, 4, 178–200. [Google Scholar] [CrossRef]
- Ramesh, A.; Tripathi, A.; Balaya, P. A mini review on cathode materials for sodium-ion batteries. Int. J. Appl. Ceram. Technol. 2022, 19, 913–923. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Passerini, S. Non-aqueous potassium-ion batteries: A review. Curr. Opin. Electrochem. 2018, 9, 41–48. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Li, Y.; Liu, M.; Feng, X.; Bai, Y.; Wu, C. Ether-based electrolytes for sodium ion batteries. Chem. Soc. Rev. 2022, 51, 4484–4536. [Google Scholar] [CrossRef]
- Mao, J.; Wang, C.; Lyu, Y.; Zhang, R.; Wang, Y.; Liu, S.; Wang, Z.; Zhang, S. Organic electrolyte design for practical potassium-ion batteries. J. Mater. Chem. A. 2022, 10. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, W.; Zhang, Q. Organic Materials as Electrodes in Potassium-Ion Batteries. Chem. Eur. J. 2021, 27, 6131–6144. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, Y.; Wang, C. Emerging organic potassium-ion batteries: Electrodes and electrolytes. J. Mater. Chem. A 2020, 8, 15547–15574. [Google Scholar] [CrossRef]
- Min, X.; Xiao, J.; Fang, M.; Wang, W.; Zhao, Y.; Liu, Y.; Abdelkader, A.M.; Xi, K. Potassium-ion batteries: Outlook on present and future technologies. Energy Environ. Sci. 2021, 14, 2186–2243. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, K.; Pei, P.; Wei, M.; Liu, X.; Xiao, Y.; Zhang, P. Zinc dendrite growth and inhibition strategies. Mater. Today Energy 2021, 20, 100692. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X. Toward Long-Life Aqueous Zinc Ion Batteries by Constructing Stable Zinc Anodes. Chem. Rec. 2022, 22, e202200088. [Google Scholar] [CrossRef]
- Huang, J.; Qiu, X.; Wang, N.; Wang, Y. Aqueous rechargeable zinc batteries: Challenges and opportunities. Curr. Opin. Electrochem. 2021, 30, 100801. [Google Scholar] [CrossRef]
- Yang, J.; Yin, B.; Sun, Y.; Pan, H.; Sun, W.; Jia, B.; Zhang, S.; Ma, T. Zinc Anode for Mild Aqueous Zinc-Ion Batteries: Challenges, Strategies, and Perspectives. Nano Micro Lett. 2022, 14, 42. [Google Scholar] [CrossRef]
- Zuo, S.; Xu, X.; Ji, S.; Wang, Z.; Liu, Z.; Liu, J. Cathodes for Aqueous Zn-Ion Batteries: Materials, Mechanisms, and Kinetics. Chem. Eur. J. 2021, 27, 830–860. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; He, W.; Xu, G.; Sun, R. Cathode materials for rechargeable zinc-ion batteries: From synthesis to mechanism and applications. J. Power Sources 2020, 449, 227596. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S. Advances and Perspectives of Cathode Storage Chemistry in Aqueous Zinc-Ion Batteries. ACS Nano 2021, 15, 9244–9272. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, F.; Arandiyan, H.; Guan, P.; Liu, Y.; Wang, Y.; Zhao, C.; Wang, D. Oxide-based cathode materials for rechargeable zinc ion batteries: Progresses and challenges. J. Energy Chem. 2021, 57, 516–542. [Google Scholar] [CrossRef]
- Ma, N.; Wu, P.; Wu, Y.; Jiang, D.; Lei, G. Progress and perspective of aqueous zinc-ion battery. Funct. Mater. Lett. 2019, 12, 1930003. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y. Recent Progress on Zinc-Ion Rechargeable Batteries. Nano Micro Lett. 2019, 11, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.P.; Fu, N.; Zhou, W.B.; Liu, B.; Deng, Q.; Wu, X.W. Comprehensive review on zinc-ion battery anode: Challenges and strategies. InfoMat 2022, 4, e12306. [Google Scholar] [CrossRef]
- Wang, N.; Wan, H.; Duan, J.; Wang, X.; Tao, L.; Zhang, J.; Wang, H. A review of zinc-based battery from alkaline to acid. Mater. Today Adv. 2021, 11, 100149. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, Y.; Ma, L.; Zhi, C.; Chen, S. Recent Advances in Electrolytes for “Beyond Aqueous” Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2106409. [Google Scholar] [CrossRef]
- Borchers, N.; Clark, S.; Horstmann, B.; Jayasayee, K.; Juel, M.; Stevens, P. Innovative zinc-based batteries. J. Power Sources 2021, 484, 229309. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Guo, M.; Zhang, G.; Wang, Y. Research Progresses and Challenges of Flexible Zinc Battery. Front. Chem. 2022, 10, 827563. [Google Scholar] [CrossRef]
- Ouchi, T.; Kim, H.; Spatocco, B.L.; Sadoway, D.R. Calcium-based multi-element chemistry for grid-scale electrochemical energy storage. Nat. Commun. 2016, 7, 10999. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; He, H.; Yao, W.; Tang, Y. Recent Advances and Perspectives on Calcium-Ion Storage: Key Materials and Devices. Adv. Mater. 2021, 33, 2005501. [Google Scholar] [CrossRef] [PubMed]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-Ion Batteries: Current State-of-the-Art and Future Perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef] [PubMed]
- Huie, M.M.; Bock, D.C.; Takeuchi, E.S.; Marschilok, A.C.; Takeuchi, K.J. Cathode materials for magnesium and magnesium-ion based batteries. Coord. Chem. Rev. 2015, 287, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.; Datta, M.K.; Velikokhatnyi, O.I.; Manivannan, A.; Alman, D.; Kumta, P.N. Rechargeable magnesium battery: Current status and key challenges for the future. Prog. Mater. Sci. 2014, 66, 1–86. [Google Scholar] [CrossRef]
- Foot, P.J.S. Principles and prospects of high-energy magnesium-ion batteries. Sci. Prog. 2015, 98, 264–275. [Google Scholar] [CrossRef]
- Rashad, M.; Asif, M.; Wang, Y.; He, Z.; Ahmed, I. Recent advances in electrolytes and cathode materials for magnesium and hybrid-ion batteries. Energy Storage Mater. 2020, 25, 342–375. [Google Scholar] [CrossRef]
- Medina, A.; Pérez-Vicente, C.; Alcántara, R. Advancing towards a practical magnesium ion battery. Materials 2021, 14, 7488. [Google Scholar] [CrossRef]
- Guo, M.; Yuan, C.; Zhang, T.; Yu, X. Solid-State Electrolytes for Rechargeable Magnesium-Ion Batteries: From Structure to Mechanism. Small 2022, 18, 2106981. [Google Scholar] [CrossRef]
- Jaschin, P.W.; Gao, Y.; Li, Y.; Bo, S.H. A materials perspective on magnesium-ion-based solid-state electrolytes. J. Mater. Chem. A 2020, 8, 2875–2897. [Google Scholar] [CrossRef]
- Rubio, S.; Medina, A.; Cabello, M.; Lavela, P.; Alcántara, R.; Vicénte, C.P.; Ortiz, G.F.; Tirado, J.L. Inorganic solids for dual magnesium and sodium battery electrodes. J. Solid State Electr. 2020, 24, 2565–2573. [Google Scholar] [CrossRef]
- Craig, B.; Schoetz, T.; Cruden, A.; Ponce de Leon, C. Review of current progress in non-aqueous aluminium batteries. Renew. Sustain. Energy Rev. 2020, 133, 110100. [Google Scholar] [CrossRef]
- Das, S.K.; Mahapatra, S.; Lahan, H. Aluminium-ion batteries: Developments and challenges. J. Mater. Chem. A 2017, 5, 6347–6367. [Google Scholar] [CrossRef]
- Ru, Y.; Zheng, S.; Xue, H.; Pang, H. Different positive electrode materials in organic and aqueous systems for aluminium ion batteries. J. Mater. Chem. A 2019, 7, 14391–14418. [Google Scholar] [CrossRef]
- Pang, X.; An, B.; Zheng, S.; Wang, B. Cathode materials of metal-ion batteries for low-temperature applications. J. Alloys Compd. 2022, 912, 165142. [Google Scholar] [CrossRef]
- Suo, G.; Cheng, Y.; Zhang, J.; Ahmed, S.M. Recent Progress and Perspectives on Alloying Anodes for Potassium-Ion Batteries. Chem. Nano Mat. 2021, 7, 1291–1308. [Google Scholar] [CrossRef]
- Zheng, S.M.; Tian, Y.R.; Liu, Y.X.; Wang, S.; Hu, C.Q.; Wang, B.; Wang, K.M. Alloy anodes for sodium-ion batteries. Rare Met. 2021, 40, 272–289. [Google Scholar] [CrossRef]
- Li, X.Y.; Qu, J.K.; Yin, H.Y. Electrolytic alloy-type anodes for metal-ion batteries. Rare Met. 2021, 40, 329–352. [Google Scholar] [CrossRef]
- Erickson, E.M.; Markevich, E.; Salitra, G.; Sharon, D.; Hirshberg, D.; De La Llave, E.; Shterenberg, I.; Rozenman, A.; Frimer, A.; Aurbach, D. Development of advanced rechargeable batteries: A continuous challenge in the choice of suitable electrolyte solutions. J. Electrochem. Soc. 2015, 162, A2424–A2438. [Google Scholar] [CrossRef]
- Lu, J.; Jaumaux, P.; Wang, T.; Wang, C.; Wang, G. Recent progress in quasi-solid and solid polymer electrolytes for multivalent metal-ion batteries. J. Mater. Chem. A 2021, 9, 24175–24194. [Google Scholar] [CrossRef]
- Liu, C.; Xie, X.; Lu, B.; Zhou, J.; Liang, S. Electrolyte Strategies toward Better Zinc-Ion Batteries. ACS Energy Lett. 2021, 6, 1015–1033. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, X.; Yang, Z.; Xia, M.; Xu, C.; Liu, Y.; Yu, H.; Zhang, L. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coord. Chem. Rev. 2022, 452, 214297. [Google Scholar] [CrossRef]
- Wu, X.; Dou, Y.; Lian, R.; Wang, Y.; Wie, Y. Understanding rechargeable magnesium ion batteries via first-principles computations: A comprehensive review. Energy Storage Mater. 2022, 48, 344–355. [Google Scholar] [CrossRef]

| Element | Atomic Mass | E00, SHE/V | Gravimetric Capacity/mAh·g−1 | Volumetric Capacity/mAh·cm−3 |
|---|---|---|---|---|
| Li | 6.94 | −3.040 | 3860 | 2061 |
| Na | 23.0 | −2.713 | 1165 | 1129 |
| K | 39.1 | −2.924 | 685 | 610 |
| Mg | 24.31 | −2.356 | 2206 | 3834 |
| Ca | 40.08 | −2.840 | 1337 | 2072 |
| Zn | 65.41 | −0.763 | 820 | 5855 |
| Al | 26.98 | −1.676 | 2980 | 8046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Holze, R. Metal-Ion Batteries. Encyclopedia 2022, 2, 1611-1623. https://doi.org/10.3390/encyclopedia2030110
Liu Y, Holze R. Metal-Ion Batteries. Encyclopedia. 2022; 2(3):1611-1623. https://doi.org/10.3390/encyclopedia2030110
Chicago/Turabian StyleLiu, Yi, and Rudolf Holze. 2022. "Metal-Ion Batteries" Encyclopedia 2, no. 3: 1611-1623. https://doi.org/10.3390/encyclopedia2030110
APA StyleLiu, Y., & Holze, R. (2022). Metal-Ion Batteries. Encyclopedia, 2(3), 1611-1623. https://doi.org/10.3390/encyclopedia2030110








